Correlation of sLOX-1 Levels and MR Characteristics of Culprit Plaques in Intracranial Arteries with Stroke Recurrence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Data and Biochemical Indicators
2.2. HR-VWI Examination
2.3. Image Analysis
the reference site) × 100%;
2.4. Statistical Analysis
3. Results
3.1. Comparison of Baseline Demographic Data and Differences in HR-VWI Characteristics between the Recurrence Group and the Non-Recurrence Group
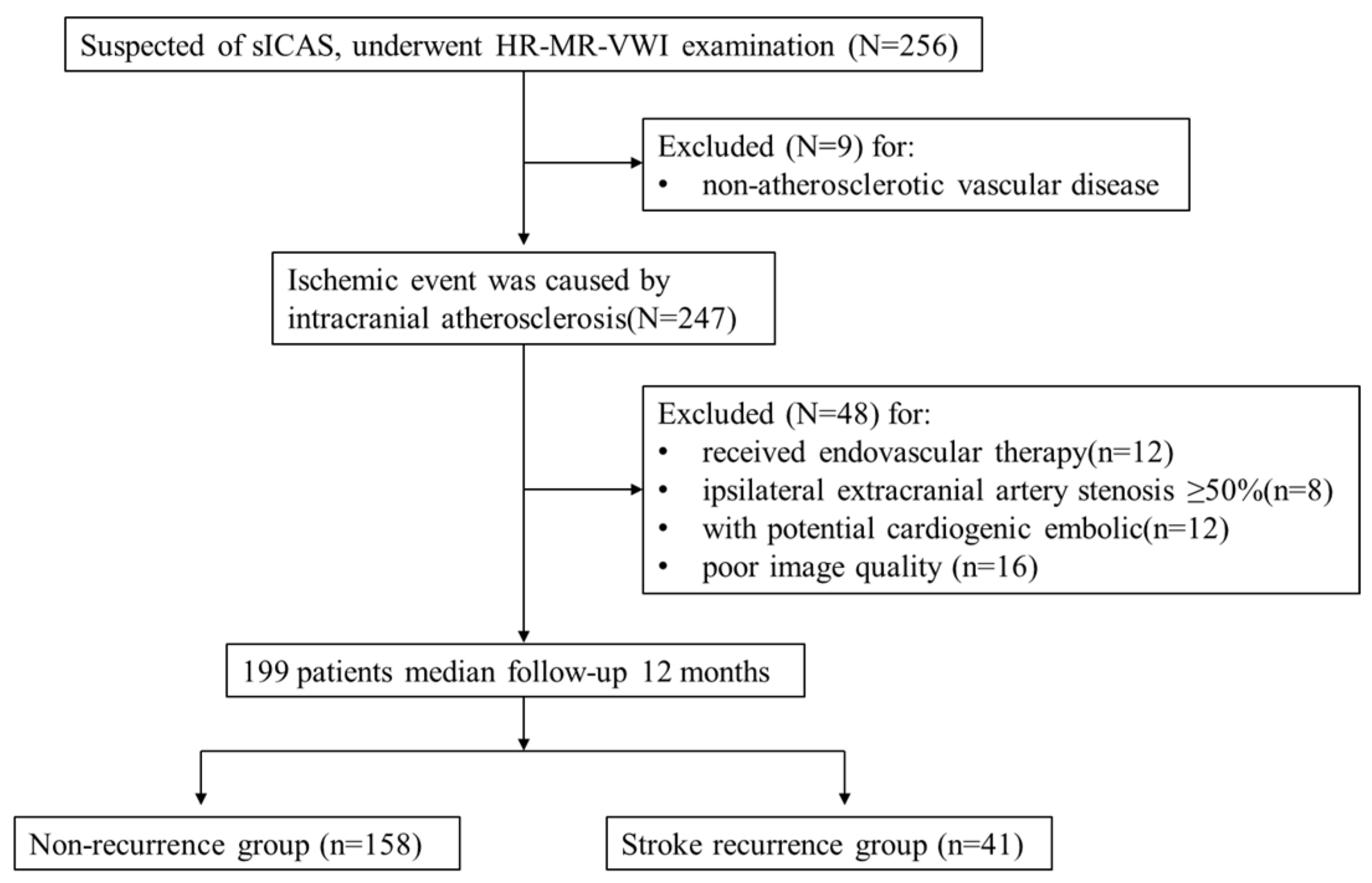
- A total of 199 patients were included in this study, and 41 developed stroke recurrence during the 12-month follow-up period. Overall, 30 patients had a new acute infarct focus in the same vascular supply area on DWI and 11 patients were diagnosed as new neurological deficit symptoms. The mean age of the patients was 67 ± 10 years in the recurrence group and 64 ± 13 years in the non-recurrence group. The proportion of men in the recurrence group, history of smoking, and history of diabetes were higher than those in the non-recurrence group, but the difference was not statistically significant. Serum glycated haemoglobin, triglyceride, total cholesterol, apolipoprotein B, LDL, homocysteine, and cystatin C levels were higher in the recurrence group than in the non-recurrence group, while serum apolipoprotein A and high-density lipoprotein (HDL) levels were higher in the non-recurrence group than that in the recurrence group, but the differences were not statistically significant. Serum LOX-1 levels were significantly higher in the recurrence group than in the non-recurrence group, and the difference was statistically significant (t = −4.29, p < 0.001) (Table 1).
