Management of Non-Melanoma Skin Cancer: Radiologists Challenging and Risk Assessment
Abstract
1. Introduction
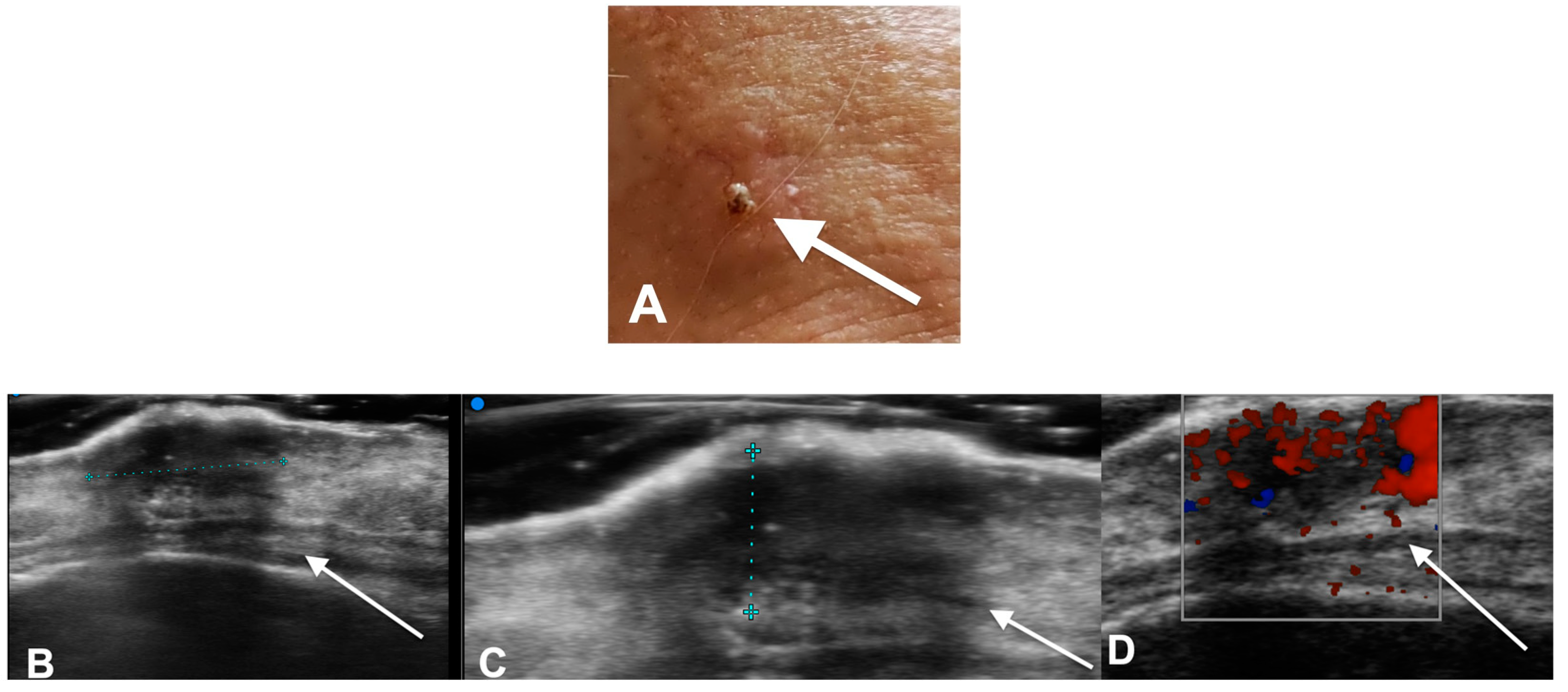
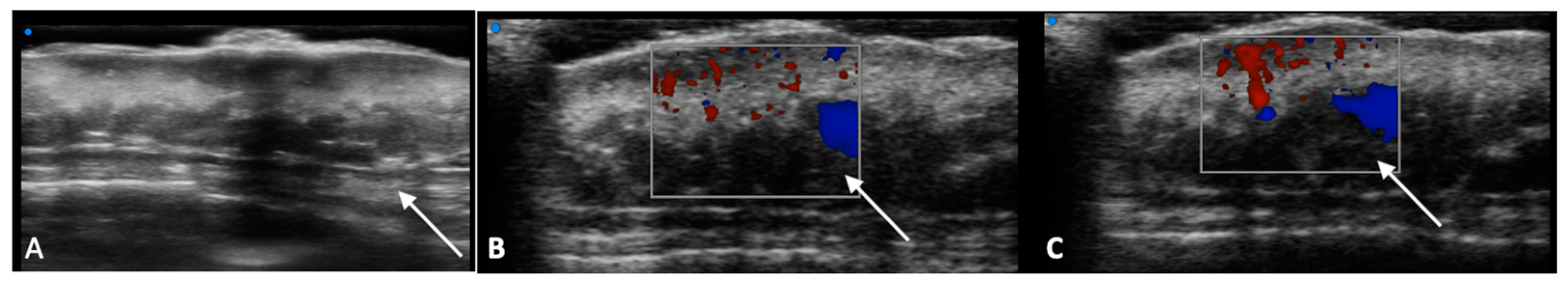
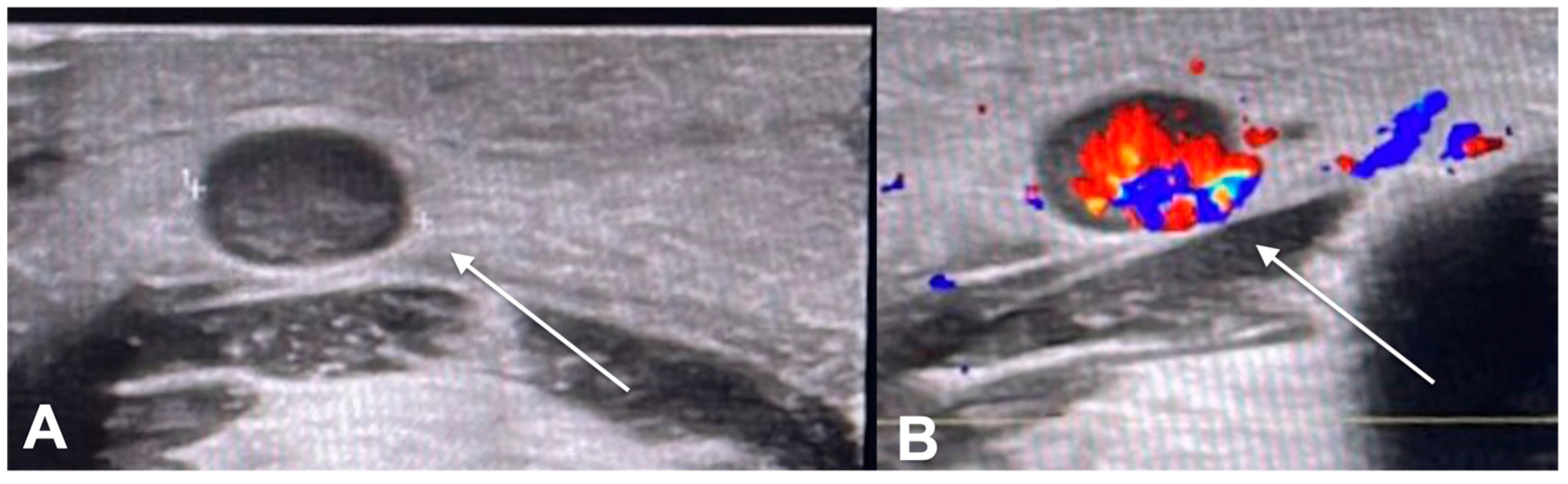
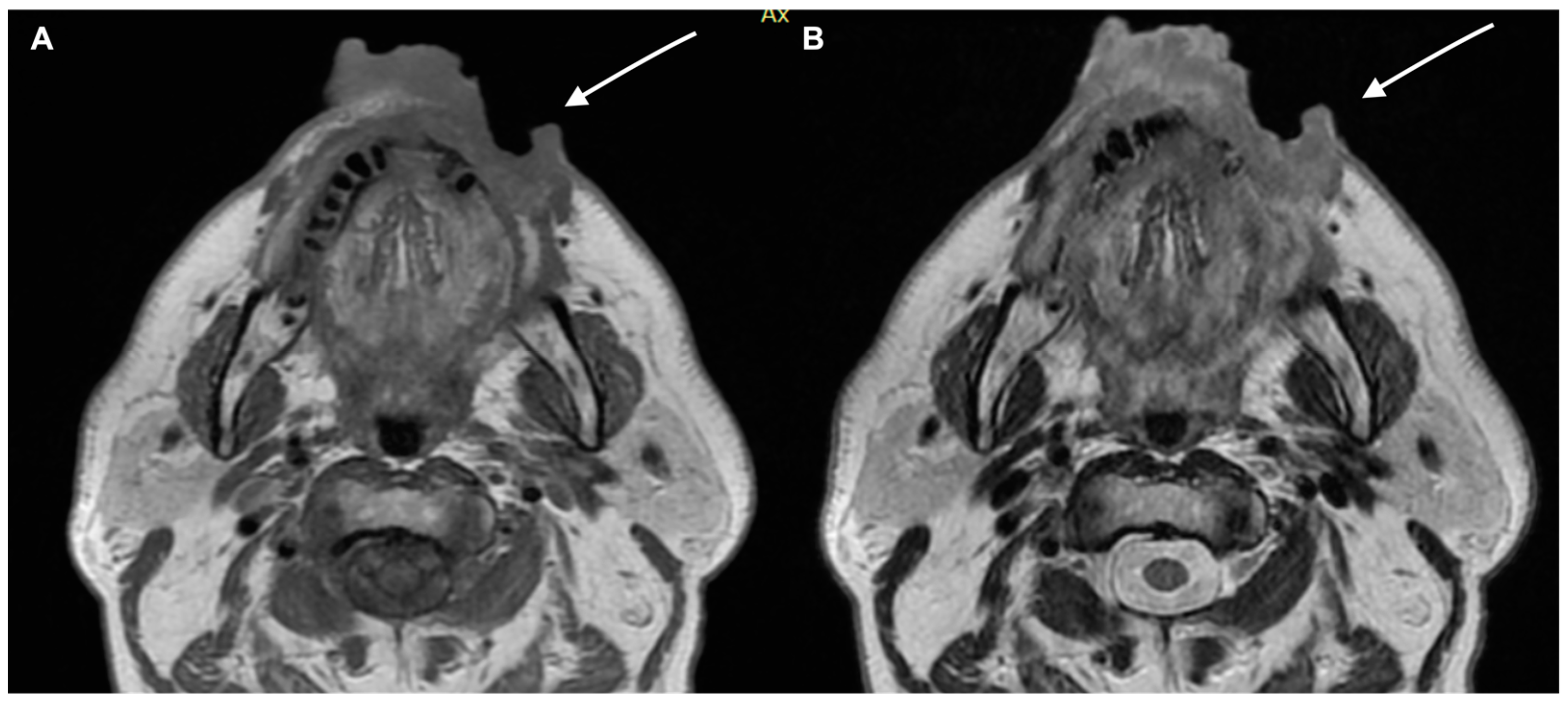
2. Diagnostic Imaging and Non-Melanoma Skin Cancer
Staging and Risk Stratification
3. Diagnostic Tools and Non-Melanoma: Staging and Surveillance
4. Follow-Up and Surveillance: Time
5. Treatment Assessment of NMSCs in Immunotherapy
6. Imaging of Immune—Related Adverse Events
7. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancers |
| AK | actinic keratosis |
| BCC | Basal cell carcinoma |
| CPD | confirmed progression disease |
| iCR | immune complete response |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DP | disease progression |
| HFUS | High-frequency US |
| ICB | immune checkpoint blockades |
| irAEs | immune-related adverse events |
| ITM | in transit |
| LN | lymph node |
| MCC | Merkel cell carcinomas |
| MSC | melanoma skin cancer |
| NCCN | National Comprehensive Cancer Network |
| NMSC | Non-melanoma skin cancer |
| PR | progression rate |
| RC | response criteria |
| RECIST | Response Evaluation Criteria in Solid Tumours |
| SCC | squamous cell cancers |
| SD | stable disease |
| SLNB | sentinel lymph node biopsy |
| SNB | sentinel node biopsy |
| SNL | sentinel lymph node |
| SUV | standardized uptake value |
| TNM | tumour-node-metastasis |
| UEP | unequivocal progression |
| UPD | unconfirmed progression disease |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Estimates: Leading Causes of Death. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 15 January 2023).
