Handheld Echocardiography Measurements Concordance and Findings Agreement: An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
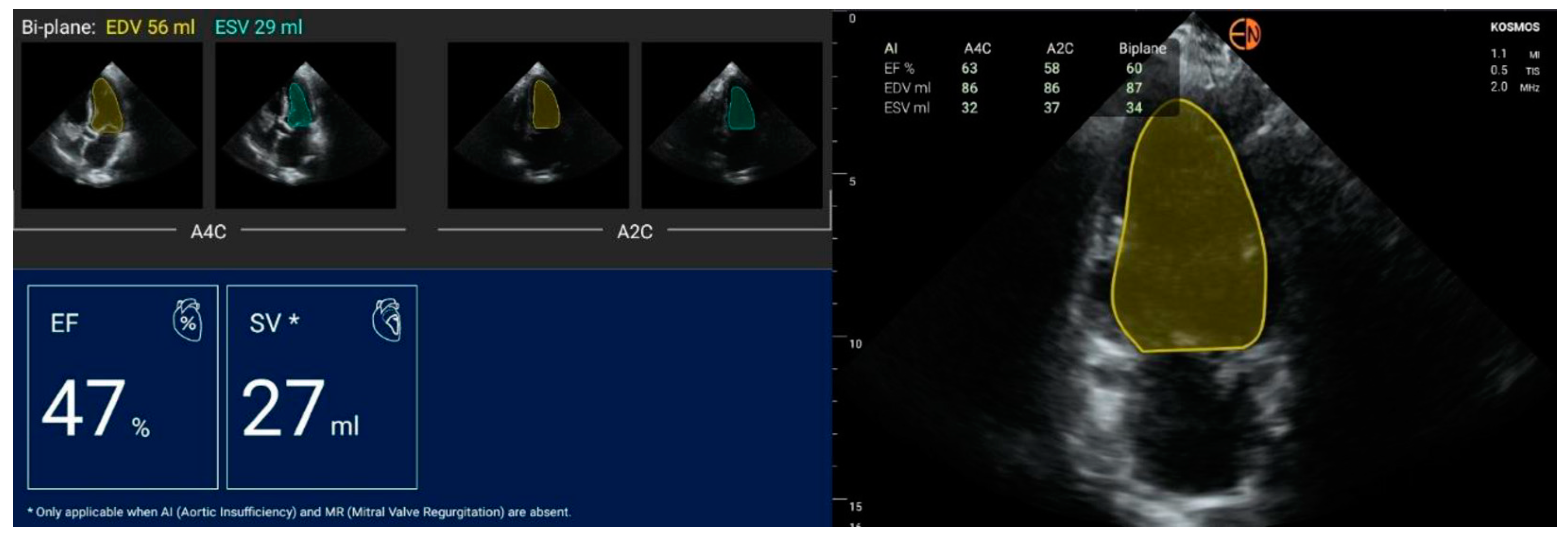
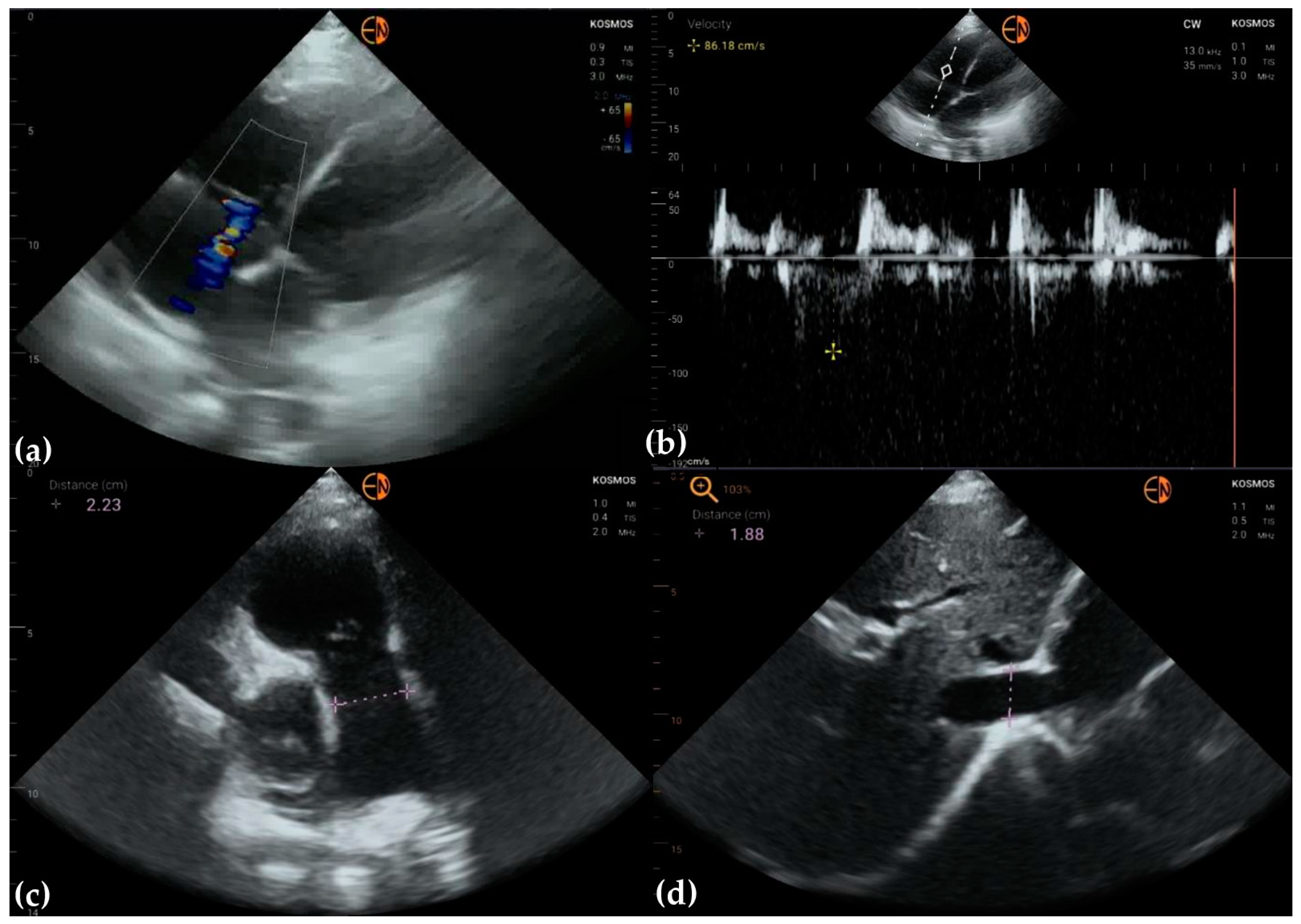
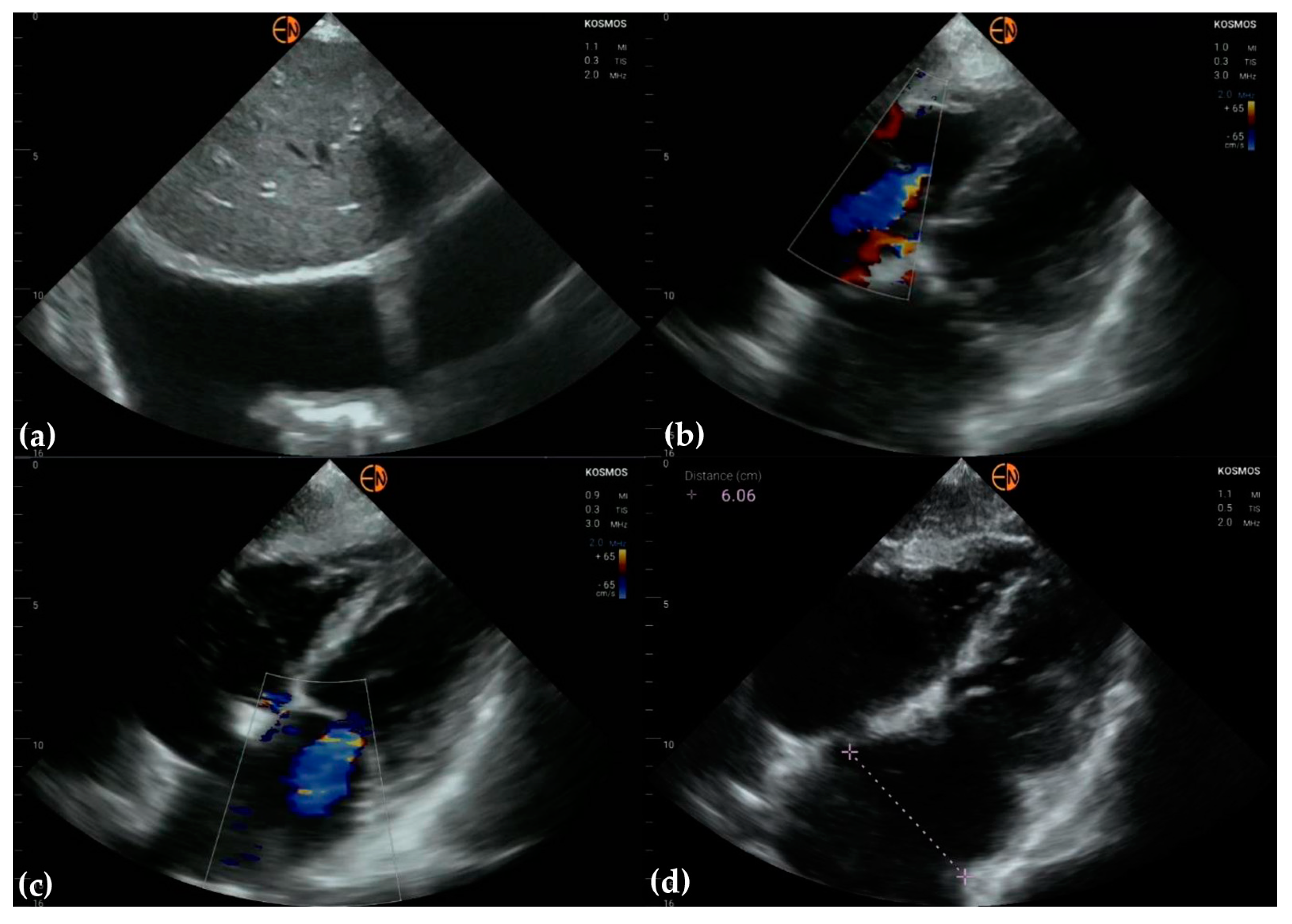
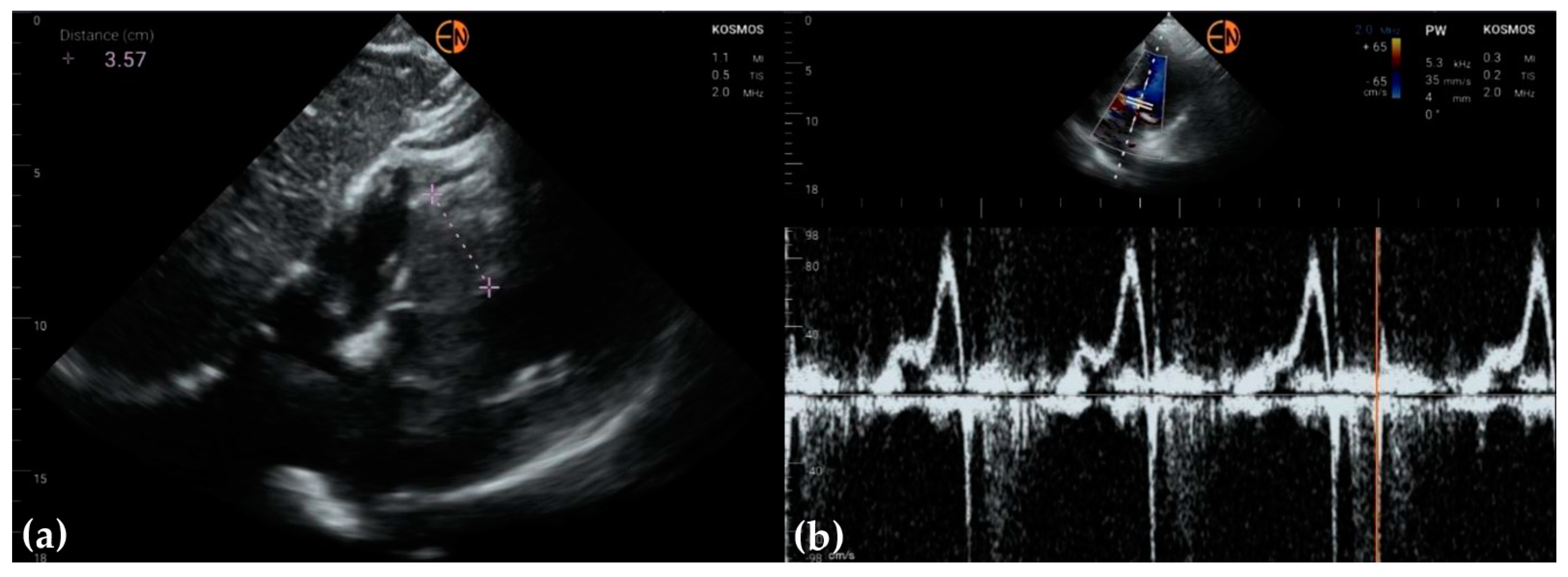
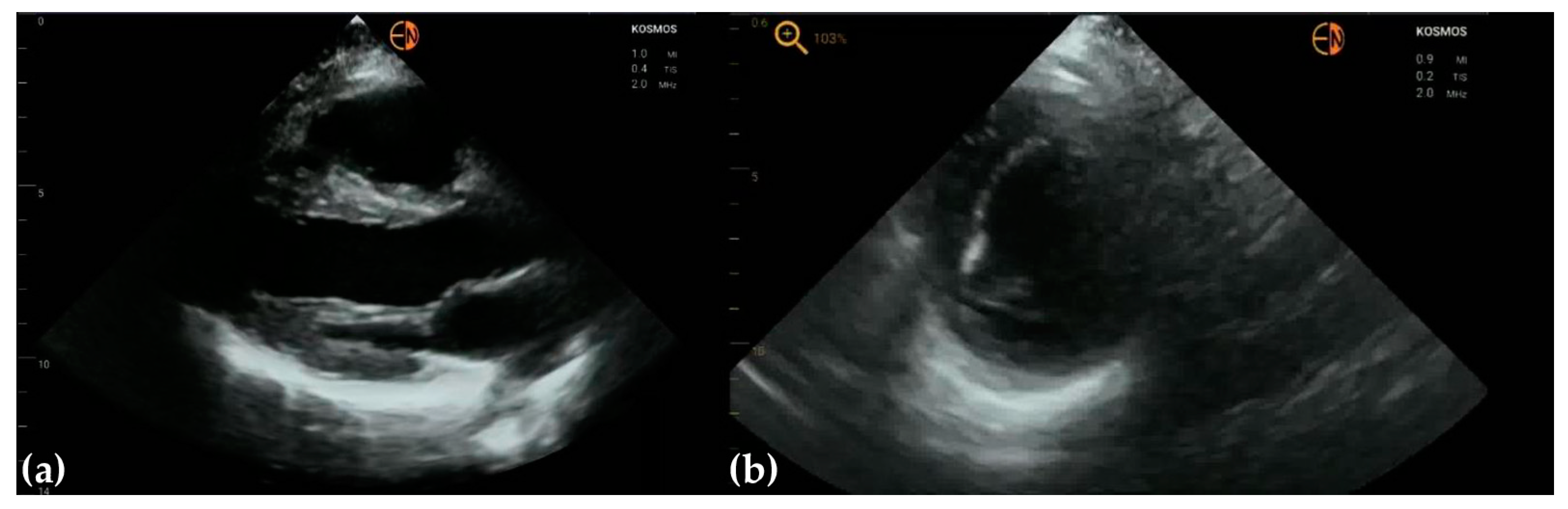
2.2. Echocardiography Measurements
2.3. Statistical Methods
3. Results
3.1. Profile of the Patients
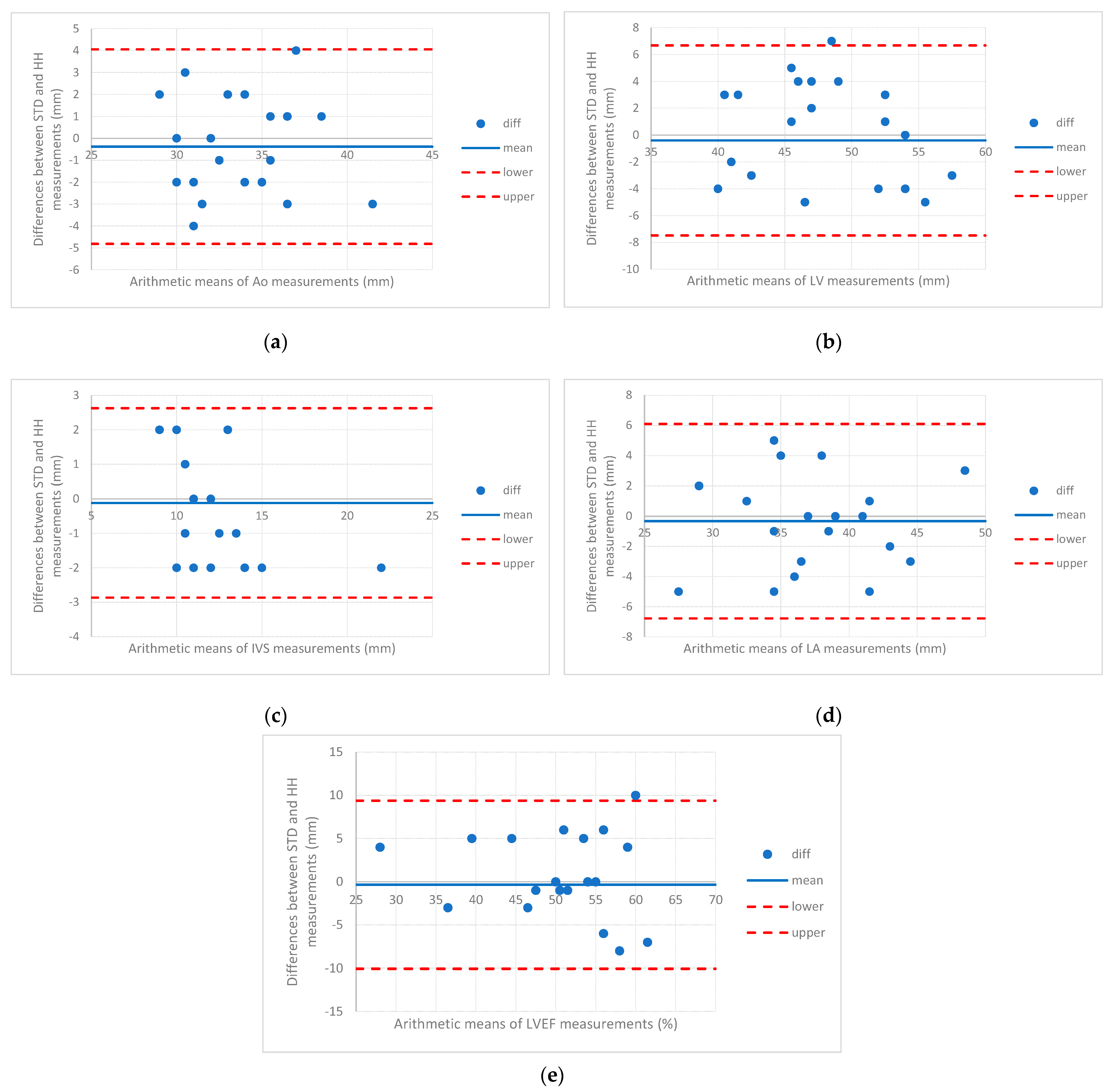
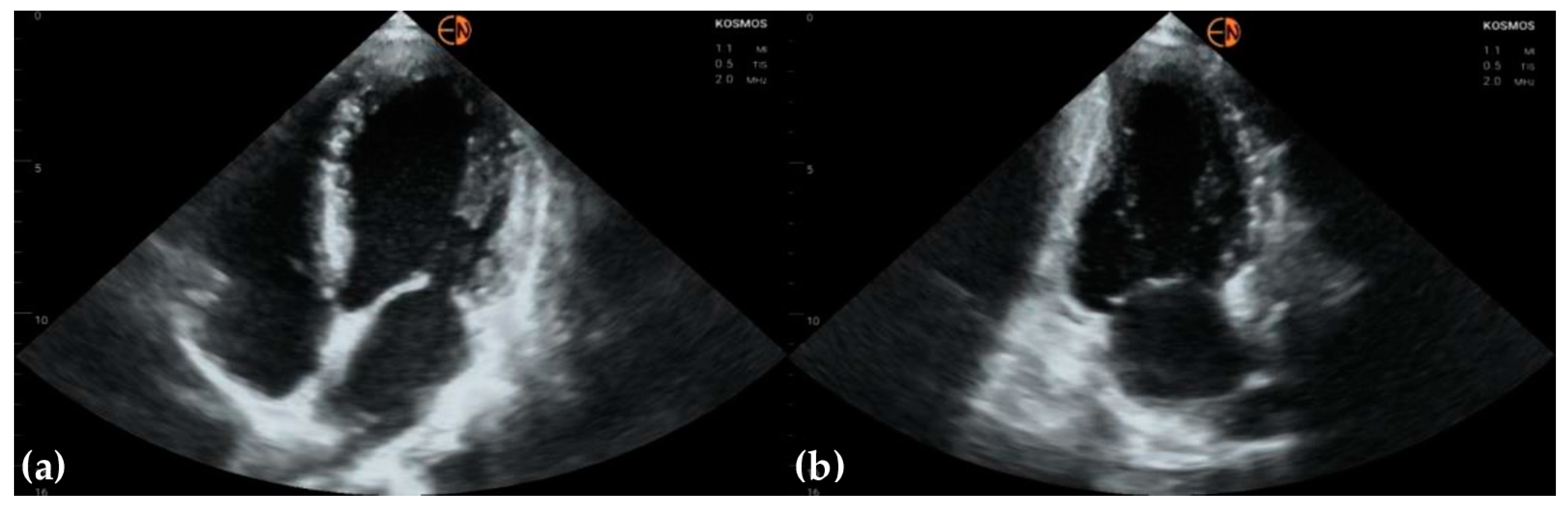
