Examining the Utility of Rapid Salivary C-Reactive Protein as a Predictor for Neonatal Sepsis: An Analytical Cross-Sectional Pilot Study
Abstract
1. Introduction
2. Materials and Methods
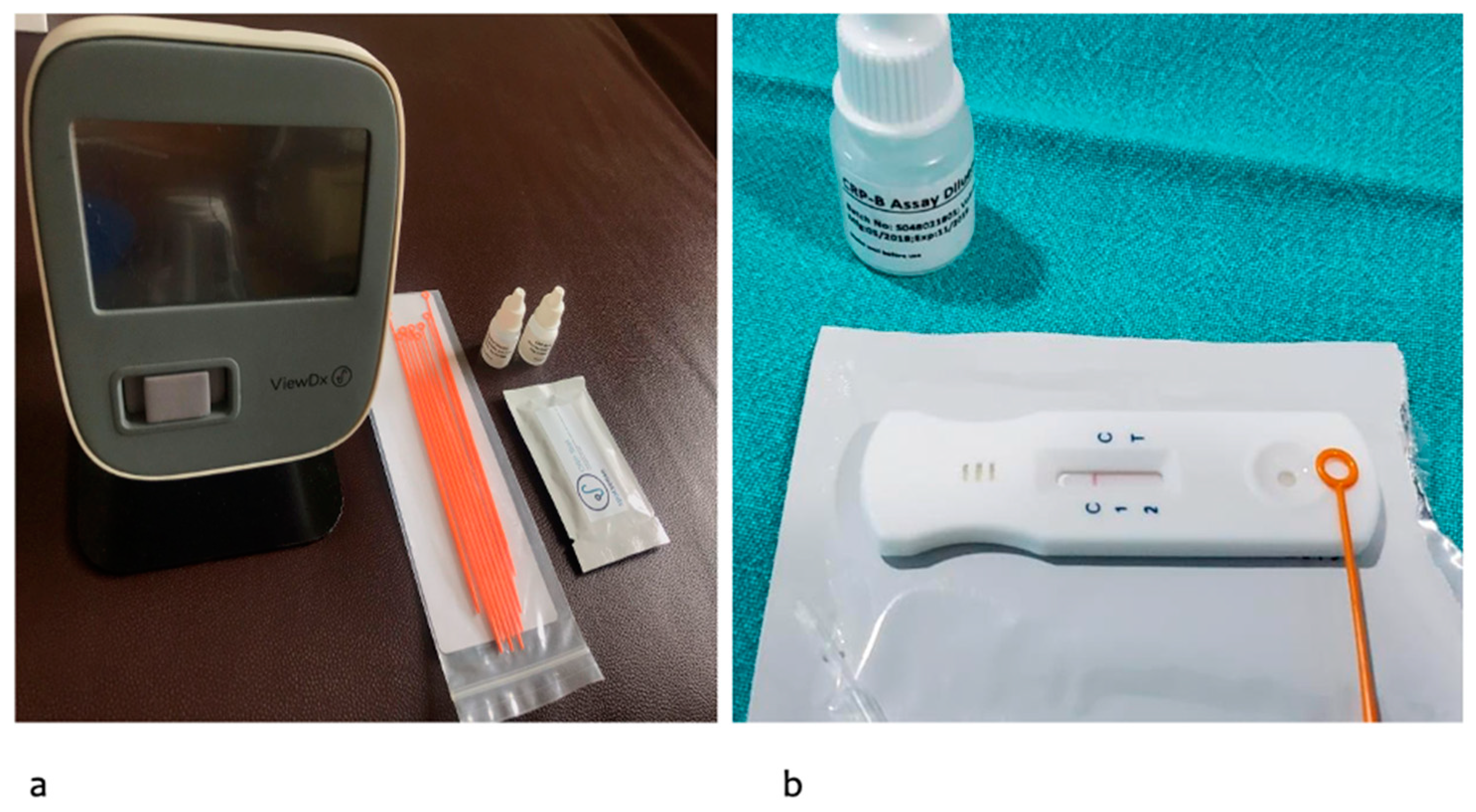
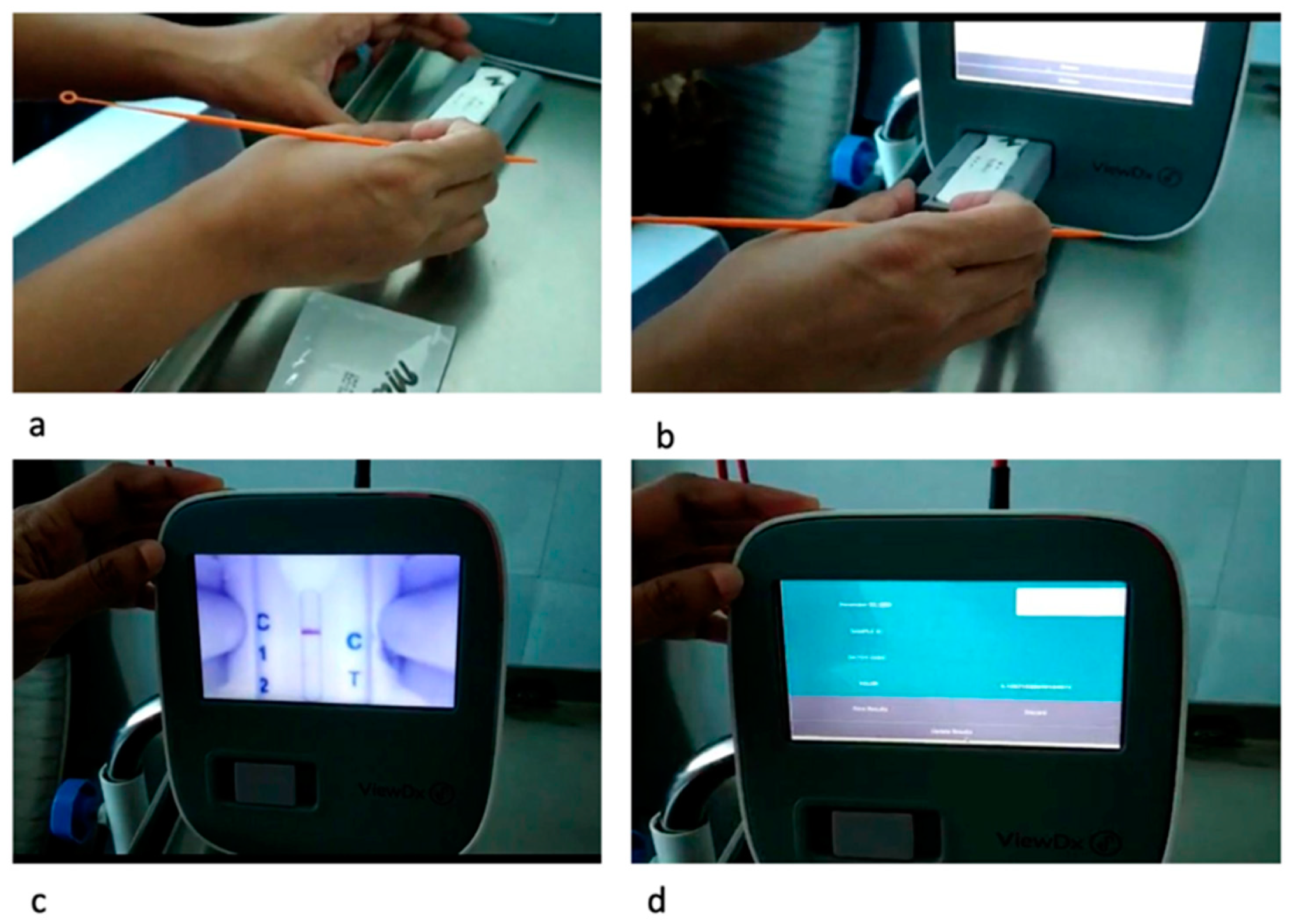
2.1. Salivary CRP Diagnostic Kit and Measurement
2.2. Serum CRP Measurements
2.3. Statistical Analysis
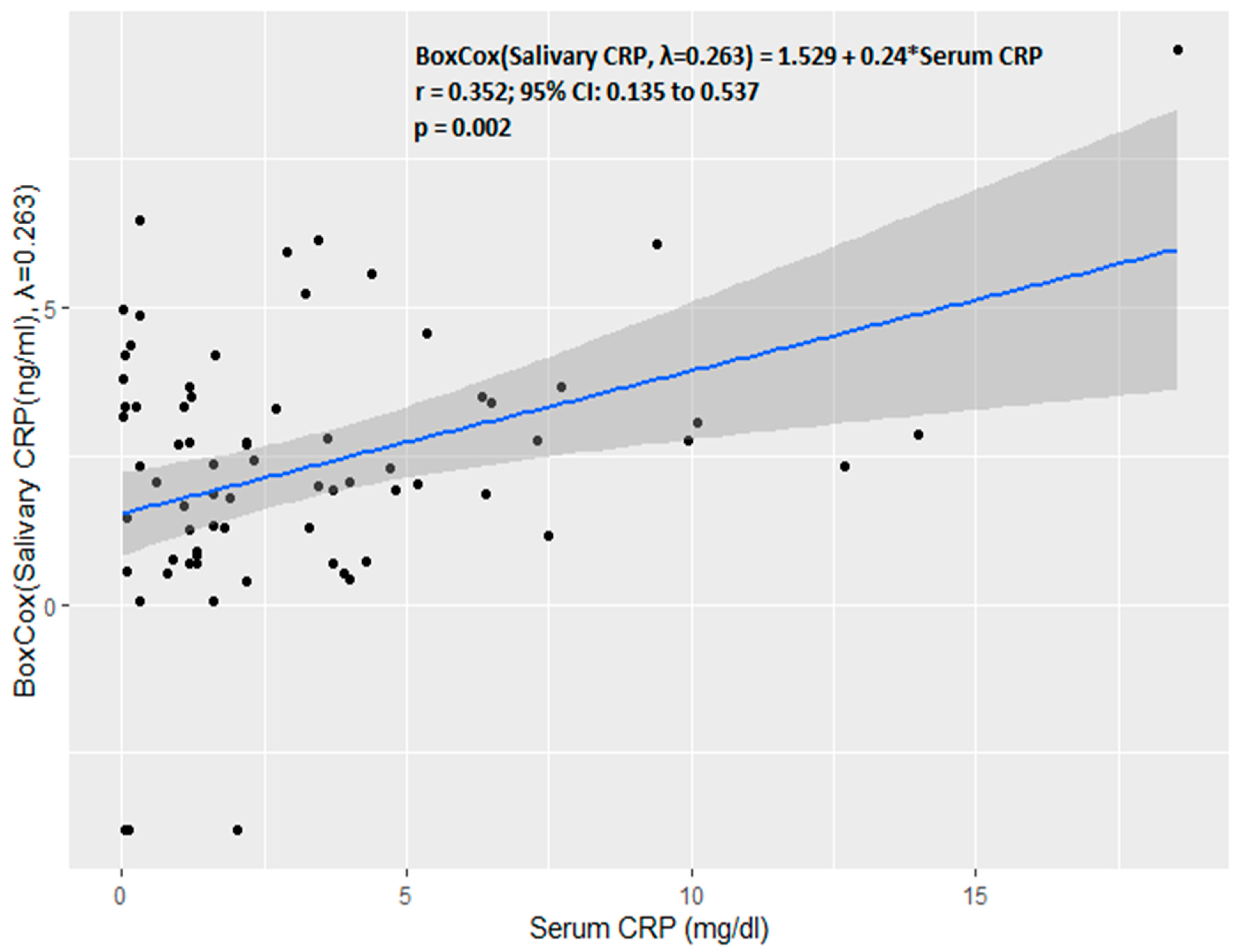
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2015, 385, 430–440. [Google Scholar] [CrossRef]
- Jain, K.; Sankar, M.J.; Nangia, S.; Ballambattu, V.B.; Sundaram, V.; Ramji, S.; Plakkal, N.; Kumar, P.; Jain, A.; Sivanandan, S.; et al. Causes of death in preterm neonates (<33 weeks) born in tertiary care hospitals in India: Analysis of three large prospective multicentric cohorts. J. Perinatol. 2019, 39, 13–19. [Google Scholar] [CrossRef]
- Fleischmann-Struzek, C.; Goldfarb, D.M.; Schlattmann, P.; Schlapbach, L.J.; Reinhart, K.; Kissoon, N. The global burden of pediatric and neonatal sepsis: A systematic review. Lancet Respir. Med. 2018, 6, 223–230. [Google Scholar] [CrossRef]
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef]
- Neonatal Infection: Antibiotics for Prevention and Treatment. NICE Guideline [NG195] Published: 20 April 2021. Available online: www.nice.org.uk (accessed on 10 October 2021).