- Urban, K.; Mehrmal, S.; Uppal, P.; Giesey, R.L.; Delost, G.R. The global burden of skin cancer: A longitudinal analysis from the Global Burden of Disease Study, 1990–2017. JAAD Int. 2021, 2, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, Z.; Ghorat, F.; Jarrahi, A.; Adineh, H.; Sohrabivafa, M.; Goodarzi, E.J.W.C.R.J. Global incidence and mortality of skin cancer by histological subtype and its relationship with the human development index (HDI); an ecology study in 2018. World Cancer Res. J. 2019, 6, e13. [Google Scholar]
- Eide, M.J.; Krajenta, R.; Johnson, D.; Long, J.J.; Jacobsen, G.; Asgari, M.M.; Lim, H.W.; Johnson, C.C. Identification of Patients with Nonmelanoma Skin Cancer Using Health Maintenance Organization Claims Data. Am. J. Epidemiol. 2010, 171, 123–128. [Google Scholar] [CrossRef]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Tendencias epidemiológicas en cáncer de piel. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar]
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M.; et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef]
- Miligi, L. Ultraviolet Radiation Exposure: Some Observations and Considerations, Focusing on Some Italian Experiences, on Cancer Risk, and Primary Prevention. Environments 2020, 7, 10. [Google Scholar] [CrossRef]
- Bais, A.F.; Lucas, R.M.; Bornman, J.F.; Williamson, C.E.; Sulzberger, B.; Austin, A.T.; Wilson, S.R.; Andrady, A.L.; Bernhard, G.; McKenzie, R.L.; et al. Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017. Photochem. Photobiol. Sci. 2018, 17, 127–179. [Google Scholar] [CrossRef]
- Griffin, L.L.; Ali, F.R.; Lear, J.T. Non-melanoma skin cancer. Clin. Med. 2016, 16, 62–65. [Google Scholar] [CrossRef]
- Xu, Y.G.; Aylward, J.L.; Swanson, A.M.; Spiegelman, V.S.; Vanness, E.R.; Teng, J.M.; Snow, S.N.; Wood, G.S. Nonmelanoma skin cancers: Basal cell and squamous cell carcinomas. In Abeloff’s Clinical Oncology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1052–1073. [Google Scholar]
- Warner, C.L.; Cockerell, C.J. The new seventh edition American joint committee on cancer staging of cutaneous non-melanoma skin cancer. Am. J. Clin. Dermatol. 2011, 12, 147–154. [Google Scholar] [CrossRef]
- Newlands, C.; Currie, R.; Memon, A.; Whitaker, S.; Woolford, T. Non-melanoma skin cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S125–S132. [Google Scholar] [CrossRef] [PubMed]
- Kučinskienė, V.; Samulėnienė, D.; Gineikienė, A.; Raišutis, R.; Kažys, R.; Valiukevičienė, S. Preoperative assessment of skin tumor thickness and structure using 14-MHz ultrasound. Medicina 2014, 50, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Loescher, L.J.; Janda, M.; Soyer, H.P.; Shea, K.; Curiel-Lewandrowski, C. Advances in Skin Cancer Early Detection and Diagnosis. Semin. Oncol. Nurs. 2013, 29, 170–181. [Google Scholar] [CrossRef]
- Humphreys, T.R.; Shah, K.; Wysong, A.; Lexa, F.; MacFarlane, D. The role of imaging in the management of patients with nonmelanoma skin cancer: When is imaging necessary? J. Am. Acad. Dermatol. 2017, 76, 591–607. [Google Scholar] [CrossRef]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef]
- Ting, P.T.; Kasper, R.; Arlette, J.P. Metastatic basal cell carcinoma: Report of two cases and literature review. J. Cutan. Med. Surg. 2005, 9, 10–15. [Google Scholar] [CrossRef]
- Toll, A.; Margalef, P.; Masferrer, E.; Ferrándiz-Pulido, C.; Gimeno, J.; Pujol, R.M.; Bigas, A.; Espinosa, L. Active nuclear IKK correlates with metastatic risk in cutaneous squamous cell carcinoma. Arch. Dermatol. Res. 2015, 307, 721–729. [Google Scholar] [CrossRef]
- Heuke, S.; Vogler, N.; Meyer, T.; Akimov, D.; Kluschke, F.; Röwert-Huber, H.-J.; Lademann, J.; Dietzek, B.; Popp, J. Detection and Discrimination of Non-Melanoma Skin Cancer by Multimodal Imaging. Healthcare 2013, 1, 64–83. [Google Scholar] [CrossRef]
- Avallone, A.; Pecori, B.; Bianco, F.; Aloj, L.; Tatangelo, F.; Romano, C.; Granata, V.; Marone, P.; Leone, A.; Botti, G.J.O.; et al. Critical role of bevacizumab scheduling in combination with pre-surgical chemo-radiotherapy in MRI-defined high-risk locally advanced rectal cancer: Results of the Branch trial. Oncotarget 2015, 6, 30394–30407. [Google Scholar] [CrossRef] [PubMed]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.M.; Cafarelli, F.P.; Paparella, M.T.; Rennie, W.J.; Guglielmi, G. Phosphaturic mesenchymal tumors: Radiological aspects and suggested imaging pathway. La Radiol. Medica 2021, 126, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Cellini, F.; Di Franco, R.; Manfrida, S.; Borzillo, V.; Maranzano, E.; Pergolizzi, S.; Morganti, A.G.; Fusco, V.; Deodato, F.; Santarelli, M.; et al. Palliative radiotherapy indications during the COVID-19 pandemic and in future complex logistic settings: The NORMALITY model. Radiol. Med. 2021, 126, 1619–1656. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Filice, S.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Petrillo, A. The current role and future prospectives of functional parameters by diffusion weighted imaging in the assessment of histologic grade of HCC. Infect. Agents Cancer 2018, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, A.; Fusco, R.; Petrillo, M.; Granata, V.; Delrio, P.; Bianco, F.; Pecori, B.; Botti, G.; Tatangelo, F.; Caracò, C.J.O. Standardized index of shape (DCE-MRI) and standardized uptake value (PET/CT): Two quantitative approaches to discriminate chemo-radiotherapy locally advanced rectal cancer responders under a functional profile. Oncotarget 2017, 8, 8143. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Filice, F.; Tatangelo, F.; Piccirillo, M.; Grassi, R.; Izzo, F.; Petrillo, A. Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: Lights and shadows. Oncotarget 2017, 8, 51224–51237. [Google Scholar] [CrossRef]