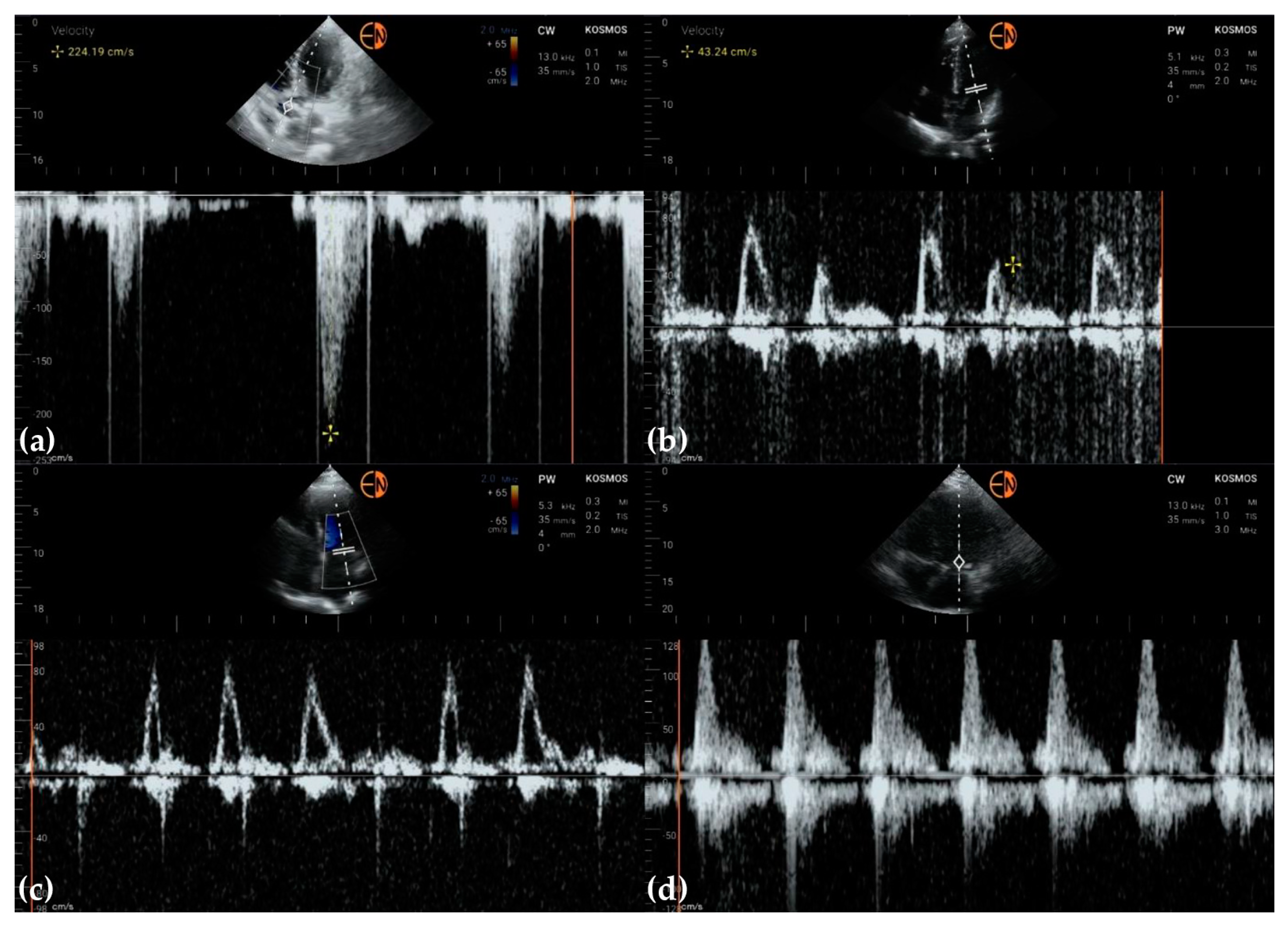
3.2. Measurements of Cardiovascular Structures
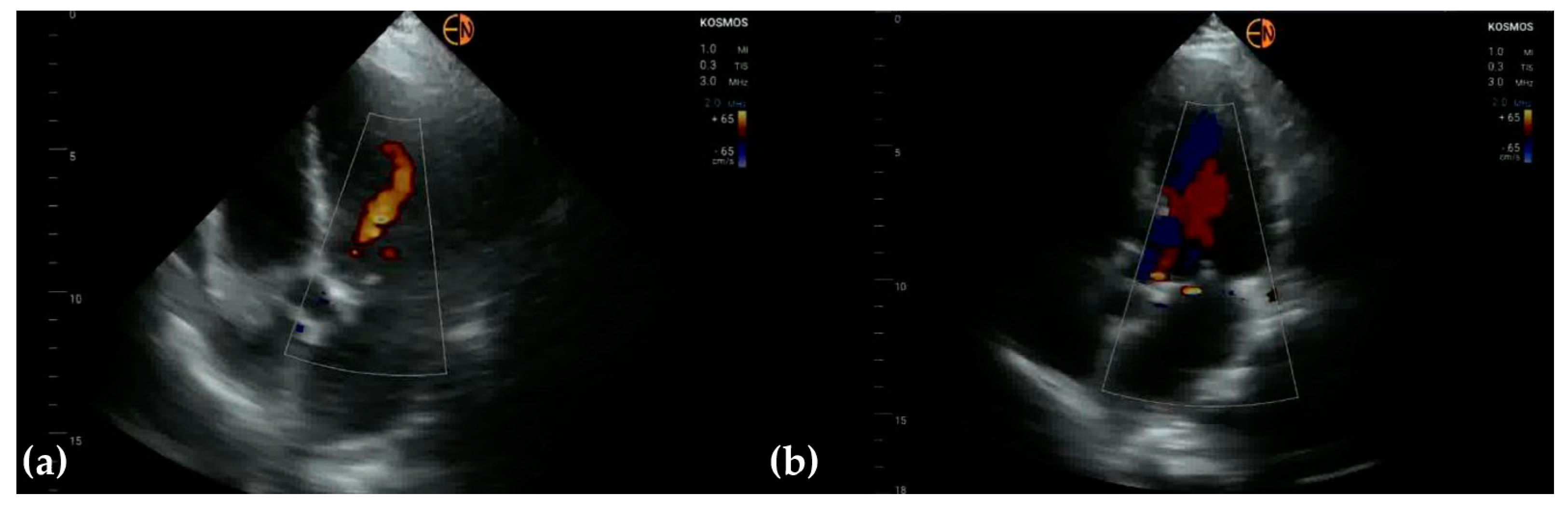
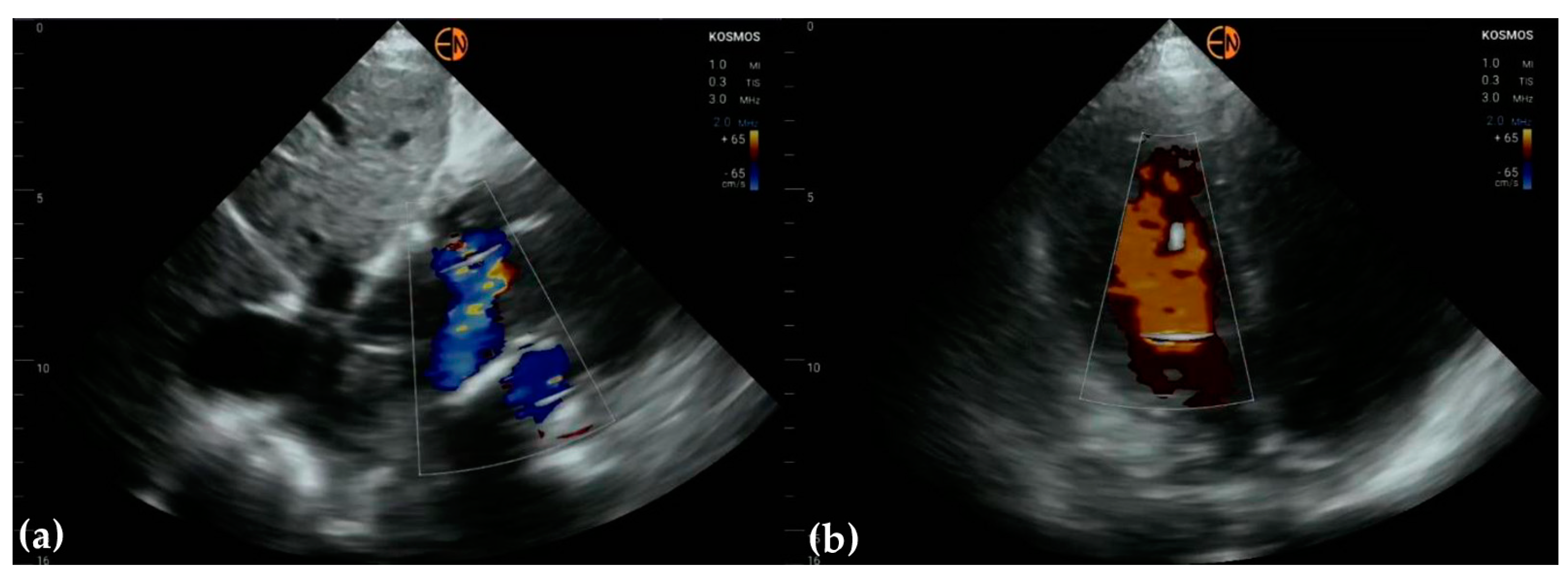
3.3. Valvular Stenosis and Regurgitation
3.4. Other Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omerovic, S.; Jain, A. Echocardiogram. In StatPearls; Updated 8 May 2022; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK558940/ (accessed on 2 September 2022).