- Philip, A.G. Detection of neonatal sepsis of late onset. JAMA 1982, 247, 489–492. [Google Scholar] [CrossRef]
- Hofer, N.; Zacharias, E.; Müller, W.; Resch, B. An Update on the Use of C-Reactive Protein in Early-Onset Neonatal Sepsis: Current Insights and New Tasks. Neonatology 2012, 102, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Benitz, W.E. Adjunct Laboratory Tests in the Diagnosis of Early-Onset Neonatal Sepsis. Clin. Perinatol. 2010, 37, 421–438. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Al-Matary, A.; Al Sulaiman, M.; Al-Otaiby, S.; Qaraqei, M.; Al-Matary, M. Association between the timing of antibiotics admin-istration and outcome of neonatal sepsis. J. Infect. Public Health 2022, 15, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Anne, R.P.; Deshabhotla, S.; Ahmed, S.W.; Ahmed, S.J.; Reddy, N.; Farooqui, D.; Oleti, T.P. A quality improvement initiative to improve management of procedural pain in preterm neonates. Pediatr. Anesthesia 2020, 31, 221–229. [Google Scholar] [CrossRef]
- Lemyre, B.; Sample, M.; Lacaze-Masmonteil, T.; Society, C.P. Fetus and Newborn Committee Minimizing blood loss and the need for transfusions in very premature infants. Paediatr. Child Health 2015, 20, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Küng, E.; Smajlhodzic, M.; Domazet, S.; Friedl, H.; Angerer, J.; Wisgrill, L.; Berger, A.; Bingle, L.; Peham, J.; et al. Directed Transport of CRP Across In Vitro Models of the Blood-Saliva Barrier Strengthens the Feasibility of Salivary CRP as Biomarker for Neonatal Sepsis. Pharmaceutics 2021, 13, 256. [Google Scholar] [CrossRef]
- Weitkamp, J.-H.; Aschner, J.L. Diagnostic Use of C-Reactive Protein (CRP) in Assessment of Neonatal Sepsis. Neoreviews 2005, 6, e508–e515. [Google Scholar] [CrossRef]
- Iyengar, A.; Paulus, J.K.; Gerlanc, D.J.; Maron, J.L. Detection and Potential Utility of C-Reactive Protein in Saliva of Neonates. Front. Pediatr. 2014, 2, 131. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.; Maaroof, A.; Saleh, M.H.; Abdelwahab, A. Salivary C-reactive protein, mean platelet volume and neutrophil lym-phocyte ratio as diagnostic markers for neonatal sepsis. J. Pediatr. 2018, 94, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Datla, S.; Kitchanan, S.; Sethuraman, G. Diagnostic Reliability of Salivary C-Reactive Protein as an Alternative Noninvasive Biomarker of Neonatal Sepsis. Indian Pediatr. 2021, 58, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Pay, J.B.; Shaw, A.M. Towards salivary C-reactive protein as a viable biomarker of systemic inflammation. Clin. Biochem. 2019, 68, 1–8. [Google Scholar] [CrossRef]
- Galhardo, L.F.; Ruivo, G.F.; De Oliveira, L.D.; Parize, G.; Dos Santos, S.S.F.; Pallos, D.; Leão, M.V.P. Inflammatory markers in saliva for diagnosis of sepsis of hospitalizes patients. Eur. J. Clin. Investig. 2020, 50, e13219. [Google Scholar] [CrossRef]
- Tosson, A.M.; Koptan, D.; Aal, R.A.; Elhady, M.A. Evaluation of serum and salivary C-reactive protein for diagnosis of late-onset neonatal sepsis: A single center cross-sectional study. J. Pediatr. 2021, 97, 623–628. [Google Scholar] [CrossRef]