- De Filippo, M.; Puglisi, S.; D’Amuri, F.; Gentili, F.; Paladini, I.; Carrafiello, G.; Maestroni, U.; Del Rio, P.; Ziglioli, F.; Pagnini, F. CT-guided percutaneous drainage of abdominopelvic collections: A pictorial essay. Radiol. Med. 2021, 126, 1561–1570. [Google Scholar] [CrossRef]
- Pampena, R.; Palmieri, T.; Kyrgidis, A.; Ramundo, D.; Iotti, C.; Lallas, A.; Moscarella, E.; Borsari, S.; Argenziano, G.; Longo, C. Orthovoltage radiotherapy for nonmelanoma skin cancer (NMSC): Comparison between 2 different schedules. J. Am. Acad. Dermatol. 2016, 74, 341–347. [Google Scholar] [CrossRef]
- Baheti, A.D.; Tirumani, S.H.; Giardino, A.; Rosenthal, M.H.; Tirumani, H.; Krajewski, K.; Ramaiya, N.H. Basal Cell Carcinoma: A Comprehensive Review for the Radiologist. Am. J. Roentgenol. 2015, 204, W132–W140. [Google Scholar] [CrossRef]
- Aubry, S.; Leclerc, O.; Tremblay, L.; Rizcallah, E.; Croteau, F.; Orfali, C.; Lepage, M. 7-Tesla MR imaging of non-melanoma skin cancer samples: Correlation with histopathology. Ski. Res. Technol. 2012, 18, 413–420. [Google Scholar] [CrossRef]
- Veness, M.J.; Oncology, R. Hypofractionated radiotherapy in patients with non-melanoma skin cancer in the post COVID-19 era: Time to reconsider its role for most patients. J. Med. Imaging Radiat. Oncol. 2020, 64, 591–594. [Google Scholar] [CrossRef]
- Silk, A.W.; Barker, C.A.; Bhatia, S.; Bollin, K.B.; Chandra, S.; Eroglu, Z.; Gastman, B.R.; Kendra, K.L.; Kluger, H.; Lipson, E.J.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of nonmelanoma skin cancer. J. Immunother. Cancer 2022, 10, e004434. [Google Scholar] [CrossRef]
- Marka, A.; Carter, J.B.; Toto, E.; Hassanpour, S. Automated detection of nonmelanoma skin cancer using digital images: A systematic review. BMC Med. Imaging 2019, 19, 21. [Google Scholar] [CrossRef]
- Malvehy, J.; Pellacani, G. Dermoscopy, Confocal Microscopy and other Non-invasive Tools for the Diagnosis of Non-Melanoma Skin Cancers and Other Skin Conditions. Acta Dermato-Venereol. 2017, 97, 22–30. [Google Scholar] [CrossRef]
- Fujimura, T.; Fujisawa, Y.; Otsuka, A.; Haass, N.K.J.F. Editorial: Recent Developments in Therapies and Diagnostic Tools for Melanoma and Non-melanoma Skin Cancer. Front. Med. 2020, 7, 613152. [Google Scholar] [CrossRef]
- Bezugly, A.; Rembielak, A. The use of high frequency skin ultrasound in non-melanoma skin cancer. J. Contemp. Brachyther. 2021, 13, 483–491. [Google Scholar] [CrossRef]
- Rohrbach, D.J.; Muffoletto, D.; Huihui, J.; Saager, R.; Keymel, K.; Paquette, A.; Morgan, J.; Zeitouni, N.; Sunar, U. Preoperative Mapping of Nonmelanoma Skin Cancer Using Spatial Frequency Domain and Ultrasound Imaging. Acad. Radiol. 2014, 21, 263–270. [Google Scholar] [CrossRef]
- Pasquali, P.; Freites-Martinez, A.; Fortuño-Mar, A. Ex vivo high-frequency ultrasound: A novel proposal for management of surgical margins in patients with non-melanoma skin cancer. J. Am. Acad. Dermatol. 2016, 74, 1278–1280. [Google Scholar] [CrossRef]
- Piłat, P.; Borzęcki, A.; Jazienicki, M.; Gerkowicz, A.; Krasowska, D. High-frequency ultrasound in the diagnosis of selected non-melanoma skin nodular lesions. Adv. Dermatol. Allergol. 2019, 36, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; Brown, M.; Busam, K.J.; Eisen, D.B.; Iyengar, V.; Lober, C.; Margolis, D.J. Guidelines of care for the management of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef]
- Fahradyan, A.; Howell, A.; Wolfswinkel, E.; Tsuha, M.; Sheth, P.; Wong, A. Updates on the Management of Non-Melanoma Skin Cancer (NMSC). Healthcare 2017, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Bichakjian, C.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; Brown, M.; Busam, K.J.; Eisen, D.B.; Iyengar, V.; Lober, C.; Margolis, D.J.; et al. Guidelines of care for the management of basal cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 540–559. [Google Scholar] [CrossRef]
- Califano, J.; Lydiatt, W.; Nehal, K.S.; O’Sullivan, B.; Schmults, C.; Seethala, R.; Weber, R.; Shah, J. Cutaneous squamous cell carcinoma of the head and neck. In AJCC Cancer Staging Manual, 8th ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Motaparthi, K.; Kapil, J.P.; Velazquez, E.F. Cutaneous Squamous Cell Carcinoma: Review of the Eighth Edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv. Anat. Pathol. 2017, 24, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Compton, C.C.; Byrd, D.R.; Garcia-Aguilar, J.; Kurtzman, S.H.; Olawaiye, A.; Washington, M.K. Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In AJCC Cancer Staging Atlas; Springer: New York, NY, USA, 2012; pp. 357–370. [Google Scholar]