- In the recurrence group, 21 patients (51.2%) had culprit plaques in the posterior circulation, which was higher than that in the non-recurrence group (44.3%). The lumen area at the culprit plaque in the recurrence group was smaller than that in the non-recurrence group, but the differences were not statistically significant. As for the quantitative indices, the culprit plaque thickness (t = −2.19, p = 0.003), stenosis (t = −2.48, p = 0.014), and plaque burden (t = −2.57, p = 0.010) were higher in the recurrence group than in the non-recurrence group, and the differences were statistically significant. Regarding the qualitative indices, the incidence of hyperintensity on T1WI (χ2 = 21.31, p < 0.001), positive remodelling (χ2 = 9.33, p = 0.003), and significant enhancement (χ2 = 5.83, p = 0.027) in the culprit plaques were higher in the recurrence group than that in the non-recurrence group, and the difference was statistically significant (Table 2).
- For the intra-observer agreement in the identification of the presence of hyperintensity on T1WI, which was significantly enhanced, the positive remodelling and the kappa value were 1.00, 0.96, and 0.93 (all p < 0.001), respectively. For the inter-observer agreement, the values of the same parameters were 1.00, 0.92 and 0.89 (all p < 0.001), respectively. The intra-observer ICC of plaque thickness, remaining lumen area, degree of stenosis and plaque burden were 0.90 (95% CI, 0.76–0.95, p < 0.001), 0.95 (95% CI, 0.91–0.98, p < 0.001), 0.88 (95% CI, 0.74–0.95, p < 0.001) and 0.80 (95% CI, 0.63–0.90, p < 0.001), respectively. The inter-observer ICC values for the above-described parameters were as follows: 0.94 (95% CI, 0.84–0.97, p < 0.001), 0.96 (95% CI, 0.93–0.99, p < 0.001), 0.87 (95% CI, 0.75–0.93, p < 0.001) and 0.86 (95% CI, 0.75–0.94, p < 0.001).