- Steeds, R.P.; Garbi, M.; Cardim, N.; Kasprzak, J.D.; Sade, E.; Nihoyannopoulos, P.; Popescu, B.A.; Stefanidis, A.; Cosyns, B.; Monaghan, M.; et al. EACVI appropriateness criteria for the use of transthoracic echocardiography in adults: A report of literature and current practice review. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 1191–1204. [Google Scholar] [CrossRef]
- Wilkinson, J.N.; Saxhaug, L.M. Handheld ultrasound in training—The future is getting smaller! J. Intensiv. Care Soc. 2020, 22, 220–229. [Google Scholar] [CrossRef]
- Hellmann, D.B.; Whiting-O’Keefe, Q.; Shapiro, E.P.; Martin, L.D.; Martire, C.; Ziegelstein, R.C. The rate at which residents learn to use hand-held echocardiography at the bedside. Am. J. Med. 2005, 118, 1010–1018. [Google Scholar] [CrossRef]
- Pathan, F.; Fonseca, R.; Marwick, T.H. Usefulness of Hand-Held Ultrasonography as a Gatekeeper to Standard Echocardiography for “Rarely Appropriate” Echocardiography Requests. Am. J. Cardiol. 2016, 118, 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Vscan. Available online: https://vscan.rocks/product/vscanextend (accessed on 10 October 2022).
- Clarius. Available online: https://clarius.com/ (accessed on 10 October 2022).
- Medical Device Store. Available online: https://medicaldevicestore.ro/produs/ecograf-portabil-philips-lumify/ (accessed on 10 October 2022).
- Butterfly iQ. Available online: https://www.butterflynetwork.com/ (accessed on 10 October 2022).
- Le, M.-P.T.; Voigt, L.; Nathanson, R.; Maw, A.M.; Johnson, G.; Dancel, R.; Mathews, B.; Moreira, A.; Sauthoff, H.; Gelabert, C.; et al. Comparison of four handheld point-of-care ultrasound devices by expert users. Ultrasound J. 2022, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.; Shiha, M.G.; Yones, E.; Wardley, J.; Ryding, A.; Sawh, C.; Flather, M.; Morris, P.; Swift, A.J.; Vassiliou, V.S.; et al. Cardiovascular examination using hand-held cardiac ultrasound. J. Echocardiogr. 2021, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Echonous Platform. Available online: https://echonous.com/en_us/ (accessed on 8 October 2022).