- Barile, A. Some thoughts and greetings from the new Editor-in-Chief. Radiol. Med. 2021, 126, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Laverde-Saad, A.; Simard, A.; Nassim, D.; Jfri, A.; Alajmi, A.; O’Brien, E.; Wortsman, X.J.D. Performance of Ultrasound for Identifying Morphological Characteristics and Thickness of Cutaneous Basal Cell Carcinoma: A Systematic Review. Dermatology 2022, 238, 692–710. [Google Scholar] [CrossRef]
- Ossola, C.; Curti, M.; Calvi, M.; Tack, S.; Mazzoni, S.; Genesio, L.; Venturini, M.; Genovese, E.A. Role of ultrasound and magnetic resonance imaging in the prognosis and classification of muscle injuries in professional football players: Correlation between imaging and return to sport time. Radiol. Med. 2021, 126, 1460–1467. [Google Scholar] [CrossRef]
- Soyer Güldoğan, E.; Ergun, O.; Taşkın Türkmenoğlu, T.; Yılmaz, K.B.; Akdağ, T.; Özbal Güneş, S.; Durmaz, H.A.; Hekimoğlu, B.J.L.r.m. The impact of TI-RADS in detecting thyroid malignancies: A prospective study. Radiol. Med. 2021, 126, 1335–1344. [Google Scholar] [CrossRef]
- Keohane, S.G.; Proby, C.M.; Newlands, C.; Motley, R.J.; Nasr, I.; Mustapa, M.F.M.; Slater, D.N.; the British Association of Dermatologists (Squamous and Basal Cell Carcinoma Guideline Development Groups); the Royal College of Pathologists (Skin Cancer Lead). The new 8th edition of TNM staging and its implications for skin cancer: A review by the British Association of Dermatologists and the Royal College of Pathologists, U.K. Br. J. Dermatol. 2018, 179, 824–828. [Google Scholar] [CrossRef]
- MacFarlane, D.; Shah, K.; Wysong, A.; Wortsman, X.; Humphreys, T.R. The role of imaging in the management of patients with nonmelanoma skin cancer: Diagnostic modalities and applications. J. Am. Acad. Dermatol. 2017, 76, 579–588. [Google Scholar] [CrossRef]
- Mlosek, R.K.; Migda, B.; Migda, M. High-frequency ultrasound in the 21st century. J. Ultrason. 2020, 20, 233–241. [Google Scholar] [CrossRef]
- Kleinerman, R.; Marmur, E.; Whang, T.B.; Bard, R.L. Ultrasound in dermatology: Principles and applications. J. Am. Acad. Dermatol. 2012, 67, 478–487. [Google Scholar] [CrossRef]
- Roldán, F.A. Ultrasound Skin Imaging. Actas Dermo-Sifiliográficas 2014, 105, 891–899. [Google Scholar] [CrossRef]
- Zhu, A.Q.; Wang, L.F.; Li, X.L.; Wang, Q.; Li, M.X.; Ma, Y.Y.; Xiang, L.H.; Guo, L.H.; Xu, H.X. High-frequency ultrasound in the diagnosis of the spectrum of cutaneous squamous cell carcinoma: Noninvasively distinguishing actinic keratosis, Bowen’s Disease, and invasive squamous cell carcinoma. Ski. Res. Technol. 2021, 27, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Cho, J.Y.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Won, C.H. Emerging Minimally Invasive Technologies for the Detection of Skin Cancer. J. Pers. Med. 2021, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Roldán, F.A.; Varelli, C.; Bard, R.; Corvino, A.; Wortsman, X. Skin cancer: Findings and role of high-resolution ultrasound. J. Ultrasound 2019, 22, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Marmur, E.S.; Berkowitz, E.Z.; Fuchs, B.S.; Singer, G.K.; Yoo, J.Y. Use of High-Frequency, High-Resolution Ultrasound Before Mohs Surgery. Dermatol. Surg. 2010, 36, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, A.K.; Keyal, U.; Liu, Y. Application of high frequency ultrasound in dermatology. Discov. Med. 2018, 26, 237–242. [Google Scholar] [PubMed]
- Khlebnikova, A.; Molochkov, V.; Selezneva, E.; Belova, L.; Bezugly, A.; Sedova, T.; Molochkov, A. Basal cell carcinoma invasion depth determined with 30 and 75 MHz high-frequency ultrasound and histopathology—A comparative study. Med. Ultrason. 2020, 22, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.; Stockfleth, E.; Connolly, S.; Correia, O.; Erdmann, R.; Foley, P.; Gupta, A.; Jacobs, A.; Kerl, H.; Lim, H.; et al. Evidence- and consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum—Short version. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2069–2079. [Google Scholar] [CrossRef]
- Tambe, S.; Bhatt, K.; Jerajani, H.; Dhurat, R. Utility of high-frequency ultrasonography in the diagnosis of benign and malignant skin tumors. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 162–182. [Google Scholar] [CrossRef]
- Crisan, M.; Crisan, D.; Sannino, G.; Lupsor, M.; Badea, R.; Amzica, F.; Lupsor-Platon, M. Ultrasonographic staging of cutaneous malignant tumors: An ultrasonographic depth index. Arch. Dermatol. Res. 2013, 305, 305–313. [Google Scholar] [CrossRef]
- Wortsman, X.; Wortsman, J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J. Am. Acad. Dermatol. 2010, 62, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Wassef, C.; Rao, B.K. Uses of non-invasive imaging in the diagnosis of skin cancer: An overview of the currently available modalities. Int. J. Dermatol. 2013, 52, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- McLean, T.; Brunner, M.; Ebrahimi, A.; Gao, K.; Ch’Ng, S.; Veness, M.J.; Clark, J.R. Concurrent primary and metastatic cutaneous head and neck squamous cell carcinoma: Analysis of prognostic factors. Head Neck 2013, 35, 1144–1148. [Google Scholar] [CrossRef]