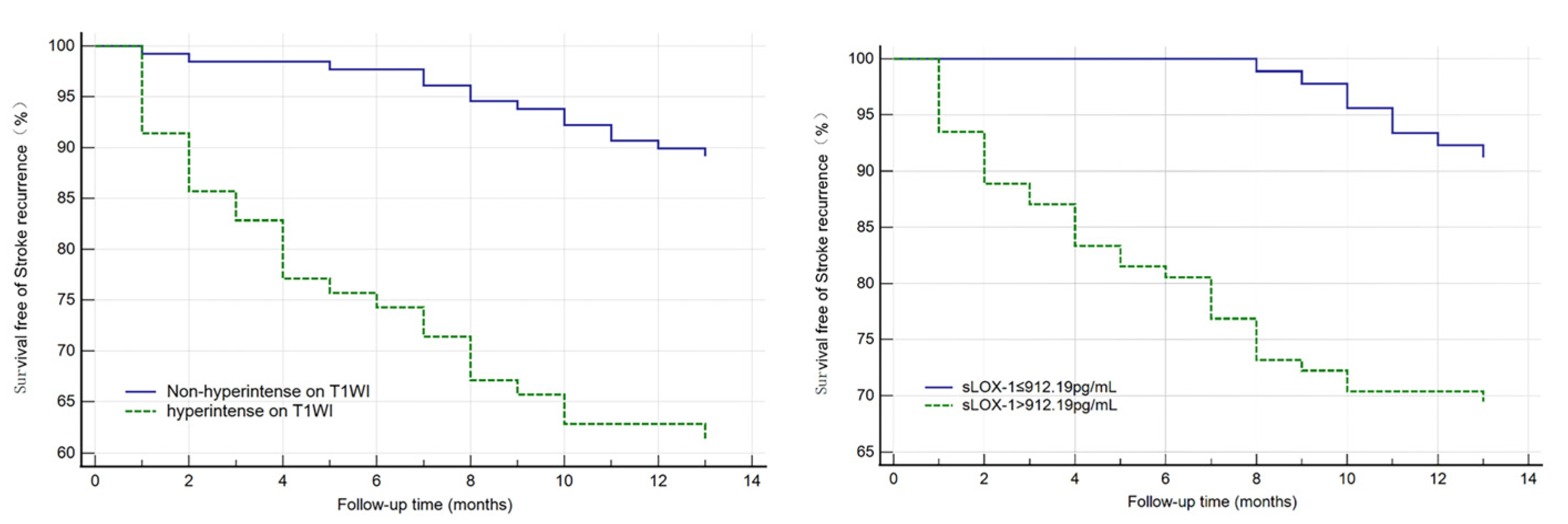
3.2. Independent Risk Factor Analysis for Stroke Recurrence and Survival Curve Analysis
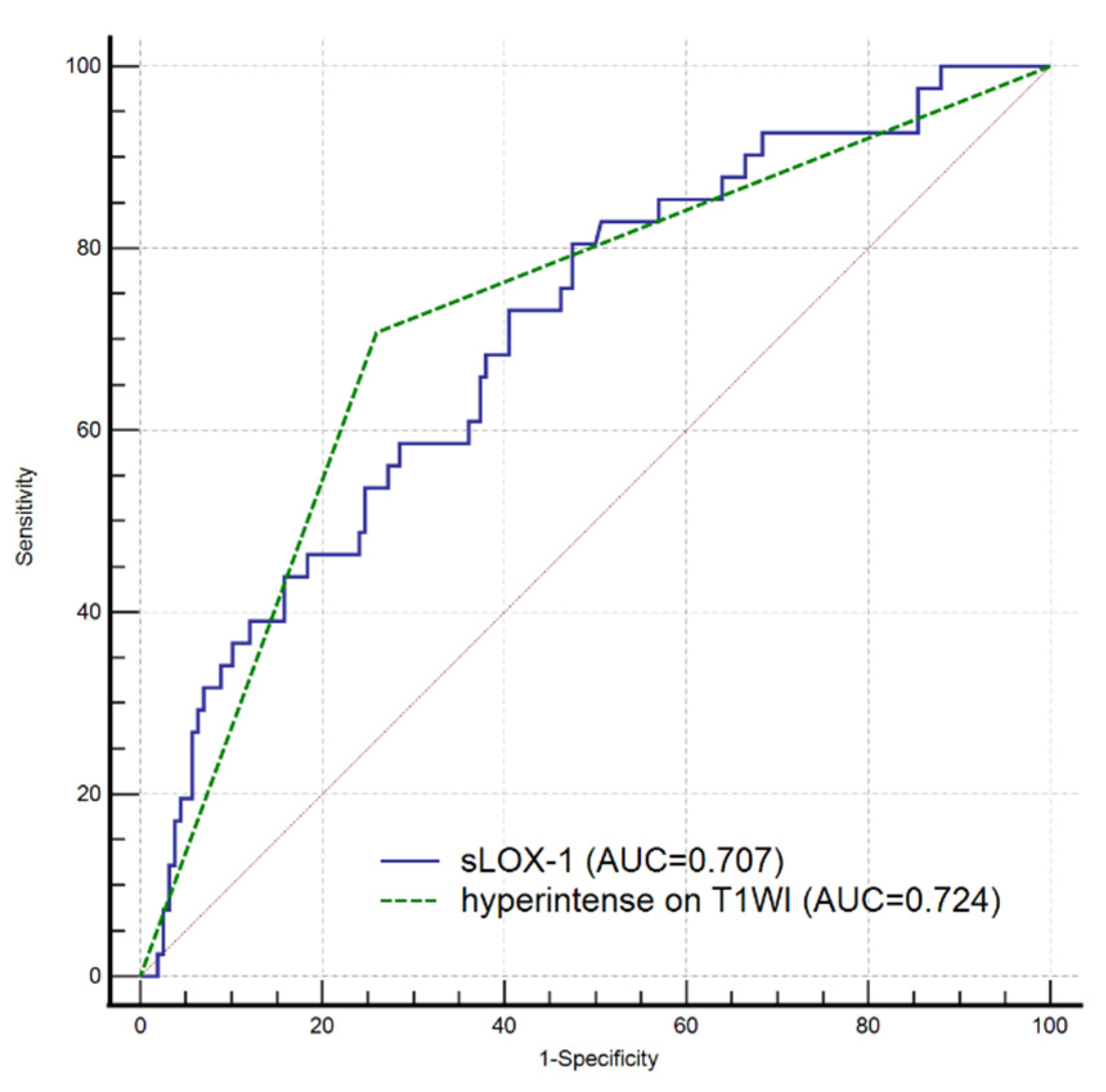
3.3. Relationship between sLOX-1 Levels and Culprit Plaque Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hankey, G.J. Secondary stroke prevention. Lancet Neurol. 2014, 13, 178–194. [Google Scholar] [CrossRef]
- Akhmedov, A.; Bonetti, N.R.; Reiner, M.F.; Spescha, R.D.; Amstalden, H.; Merlini, M.; Gaul, D.S.; Diaz-Cañestro, C.; Briand-Schumacher, S.; Spescha, R.S.; et al. Deleterious role of endothelial lectin-like oxidized low-density lipoprotein receptor-1 in ischaemia/reperfusion cerebral injury. J. Cereb. Blood Flow Metab. 2018, 39, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Khasiyev, F.; Liu, M.; DeRosa, J.T.; Tom, S.E.; Rundek, T.; Cheung, K.; Wright, C.B.; Sacco, R.L.; Elkind, M.S. Determinants and Outcomes of Asymptomatic Intracranial Atherosclerotic Stenosis. J. Am. Coll. Cardiol. 2021, 78, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-J.; Lu, Y.; Cui, J.-Y.; Ma, L.-Q.; Zhang, R.-P.; Xu, Z. Characteristics of symptomatic plaque on high-resolution magnetic resonance imaging and its relationship with the occurrence and recurrence of ischemic stroke. Neurol. Sci. 2021, 42, 3605–3613. [Google Scholar] [CrossRef]
- Ren, K.; Jiang, H.; Li, T.; Qian, C.; Gong, S.; Wang, T.; Zhu, L. Predictive value of the combination between the intracranial arterial culprit plaque characteristics and the Essen Stroke Risk Score for short-term stroke recurrence. J. Stroke Cerebrovasc. Dis. 2022, 31, 106624. [Google Scholar] [CrossRef]
- Roldan, P.C.; Greene, E.R.; Qualls, C.R.; Sibbitt, W.L.; Roldan, C.A. Progression of atherosclerosis versus arterial stiffness with age within and between arteries in systemic lupus erythematosus. Rheumatol. Int. 2019, 39, 1027–1036. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis is an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Liang, Y.-Q.; Kakino, A.; Matsuzaka, Y.; Mashimo, T.; Isono, M.; Akamatsu, T.; Shimizu, H.; Tajima, M.; Kaneko, T.; Li, L.; et al. LOX-1 (Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1) Deletion Has Protective Effects on Stroke in the Genetic Background of Stroke-Prone Spontaneously Hypertensive Rat. Stroke 2020, 51, 1835–1843. [Google Scholar] [CrossRef]