- Neskovic, A.N.; Edvardsen, T.; Galderisi, M.; Garbi, M.; Gullace, G.; Jurcut, R.; Dalen, H.; Hagendorff, A.; Lancellotti, P.; Popescu, B.A.; et al. Focus cardiac ultrasound: The European Association of Cardiovascular Imaging viewpoint. Eur. Heart J.-Cardiovasc. Imaging 2014, 15, 956–960. [Google Scholar] [CrossRef]
- American College of Emergency Physicians. Emergency Ultrasound Guidelines. Ann. Emerg. Med. 2009, 53, 550–570. [Google Scholar] [CrossRef]
- Labovitz, A.J.; Noble, V.E.; Bierig, M.; Goldstein, S.A.; Jones, R.; Kort, S.; Porter, T.R.; Spencer, K.T.; Tayal, V.S.; Wei, K. Focused Cardiac Ultrasound in the Emergent Setting: A Consensus Statement of the American Society of Echocardiography and American College of Emergency Physicians. J. Am. Soc. Echocardiogr. 2010, 23, 1225–1230. [Google Scholar] [CrossRef]
- Chamsi-Pasha, M.A.; Sengupta, P.P.; Zoghbi, W.A. Handheld Echocardiography: Current State and Future Perspectives. Circulation 2017, 136, 2178–2188. [Google Scholar] [CrossRef]
- American College of Emergency Physicians. Appropriate use criteria for handheld pocket ultrasound devices: ACEP Policy Statement. 2018. Available online: https://www.acep.org/globalassets/new-pdfs/policy-statements/appropriate-use-criteria-for-handheld-pocket-ultrasound-devices.pdf (accessed on 19 December 2022).
- Clevert, D.A.; Schwarze, V.; Nyhsen, C.; D’Onofrio, M.; Sidhu, P.; Brady, A.P. ESR statement on portable ultrasound devices. Insights Imaging 2019, 10, 89. [Google Scholar] [CrossRef]
- Oliver, C.M.; Hunter, S.A.; Ikeda, T.; Galletly, D.C. Junior doctor skill in the art of physical examination: A retrospective study of the medical admission note over four decades. BMJ Open 2013, 3, e002257. [Google Scholar] [CrossRef] [PubMed]
- Roelandt, J.R.T.C. The decline of our physical examination skills: Is echocardiography to blame? Eur. Heart J.-Cardiovasc. Imaging 2013, 15, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Prinz, C.; Dohrmann, J.; Van Buuren, F.; Bitter, T.; Bogunovic, N.; Horstkotte, D.; Faber, L. Diagnostic Performance of Handheld Echocardiography for the Assessment of Basic Cardiac Morphology and Function: A Validation Study in Routine Cardiac Patients. Echocardiography 2012, 29, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.W.; Blauwet, L.A.; Vatury, O.M.; Mulvagh, S.L.; Behrenbeck, T.R.; Scott, C.; Pellikka, P.A. Diagnostic Capability of Comprehensive Handheld vs Transthoracic Echocardiography. Mayo Clin. Proc. 2014, 89, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.W.; Geske, J.B.; Anavekar, N.S.; Askew, J.W.; Lewis, B.R.; Oh, J.K. Handheld echocardiography during hospitalization for acute myocardial infarction. Clin. Cardiol. 2017, 40, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, B.; Parra, D.A.; Aliyu, M.; Moon, T.; Soslow, J.H. Smartphone interfaced handheld echocardiography for focused assessment of ventricular function and structure in children: A pilot study. Echocardiography 2019, 37, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Blume, G.G.; Lechinewski, L.D.; Vieira, I.P.; Clausell, N.; Bertinato, G.P.; Machado-Júnior, P.A.B.; Berro, P.G.; Moura, L.A.Z.; Tsang, T. Handheld Echocardiography in a Clinical Practice Scenario: Concordances Compared to Standard Echocardiographic Reports. J. Cardiovasc. Imaging 2022, 30, 25–34. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Jenkins, S.; Alabed, S.; Swift, A.; Marques, G.; Ryding, A.; Sawh, C.; Wardley, J.; Shah, B.N.; Swoboda, P.; Senior, R.; et al. Diagnostic accuracy of handheld cardiac ultrasound device for assessment of left ventricular structure and function: Systematic review and meta-analysis. Heart 2021, 107, 1826–1834. [Google Scholar] [CrossRef]
- Cardim, N.; Dalen, H.; Voigt, J.-U.; Ionescu, A.; Price, S.; Neskovic, A.N.; Edvardsen, T.; Galderisi, M.; Sicari, R.; Donal, E.; et al. The use of handheld ultrasound devices: A position statement of the European Association of Cardiovascular Imaging (2018 update). Eur. Heart J.-Cardiovasc. Imaging 2018, 20, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Medgadget. Available online: https://www.medgadget.com/2020/09/echonous-kosmos-3-in-1-ultrasound-electronic-stethoscope-and-ecg-helps-with-covid-19.html (accessed on 11 October 2022).
- Papadopoulou, S.-L.; Sachpekidis, V.; Kantartzi, V.; Styliadis, I.; Nihoyannopoulos, P. Clinical validation of an artificial intelligence-assisted algorithm for automated quantification of left ventricular ejection fraction in real time by a novel handheld ultrasound device. Eur. Heart J.-Digit. Health 2022, 3, 29–37. [Google Scholar] [CrossRef] [PubMed]
| HH Device [Ref] | Price | Features |
|---|---|---|
| Vscan Extend with Dual Probe (GE’s Vscan) [6] * | around €5100 (plus taxes) | B-mode, M-mode, and color flow imaging at a frequency between 1.7 and 3.8 MHz, with a scan time of 60 min and a recharge time of 75 min |
| Vscan Air (GE’s Vscan) [6] * | €4.250 (plus taxes) | similar to Vscan Extend with Dual Probe but has a wireless probe |