- Durham, A.B.; Wong, S.L. Sentinel lymph node biopsy in melanoma: Controversies and current guidelines. Futur. Oncol. 2014, 10, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Foy, J.; Kolb, F.; Routier, E.; Cavalcanti, A.; Lumbroso, J.; Tomasic, G.; Mateus, C.; Temam, S.; Robert, C.; Moya-Plana, A.; et al. Sentinel lymph node biopsy in 33 non-melanoma skin cancers of the head and neck: A twelve-year experience with long-term follow-up. Clin. Otolaryngol. 2018, 43, 1148–1152. [Google Scholar] [CrossRef]
- Ji, R.-C. Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses. Int. J. Mol. Sci. 2016, 18, 51. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Liu, J.; Zhu, Q.-L.; Zhao, C.-Y.; Qu, T.; Li, F.; Wortsman, X.; Jin, H.-Z. High-frequency ultrasound features of basal cell carcinoma and its association with histological recurrence risk. Chin. Med. J. 2019, 132, 2021–2026. [Google Scholar] [CrossRef]
- Dinnes, J.; Bamber, J.; Chuchu, N.; Bayliss, S.E.; Takwoingi, Y.; Davenport, C.; Godfrey, K.; O’Sullivan, C.; Matin, R.N.; Deeks, J.J.; et al. High-frequency ultrasound for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 2018, CD013188. [Google Scholar] [CrossRef]
- Reginelli, A.; Belfiore, M.P.; Russo, A.; Turriziani, F.; Moscarella, E.; Troiani, T.; Brancaccio, G.; Ronchi, A.; Giunta, E.F.; Sica, A.; et al. A Preliminary Study for Quantitative Assessment with HFUS (High- Frequency Ultrasound) of Nodular Skin Melanoma Breslow Thickness in Adults Before Surgery: Interdisciplinary Team Experience. Curr. Radiopharm. 2020, 13, 48–55. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Reginelli, A.; Russo, A.; Russo, G.M.; Rocco, M.P.; Moscarella, E.; Ferrante, M.; Sica, A.; Grassi, R.; Cappabianca, S. Usefulness of High-Frequency Ultrasonography in the Diagnosis of Melanoma: Mini Review. Front. Oncol. 2021, 11, 673026. [Google Scholar] [CrossRef]
- Sun, J.; Yang, L.; Zhou, Z.; Zhang, D.; Han, W.; Zhang, Q.; Peng, Y. Performance evaluation of two iterative reconstruction algorithms, MBIR and ASIR, in low radiation dose and low contrast dose abdominal CT in children. Radiol. Med. 2020, 125, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef]
- Fosko, S.W.; Hu, W.; Cook, T.F.; Lowe, V.J. Positron Emission Tomography for Basal Cell Carcinoma of the Head and Neck. Arch. Dermatol. 2003, 139, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Kato, H.; Noda, Y.; Kobayashi, K.; Miyazaki, T.; Hyodo, F.; Matsuo, M. Imaging findings of malignant skin tumors: Radiological–pathological correlation. Insights Imaging 2022, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.S.; Karia, P.S.; Morgan, F.C.; Schmults, C.D. The positive impact of radiologic imaging on high-stage cutaneous squamous cell carcinoma management. J. Am. Acad. Dermatol. 2017, 76, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Kato, H.; Matsuyama, K.; Noda, Y.; Hyodo, F.; Matsuo, M. Prognostic value of 18F-FDG PET/CT and MRI features in patients with high-risk and very-high-risk cutaneous squamous cell carcinoma. Br. J. Radiol. 2022, 95, 20211003. [Google Scholar] [CrossRef]
- Forouzan, P.; Calame, A.; Uebelhoer, N.S.; Cohen, P.R. Basal Cell Carcinoma with Calcification: Case Report of Calcifying Basal Cell Carcinoma and Review of Calcinosis Cutis Associated with Basal Cell Carcinoma. Cureus 2021, 13, e12721. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kato, H.; Suzui, N.; Miyazaki, T.; Tomita, H.; Hara, A.; Matsuyama, K.; Seishima, M.; Matsuo, M. Imaging findings of cutaneous angiosarcoma of the scalp: Comparison with cutaneous squamous cell carcinoma. Neuroradiol. J. 2021, 34, 329–334. [Google Scholar] [CrossRef]
- Rajesh, A.; Khan, A.; Kendall, C.; Hayter, J.; Cherryman, G. Can magnetic resonance imaging replace single photon computed tomography and computed tomography in detecting bony invasion in patients with oral squamous cell carcinoma? Br. J. Oral Maxillofac. Surg. 2008, 46, 11–14. [Google Scholar] [CrossRef]
- Arai, M.; Nozaki, T.; Matsusako, M.; Zenke, Y.; Arai, S.; Matsui, M.; Suzuki, K.; Jinzaki, M.; Kurihara, Y. MR Imaging of Mushroom-like Skin Adnexal Tumors in the Scalp: A Report of Two Cases. Magn. Reson. Med. Sci. 2020, 19, 282–285. [Google Scholar] [CrossRef]
- Akaike, G.; Akaike, T.; Fadl, S.A.; Lachance, K.; Nghiem, P.; Behnia, F. Imaging of Merkel Cell Carcinoma: What Imaging Experts Should Know. Radiographics 2019, 39, 2069–2084. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Kato, H.; Tomita, H.; Hara, A.; Suzui, N.; Miyazaki, T.; Matsuyama, K.; Seishima, M.; Matsuo, M. Magnetic Resonance Imaging Findings Differentiating Cutaneous Basal Cell Carcinoma from Squamous Cell Carcinoma in the Head and Neck Region. Korean J. Radiol. 2020, 21, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Kato, H.; Tomita, H.; Hara, A.; Suzui, N.; Miyazaki, T.; Matsuyama, K.; Seishima, M.; Matsuo, M. MR imaging findings for differentiating cutaneous malignant melanoma from squamous cell carcinoma. Eur. J. Radiol. 2020, 132, 109212. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Tang, M.; Lin, W.; Guo, L.; He, W.; Chen, W.; Li, K.; Liu, J.; Xiao, C.; Li, Y. The value of preoperative high-resolution MRI with microscopy coil for facial nonmelanoma skin cancers. Ski. Res. Technol. 2021, 27, 62–69. [Google Scholar] [CrossRef]