- Huang, W.; Li, Q.; Chen, X.; Lin, Y.; Xue, J.; Cai, Z.; Zhang, W.; Wang, H.; Jin, K.; Shao, B. Soluble lectin-like oxidized low-density lipoprotein receptor-1 as a novel biomarker for large-artery atherosclerotic stroke. Int. J. Neurosci. 2017, 127, 881–886. [Google Scholar] [CrossRef]
- Feng, X.; Chan, K.L.; Lan, L.; Abrigo, J.; Liu, J.; Fang, H.; Xu, Y.; Soo, Y.; Leng, X.; Leung, T.W. Stroke Mechanisms in Symptomatic Intracranial Atherosclerotic Disease: Classification and Clinical Implications. Stroke 2019, 50, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Lindenholz, A.; van der Kolk, A.G.; Zwanenburg, J.J.; Hendrikse, J. The Use and Pitfalls of Intracranial Vessel Wall Imaging: How We Do It. Radiology 2018, 286, 12–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimowitz, M.; Kokkinos, J.; Strong, J.; Brown, M.B.; Levine, S.R.; Silliman, S.; Pessin, M.S.; Weichel, E.; Sila, C.A.; Furlan, A.J.; et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology 1995, 45, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Samuels, O.B.; Joseph, G.J.; Lynn, M.J.; Smith, H.A.; Chimowitz, M.I. A Standardized Method for Measuring Intracranial Arterial Stenosis. Am. J. Neuroradiol. 2000, 21, 643–646. [Google Scholar]
- Kim, H.J.; Choi, E.-H.; Chung, J.-W.; Kim, J.-H.; Kim, Y.S.; Seo, W.-K.; Kim, G.-M.; Bang, O.Y. Luminal and Wall Changes in Intracranial Arterial Lesions for Predicting Stroke Occurrence. Stroke 2020, 51, 2495–2504. [Google Scholar] [CrossRef]
- Huang, J.; Jiao, S.; Zhao, X.; Zhang, J.; Zhang, C.; Chen, M.; Song, Y. Characteristics of patients with enhancing intracranial atherosclerosis and association between plaque enhancement and recent cerebrovascular ischemic events: A high-resolution magnetic resonance imaging study. Acta Radiol. 2019, 60, 1301–1307. [Google Scholar] [CrossRef]