- Omran, A.; Ali, M.; Mohammad, M.H.S.; Zekry, O. Salivary C-reactive protein and mean platelet volume in diagnosis of late-onset neonatal pneumonia. Clin. Respir. J. 2017, 12, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Barekatain, B.; HasanGhalyaei, N.; Mohammadizadeh, M.; Tavakolifard, N. Investigation of salivary C-reactive protein and in-terleukin-18 for the diagnosis of neonatal sepsis. J. Res. Med.Sci. Off. J. Isfahan Univ. Med. Sci. 2021, 26, 131. [Google Scholar]

| Parameter | Variable (n = 74) |
|---|---|
| Gestational age (weeks) a | 34.1 ± 4.8 |
| Birth weight (g) b | 2370 (1067–3182) |
| Male sex c | 44 (59.5) |
| Meconium-stained liquor c | 10 (13.5%) |
| PROM c | 23 (31) |
| Maternal urinary tract infection at delivery c | 3 (1.4) |
| Chorioamnionitis c | 14 (18.9) |
| Mode of delivery: Caesarean section c | 44 (59.5) |
| Required resuscitation c | 13 (17.6) |
| NICU admission c | 69 (93.2) |
| Leukopenia c | 8 (10.8) |
| Neutropenia c | 6 (8.1) |
| Received any respiratory support c | 64 (86) |
| CPAP/HFNC c | 49 (66) |
| Ventilation c | 15 (20) |
| Day of testing b | 1 (1–4) |
| Test Variable | Blood Culture Positive Sepsis | Probable Sepsis | Neonates with Only Risk Factors | p-Value |
|---|---|---|---|---|
| Serum CRP (mg/dL) b | 3.87 (2–8.1) | 1.8 (0.75–4) | 0.1 (0.04–0.3) | <0.001 |
| Salivary CRP | 21 (7.9–38) | 6.13 (2.2–12) | 5.1 (1.3–12.5) | <0.001 |
| (ng/mL) b |
| Serum CRP | Salivary CRP | |
|---|---|---|
| Area under the curve (AUC) | 0.72 (0.58–0.86) | 0.83 (0.70–0.97) |
| Optimal cut-off point in the study population | 2.8 mg/dL | 11.6 ng/mL |
| Sensitivity (%) | 72 (46–90) | 72 (46–90) |
| Specificity (%) | 70 (56–81) | 89 (78–96) |
| Positive predictive value (%) | 43 (32–56) | 68 (49–83) |
| Negative predictive value (%) | 89 (78–94) | 91 (83–95) |
| Accuracy (%) | 70 (59–80) | 85 (75–92) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramavath, C.; Katam, S.K.; Vardhelli, V.; Deshabhotla, S.; Oleti, T.P. Examining the Utility of Rapid Salivary C-Reactive Protein as a Predictor for Neonatal Sepsis: An Analytical Cross-Sectional Pilot Study. Diagnostics 2023, 13, 867. https://doi.org/10.3390/diagnostics13050867
Ramavath C, Katam SK, Vardhelli V, Deshabhotla S, Oleti TP. Examining the Utility of Rapid Salivary C-Reactive Protein as a Predictor for Neonatal Sepsis: An Analytical Cross-Sectional Pilot Study. Diagnostics. 2023; 13(5):867. https://doi.org/10.3390/diagnostics13050867
Chicago/Turabian StyleRamavath, Chaitra, Shravan Kumar Katam, Venkateshwarlu Vardhelli, Saikiran Deshabhotla, and Tejo Pratap Oleti. 2023. "Examining the Utility of Rapid Salivary C-Reactive Protein as a Predictor for Neonatal Sepsis: An Analytical Cross-Sectional Pilot Study" Diagnostics 13, no. 5: 867. https://doi.org/10.3390/diagnostics13050867
APA StyleRamavath, C., Katam, S. K., Vardhelli, V., Deshabhotla, S., & Oleti, T. P. (2023). Examining the Utility of Rapid Salivary C-Reactive Protein as a Predictor for Neonatal Sepsis: An Analytical Cross-Sectional Pilot Study. Diagnostics, 13(5), 867. https://doi.org/10.3390/diagnostics13050867