- Dobbs, N.; Budak, M.; White, R.; Zealley, I. MR-Eye: High-Resolution Microscopy Coil MRI for the Assessment of the Orbit and Periorbital Structures, Part 2: Clinical Applications. Am. J. Neuroradiol. 2021, 42, 1184–1189. [Google Scholar] [CrossRef]
- Juan, Y.-H.; Saboo, S.S.; Tirumani, S.H.; Khandelwal, A.; Shinagare, A.B.; Ramaiya, N.; Krajewski, K.M. Malignant Skin and Subcutaneous Neoplasms in Adults: Multimodality Imaging With CT, MRI, and 18F-FDG PET/CT. Am. J. Roentgenol. 2014, 202, W422–W438. [Google Scholar] [CrossRef]
- Mahajan, S.; Barker, C.A.; Mauguen, A.; Singh, B.; Pandit-Taskar, N. Restaging [18F] fludeoxyglucose positron emission tomography/computed tomography scan in recurrent cutaneous squamous cell carcinoma: Diagnostic performance and prognostic significance. J. Am. Acad. Dermatol. 2020, 82, 878–886. [Google Scholar] [CrossRef]
- Mahajan, S.; Barker, C.; Pandit-Taskar, N. FDG PET/CT in staging cutaneous squamous cell carcinoma. J. Nucl. Med. 2017, 58, 121. [Google Scholar]
- Schröer-Günther, M.A.; Wolff, R.F.; Westwood, M.E.; Scheibler, F.J.; Schürmann, C.; Baumert, B.G.; Sauerland, S.; Kleijnen, J. F-18-fluoro-2-deoxyglucose positron emission tomography (PET) and PET/computed tomography imaging in primary staging of patients with malignant melanoma: A systematic review. Syst. Rev. 2012, 1, 62. [Google Scholar] [CrossRef]
- Schwartz, J.L.; Wong, S.L.; McLean, S.A.; Hayman, J.A.; Lao, C.D.; Kozlow, J.H.; Malloy, K.M.; Bradford, C.R.; Frohm, M.L.; Fullen, U.R.; et al. NCCN Guidelines implementation in the multidisciplinary Merkel Cell Carcinoma Program at the University of Michigan. J. Natl. Compr. Cancer Netw. 2014, 12, 434–441. [Google Scholar] [CrossRef]
- Madan, V.; Lear, J.T.; Szeimies, R.-M.J.T.l. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef] [PubMed]
- van Loo, E.; Mosterd, K.; Krekels, G.A.; Roozeboom, M.H.; Ostertag, J.U.; Dirksen, C.D.; Steijlen, P.M.; Neumann, H.M.; Nelemans, P.J.; Kelleners-Smeets, N.W. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: A randomised clinical trial with 10year follow-up. Eur. J. Cancer 2014, 50, 3011–3020. [Google Scholar] [CrossRef] [PubMed]
- McCusker, M.; Basset-Seguin, N.; Dummer, R.; Lewis, K.; Schadendorf, D.; Sekulic, A.; Hou, J.; Wang, L.; Yue, H.; Hauschild, A. Metastatic basal cell carcinoma: Prognosis dependent on anatomic site and spread of disease. Eur. J. Cancer 2014, 50, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Ferlito, A.; Takes, R.P.; Bradford, C.R.; Corry, J.; Fagan, J.J.; Rinaldo, A.; Strojan, P.; Rodrigo, J.P. Cutaneous head and neck basal and squamous cell carcinomas with perineural invasion. Oral Oncol. 2012, 48, 918–922. [Google Scholar] [CrossRef]
- Abgral, R.; Querellou, S.; Potard, G.; Le Roux, P.-Y.; Le Duc-Pennec, A.; Marianovski, R.; Pradier, O.; Bizais, Y.; Kraeber-Bodéré, F.; Salaun, P.Y. Does 18F-FDG PET/CT Improve the Detection of Posttreatment Recurrence of Head and Neck Squamous Cell Carcinoma in Patients Negative for Disease on Clinical Follow-up? J. Nucl. Med. 2009, 50, 24–29. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Buck, A.; Krause, B.J.; Scheidhauer, K.; Schwaiger, M. Clinical Applications of FDG PET and PET/CT in Head and Neck Cancer. J. Oncol. 2009, 2009, 1–13. [Google Scholar] [CrossRef]
- Shintani, S.A.; Foote, R.L.; Lowe, V.J.; Brown, P.D.; Garces, Y.I.; Kasperbauer, J.L. Utility of PET/CT Imaging Performed Early After Surgical Resection in the Adjuvant Treatment Planning for Head and Neck Cancer. Int. J. Radiat. Oncol. 2008, 70, 322–329. [Google Scholar] [CrossRef]
- Harms, P.W. Update on Merkel cell carcinoma. Clin. Lab. Med. 2017, 37, 485–501. [Google Scholar] [CrossRef]
- Loh, T.Y.; Rubin, A.G.; Jiang, S.I.B. Basal Cell Carcinoma of the Dorsal Foot: An Update and Comprehensive Review of the Literature. Dermatol. Surg. 2017, 43, 32–39. [Google Scholar] [CrossRef]
- Tran, D.C.; Colevas, A.D.; Chang, A.L.S. Follow-up on Programmed Cell Death 1 Inhibitor for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2017, 153, 92–94. [Google Scholar] [CrossRef]
- Borradori, L.; Sutton, B.; Shayesteh, P.; Daniels, G. Rescue therapy with anti-programmed cell death protein 1 inhibitors of advanced cutaneous squamous cell carcinoma and basosquamous carcinoma: Preliminary experience in five cases. Br. J. Dermatol. 2016, 175, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Guminski, A.D.; Lim, A.M.L.; Khushalani, N.I.; Schmults, C.D.; Hernandez-Aya, L.F.; Modi, B.; Dunn, L.; Hughes, B.G.M.; Chang, A.L.S.; Hauschild, A.; et al. Phase 2 study of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with metastatic cutaneous squamous cell carcinoma (mCSCC; Group 1): 12-month follow-up. J. Clin. Oncol. 2019, 37, 9526. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Papadopoulos, K.P.; Johnson, M.L. Phase 1 Study of Cemiplimab, a Human Monoclonal Anti-PD-1, in Patients with Unresectable Locally Advanced or Metastatic Cutaneous Squamous Cell Carcinoma (CSCC): Final Efficacy and Safety Data. Ski. J. Cutan. Med. 2018, 2, S78. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.F.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, A.; Migden, M.R.; Basset-Seguin, N.; Garbe, C.; Gesierich, A.; Lao, C.D.; Miller, C.; Mortier, L.; Murrell, D.F.; Hamid, O.; et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: Final update of the pivotal ERIVANCE BCC study. BMC Cancer 2017, 17, 332. [Google Scholar] [CrossRef]