- Yamada, N.; Higashi, M.; Otsubo, R.; Sakuma, T.; Oyama, N.; Tanaka, R.; Iihara, K.; Naritomi, H.; Minematsu, K.; Naito, H. Association between Signal Hyperintensity on T1-Weighted MR Imaging of Carotid Plaques and Ipsilateral Ischemic Events. Am. J. Neuroradiol. 2007, 28, 287–292. [Google Scholar]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Finn, A.V.; Gold, H.K.; Tulenko, T.N.; Wrenn, S.P.; Narula, J. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2054–2061. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Wang, L.; Li, X.; Zhang, J.; Zhang, J.; Liu, X.; Wu, H.; Mossa-Basha, M.; Xu, J.; Zhao, B.; et al. Intracranial Atherosclerotic Plaque Characteristics and Burden Associated with Recurrent Acute Stroke: A 3D Quantitative Vessel Wall MRI Study. Front. Aging Neurosci. 2021, 13, 706544. [Google Scholar] [CrossRef]
- Song, X.; Zhao, X.; Liebeskind, D.S.; Wang, L.; Xu, W.; Xu, Y.; Hou, D.; Zheng, Z.; Wu, J. Incremental value of plaque enhancement in predicting stroke recurrence in symptomatic intracranial atherosclerosis. Neuroradiology 2020, 62, 1123–1131. [Google Scholar] [CrossRef]
- Xu, W. High-resolution MRI of intracranial large artery diseases: How to use it in clinical practice? Stroke Vasc. Neurol. 2019, 4, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Etesami, M.; Astor, B.C.; Zeiler, S.R.; Trout, H.H., 3rd; Wasserman, B.A. Carotid plaque neovascularization and hemorrhage detected by MR imaging are associated with recent cerebrovascular ischemic events. AJNR Am. J. Neuroradiol. 2012, 33, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Wang, Y.; Zhu, M.; Wu, X.; Malhotra, A.; Lei, X.; Zhang, F.; Wang, X.; Xie, S.; Zhou, J.; et al. Higher Plaque Burden of Middle Cerebral Artery Is Associated with Recurrent Ischemic Stroke: A Quantitative Magnetic Resonance Imaging Study. Stroke 2020, 51, 659–662. [Google Scholar] [CrossRef]
- Shi, Z.; Li, J.; Zhao, M.; Zhang, X.; Degnan, A.J.; Mossa-Basha, M.; Saloner, D.; Lu, J.; Liu, Q.; Zhu, C. Progression of Plaque Burden of Intracranial Atherosclerotic Plaque Predicts Recurrent Stroke/Transient Ischemic Attack: A Pilot Follow-Up Study Using Higher-Resolution MRI. J. Magn. Reson. Imaging 2021, 54, 560–570. [Google Scholar] [CrossRef]