| Clarius [7] * | around €3395 require a €595 annual membership fee | bidimensional and color Doppler imaging capabilities |
| Philips’ Lumify [8] * | around €5800 per probe + cost for the tablet | three probes: linear features pulsed wave Doppler, convex, and phased array—used for cardiac imaging, does not provide continuous wave Doppler |
| Butterfly iQ [9] ** | around $2000 plus an annual membership fee | sacrificed image quality, especially in the examination of the heart [10] |
| Who? | HH Device | TTE (Gold Standard) | n | Examiners | Reported Results |
|---|---|---|---|---|---|
| Prinz et al. [21], 2012 | Vscan (GE Vingmed Ultrasound, Horten, Norway) | Vivid 7system (GE Vingmed Ultrasound | 320 inpatients | 2 | Concordance: low for “mild” regurgitant valvular lesions; Agreement: positive and negative deviation on Bland–Altman agreement plots for LVEF |
| Cullen et al. [22], 2014 | Vscan (GE Healthcare) | iE33 (Phillips) or Vivid E9 (GE Healthcare) | 190 outpatients | 6 * | Agreement: from 0.51 (0.28 to 0.74) for increased LV wall thickness (present vs. absent) to 0.91 (0.86 to 0.95) for aortic diameter (mm) |
| Cullen et al. [23], 2017 | Vscan (GE Healthcare, Waukesha, WI) | Vivid E9 (GE Healthcare, Waukesha, WI) or iE33 (Philips Healthcare, Andover, MA) | 82 patients with acute myocardial infarction | 2 | Concordance: 0.75 (0.66 to 0.82) for LEFV; 0.69 (0.58 to 0.77) for WMSI; Agreement: 0.55 to 0.79 for mitral regurgitation and 0.48 to 0.78 for tricuspid regurgitation |
| Acheampong et al. [24], 2020 | Lumify | Philips EPIQ7 | 60 pediatric outpatients | unclear | Agreements were smaller for children ≤5 years (e.g., 0.59 (0.31 to 0.88), n = 20 for fractional shortening compared to 0.82 (0.73 to 0.90) for all patients (n = 55)) |
| Blume et al. [25], 2022 | Vscan (GE Vingmed Ultrasound AS, Horten, Norway) | Philips iE33 (Philips Ultrasound Inc., Bothell, WA, USA) | 108 patients | 2 | Concordance: from 0.25 (0.17 to 0.32) for pulmonary regurgitation (none/mild/moderate/severe) to 0.86 (0.80 to 0.90) for LEFV |
| Item | Statistics | STD | HH | Diff. | Agreement [95%CI] |
|---|---|---|---|---|---|
| Ao (mm) | mean (stdev) | 34 (3.8) | 34.4 (4) | −0.38 (2.26) | −0.4 [−4.8 to 4.1] |
| median [Q1 to Q3] | 35 [32 to 36] | 35 [32.3 to 36] | −0.5 [−2 to 1] | ||
| {min to max} | {24 to 41} | {25 to 43} | {−4 to 5} | ||
| LV (mm) | mean (stdev) | 48.1 (5.4) | 48.5 (5.7) | −0.4 (3.61) | −0.4 [−7.5 to 6.7] |
| median [Q1 to Q3] | 48 [44.3 to 52.8] | 47 [44.3 to 53.8] | −1 [−3 to 2.75] | ||
| {min to max} | {35 to 56} | {36 to 59} | {−6 to 7} | ||
| IVS (mm) | mean (stdev) | 12 (2.2) | 12.1 (2.7) | −0.12 (1.4) | −0.1 [−2.9 to 2.6] |
| median [Q1 to Q3] | 12 [10.3 to 13] | 12 [11 to 13] | 0 [−1 to 1] | ||
| {min to max} | {9 to 21} | {8 to 23} | {−2 to 3} | ||
| LA (mm) | mean (stdev) | 39.2 (5.9) | 39.5 (5.8) | −0.33 (3.28) | −0.3 [−6.8 to 6.1] |
| median [Q1 to Q3] | 39 [35 to 42] | 39.5 [36.3 to 44] | 0 [−3 to 2.75] | ||
| {min to max} | {25 to 50} | {28 to 51} | {−7 to 5} | ||
| FEVS (%) | mean (stdev) | 51.4 (9.1) | 51.7 (9.8) | −0.33 (4.96) | −0.3 [−10.1 to 9.4] |
| median [Q1 to Q3] | 53.5 [46 to 57] | 52.5 [48 to 58.5] | −1 [−3 to 4] | ||
| {min to max} | {30 to 67} | {26 to 68} | {−11 to 10} |
| STD | HH | Full Agreement n (% [95%CI]) Kappa * [95%CI] | |
|---|---|---|---|
| Aortic valve | 33 (78.6 [62.8 to 89.2]) 0.6557 [0.3862 to 0.9252] | ||
| Mild aortic regurgitation | 10 | 5 | |
| Moderate aortic regurgitation | 1 | 0 | |
| Mild aortic stenosis | 1 | 1 | |
| Severe aortic stenosis | 1 | 1 | |
| Normal | 29 | 35 | |
| Mitral valve | 26 (61.9 [45.7 to 76.0]) 0.5321 [0.3451 to 0.7191] | ||
| Mild mitral regurgitation | 16 | 14 | |
| Moderate mitral regurgitation | 10 | 7 | |
| Severe mitral regurgitation | 1 | 1 | |
| Normal | 15 | 21 | |
| Tricuspid valve | 29 (69.0 [52.8 to 81.9]) 0.6072 [0.4183 to 0.7961] | ||
| Mild tricuspid regurgitation | 12 | 11 | |
| Moderate tricuspid regurgitation | 7 | 5 | |
| Severe tricuspid regurgitation | 3 | 1 | |
| Normal | 20 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haji-Hassan, M.; Duțu, B.; Bolboacă, S.D. Handheld Echocardiography Measurements Concordance and Findings Agreement: An Exploratory Study. Diagnostics 2023, 13, 853. https://doi.org/10.3390/diagnostics13050853
Haji-Hassan M, Duțu B, Bolboacă SD. Handheld Echocardiography Measurements Concordance and Findings Agreement: An Exploratory Study. Diagnostics. 2023; 13(5):853. https://doi.org/10.3390/diagnostics13050853
Chicago/Turabian StyleHaji-Hassan, Mariam, Bogdan Duțu, and Sorana D. Bolboacă. 2023. "Handheld Echocardiography Measurements Concordance and Findings Agreement: An Exploratory Study" Diagnostics 13, no. 5: 853. https://doi.org/10.3390/diagnostics13050853
APA StyleHaji-Hassan, M., Duțu, B., & Bolboacă, S. D. (2023). Handheld Echocardiography Measurements Concordance and Findings Agreement: An Exploratory Study. Diagnostics, 13(5), 853. https://doi.org/10.3390/diagnostics13050853