- Wong, K.S.; Li, H. Long-Term Mortality and Recurrent Stroke Risk Among Chinese Stroke Patients with Predominant Intracranial Atherosclerosis. Stroke 2003, 34, 2361–2366. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Liu, H.; Pan, Y.; Jing, J.; Li, H.; Zhao, X.; Liu, L.; Wang, D.; Johnston, S.C.; Wang, Z.; et al. Elevated Neutrophil and Presence of Intracranial Artery Stenosis Increase the Risk of Recurrent Stroke. Stroke 2018, 49, 2294–2300. [Google Scholar] [CrossRef]
- Mitsuoka, H.; Kume, N.; Hayashida, K.; Inui-Hayashiada, A.; Aramaki, Y.; Toyohara, M.; Jinnai, T.; Nishi, E.; Kita, T. Interleukin 18 stimulates release of soluble lectin-like oxidized LDL receptor-1 (sLOX-1). Atherosclerosis 2009, 202, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Hou, D.; Liu, J.; Wang, T.; Luo, Y.; Sun, W.; Li, C.; Shen, L.; Liu, W.; Wu, D. Soluble Lectin-like Oxidized Low-Density Lipoprotein Receptor-1 Level is Related to Clinical Prognosis In Patients with Acute Atherosclerosis-related Ischemic Stroke. Clin. Appl. Thromb. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Singh, S.; Gautam, A.S. Upregulated LOX-1 Receptor: Key Player of the Pathogenesis of Atherosclerosis. Curr. Atheroscler. Rep. 2019, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Li, H.; Zhang, L.; Su, S.; Wang, C.; Zhang, B.; Liang, D.; Zheng, H.; Liu, X.; Zhang, N. Automated morphologic analysis of intracranial and extracranial arteries using convolutional neural networks. Br. J. Radiol. 2022, 95, 20210031. [Google Scholar] [CrossRef] [PubMed]


| Index | Non-Recurrence Group (n = 158) | Recurrence Group (n = 41) | t/z/χ2 | p Value |
|---|---|---|---|---|
| Age, years, mean ± SD | 63.56 ± 12.98 | 67.10 ± 9.96 | −1.59 | 0.113 |
| Male, n (%) | 102 (64.6) | 32 (78.0) | 2.69 | 0.134 |
| Smoking history, n (%) | 34 (21.5) | 14 (34.1) | 2.84 | 0.103 |
| History of hypertension, n (%) | 119 (75.3) | 26 (63.4) | 2.33 | 0.167 |
| History of diabetes, n (%) | 46 (29.1) | 17 (41.5) | 2.30 | 0.136 |
| Glycosylated haemoglobin, mmol/L, mean ± SD | 6.78 ± 1.50 | 7.15 ± 1.59 | −1.33 | 0.186 |
| Triglycerides, mmol/L, mean ± SD | 1.67 ± 1.08 | 1.68 ± 0.94 | −0.03 | 0.980 |
| Total cholesterol, mmol/L, mean ± SD | 3.97 ± 1.26 | 4.15 ± 1.07 | −0.89 | 0.373 |
| HDL, mmol/L, mean ± SD | 1.20 ± 0.32 | 1.12 ± 0.30 | 1.37 | 0.172 |
| LDL, mmol/L, mean ± SD | 2.48 ± 1.16 | 3.13 ± 6.32 | −0.62 | 0.537 |
| Apolipoprotein a, mmol/L, mean ± SD | 1.20 ± 0.23 | 1.15 ± 0.22 | 1.16 | 0.247 |
| Apolipoprotein b, mmol/L, mean ± SD | 0.93 ± 0.35 | 2.15 ± 17.87 | −0.50 | 0.616 |
| Hs-CRP, mmol/L, mean ± SD | 5.54 ± 15.34 | 5.48 ± 16.47 | 0.02 | 0.980 |
| Homocysteine, μmol/L, mean ± SD | 39.83 ± 103.94 | 48.99 ± 143.81 | −0.86 | 0.393 |
| Cystatin C, mmol/L, mean ± SD | 20.49 ± 110.57 | 38.05 ± 148.73 | −0.77 | 0.443 |
| sLOX-1, pg/mL, mean ± SD | 936.36 ± 552.47 | 1351.68 ± 551.05 | −4.29 | <0.001 |
| Characteristic | Non-Recurrence (n = 158) | Recurrence Group (n = 41) | t/z/χ2 | p Value |
|---|---|---|---|---|
| Posterior circulation, n (%) | 70 (44.3) | 21 (51.2) | 0.63 | 0.483 |
| Plaque thickness, mm, mean ± SD | 1.52 ± 0.40 | 1.70 ± 0.49 | −2.19 | 0.033 |
| Remaining lumen area, mm2, mean ± SD | 4.40 ± 2.55 | 4.28 ± 3.49 | 0.25 | 0.804 |
| Degree of stenosis, mean ± SD | 41.14 ± 18.30 | 49.40 ± 21.15 | −2.48 | 0.014 |
| Plaque burden, median [IQR] | 71 (65, 80) | 76 (68, 85) | −2.57 | 0.010 |
| Eccentric distribution, n (%) | 97 (61.4) | 20 (48.8) | 2.14 | 0.157 |
| Hyperintensity on T1WI, n (%) | 43 (27.2) | 27 (65.9) | 21.31 | <0.001 |
| Positive Remodelling, n (%) | 62 (39.2) | 27 (65.9) | 9.33 | 0.003 |
| Significantly enhanced, n (%) | 49 (31.0) | 21 (51.2) | 5.83 | 0.027 |
| Risk Factors | HR (95%CI) | p Value |
|---|---|---|
| sLOX-1, pg/mL | 1.001 (1.000, 1.002) | 0.002 |
| Plaque thickness | 1.515 (0.735, 3.120) | 0.260 |
| Degree of stenosis | 1.006 (0.985, 1.028) | 0.560 |
| Plaque burden | 1.003 (0.974, 1.032) | 0.858 |
| Hyperintensity on T1WI | 2.326 (1.034, 5.231) | 0.041 |
| Positive Remodelling | 1.282 (0.595, 2.763) | 0.526 |
| Significantly enhanced | 0.871 (0.419, 1.812) | 0.712 |
| Risk Factors | HR (95%CI) | p Value |
|---|---|---|
| sLOX-1 > 912.19 pg/mL | 2.583 (1.142, 5.486) | 0.023 |
| Plaque thickness | 1.342 (0.670, 2.689) | 0.407 |
| Degree of stenosis | 1.007 (0.987, 1.028) | 0.479 |
| Plaque burden | 0.999 (0.972, 1.026) | 0.916 |
| Hyperintensity on T1WI | 2.632 (1.197, 5.790) | 0.016 |
| Positive Remodelling | 1.316 (0.621, 2.791) | 0.474 |
| Significantly enhanced | 0.914 (0.972, 1.026) | 0.809 |
| sLOX-1 | Q1 | Q2 | Q3 | Q4 | F | p Value | |
|---|---|---|---|---|---|---|---|
| Characteristic | |||||||
| Posterior circulation, n (%) | 16 (32.0) | 27 (54.0) | 23 (46.9) | 25 (50.0) | 1.872 | 0.136 | |
| Eccentric distribution, n (%) | 33 (66.0) | 29 (58.0) | 27 (55.1) | 28 (56.0) | 0.501 | 0.682 | |
| Hyperintensity on T1WI, n (%) | 3 (6.0) | 15 (30.0) | 21 (42.9) | 31 (62.0) | 14.501 | <0.001 | |
| Positive remodelling, n (%) | 8 (9.0) | 23 (25.8) | 26 (29.2) | 32 (36.0) | 9.602 | <0.001 | |
| Significantly enhanced, n (%) | 6 (12.0) | 16 (32.0) | 21 (42.9) | 27 (54.0) | 7.684 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, K.; Jiang, H.; Li, T.; Qian, C.; Zhu, L.; Wang, T. Correlation of sLOX-1 Levels and MR Characteristics of Culprit Plaques in Intracranial Arteries with Stroke Recurrence. Diagnostics 2023, 13, 804. https://doi.org/10.3390/diagnostics13040804
Ren K, Jiang H, Li T, Qian C, Zhu L, Wang T. Correlation of sLOX-1 Levels and MR Characteristics of Culprit Plaques in Intracranial Arteries with Stroke Recurrence. Diagnostics. 2023; 13(4):804. https://doi.org/10.3390/diagnostics13040804
Chicago/Turabian StyleRen, Kaixuan, Huayun Jiang, Tiantian Li, Chengqun Qian, Li Zhu, and Tianle Wang. 2023. "Correlation of sLOX-1 Levels and MR Characteristics of Culprit Plaques in Intracranial Arteries with Stroke Recurrence" Diagnostics 13, no. 4: 804. https://doi.org/10.3390/diagnostics13040804
APA StyleRen, K., Jiang, H., Li, T., Qian, C., Zhu, L., & Wang, T. (2023). Correlation of sLOX-1 Levels and MR Characteristics of Culprit Plaques in Intracranial Arteries with Stroke Recurrence. Diagnostics, 13(4), 804. https://doi.org/10.3390/diagnostics13040804