- Basset-Séguin, N.; Hauschild, A.; Kunstfeld, R.; Grob, J.; Dréno, B.; Mortier, L.; Ascierto, P.; Licitra, L.; Dutriaux, C.; Thomas, L.; et al. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. Eur. J. Cancer 2017, 86, 334–348. [Google Scholar] [CrossRef]
- Aide, N.; Hicks, R.J.; Le Tourneau, C.; Lheureux, S.; Fanti, S.; Lopci, E. FDG PET/CT for assessing tumour response to immunotherapy. Eur. J. Nucl. Med. 2019, 46, 238–250. [Google Scholar] [CrossRef]
- Wang, G.X.; Kurra, V.; Gainor, J.F.; Sullivan, R.J.; Flaherty, K.T.; Lee, S.I.; Fintelmann, F.J. Immune Checkpoint Inhibitor Cancer Therapy: Spectrum of Imaging Findings. Radiographics 2017, 37, 2132–2144. [Google Scholar] [CrossRef]
- Reckamp, K.L. Real-world pseudoprogression: An uncommon phenomenon. J. Thorac. Oncol. 2018, 13, 880–882. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein Jr, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Lim, A.M.; Cavanagh, K.; Hicks, R.J.; McLean, L.; Goh, M.S.; Webb, A.; Rischin, D. Delayed Response After Confirmed Progression (DR) and Other Unique Immunotherapy-Related Treatment Concepts in Cutaneous Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 656611. [Google Scholar] [CrossRef]
- Chiou, V.L.; Burotto, M. Pseudoprogression and Immune-Related Response in Solid Tumors. J. Clin. Oncol. 2015, 33, 3541–3543. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Piccirillo, M.; Palaia, R.; Petrillo, A.; Lastoria, S.; Izzo, F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int. J. Surg. 2015, 18, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Piccirillo, M.; Leongito, M.; Palaia, R.; Granata, F.; Lastoria, S.; Izzo, F.; Petrillo, A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J. Gastroenterol. 2017, 23, 4767–4778. [Google Scholar] [CrossRef] [PubMed]
- Tirkes, T.; Hollar, M.A.; Tann, M.; Kohli, M.D.; Akisik, F.; Sandrasegaran, K. Response Criteria in Oncologic Imaging: Review of Traditional and New Criteria. Radiographics 2013, 33, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Hochmair, M.; Prosch, H. Pitfalls in the radiological response assessment of immunotherapy. memo—Mag. Eur. Med. Oncol. 2018, 11, 138–143. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1. 1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Borcoman, E.; Kanjanapan, Y.; Champiat, S.; Kato, S.; Servois, V.; Kurzrock, R.; Goel, S.; Bedard, P.; Le Tourneau, C. Novel patterns of response under immunotherapy. Ann. Oncol. 2019, 30, 385–396. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Giobbie-Hurder, A.; Gargano, M.; Suda, M.; Ramaiya, N.H.; Hodi, F.S. Developing a Common Language for Tumor Response to Immunotherapy: Immune-Related Response Criteria Using Unidimensional MeasurementsUnidimensional irRC as a Common Language for Immunotherapy. Clin. Cancer Res. 2013, 19, 3936–3943. [Google Scholar] [CrossRef] [PubMed]
- Sheikhbahaei, S.; Marcus, C.V.; Sadaghiani, M.S.; Rowe, S.P.; Pomper, M.G.; Solnes, L.B. Imaging of Cancer Immunotherapy: Response Assessment Methods, Atypical Response Patterns, and Immune-Related Adverse Events, From the AJR Special Series on Imaging of Inflammation. Am. J. Roentgenol. 2022, 218, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Cousin, S.; Italiano, A.J.C.C.R. Molecular Pathways: Immune Checkpoint Antibodies and their ToxicitiesSafety Profile of Immune Checkpoint Antibodies. Clin. Cancer Res. 2016, 22, 4550–4555. [Google Scholar] [CrossRef] [PubMed]
- Kroschinsky, F.; on behalf of the Intensive Care in Hematological and Oncological Patients (iCHOP) Collaborative Group; Stölzel, F.; von Bonin, S.; Beutel, G.; Kochanek, M.; Kiehl, M.; Schellongowski, P. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care 2017, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) V5; US Department of Health and Human Services: Washington, DC, USA, 2017.
- Arnaud-Coffin, P.; Maillet, D.; Gan, H.K.; Stelmes, J.-J.; You, B.; Dalle, S.; Péron, J. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int. J. Cancer 2019, 145, 639–648. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Tang, S.-Q.; Tang, L.-L.; Mao, Y.-P.; Li, W.-F.; Chen, L.; Zhang, Y.; Guo, Y.; Liu, Q.; Sun, Y.; Xu, C.; et al. The Pattern of Time to Onset and Resolution of Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors in Cancer: A Pooled Analysis of 23 Clinical Trials and 8,436 Patients. Cancer Res. Treat. 2021, 53, 339–354. [Google Scholar] [CrossRef]
- Delaunay, M.; Cadranel, J.; Lusque, A.; Meyer, N.; Gounaut, V.; Moro-Sibilot, D.; Michot, J.-M.; Raimbourg, J.; Girard, N.; Guisier, F.; et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur. Respir. J. 2017, 50, 1700050. [Google Scholar] [CrossRef]
- Nishino, M.; Hatabu, H.; Hodi, F.S. Imaging of Cancer Immunotherapy: Current Approaches and Future Directions. Radiology 2019, 290, 9–22. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef] [PubMed]
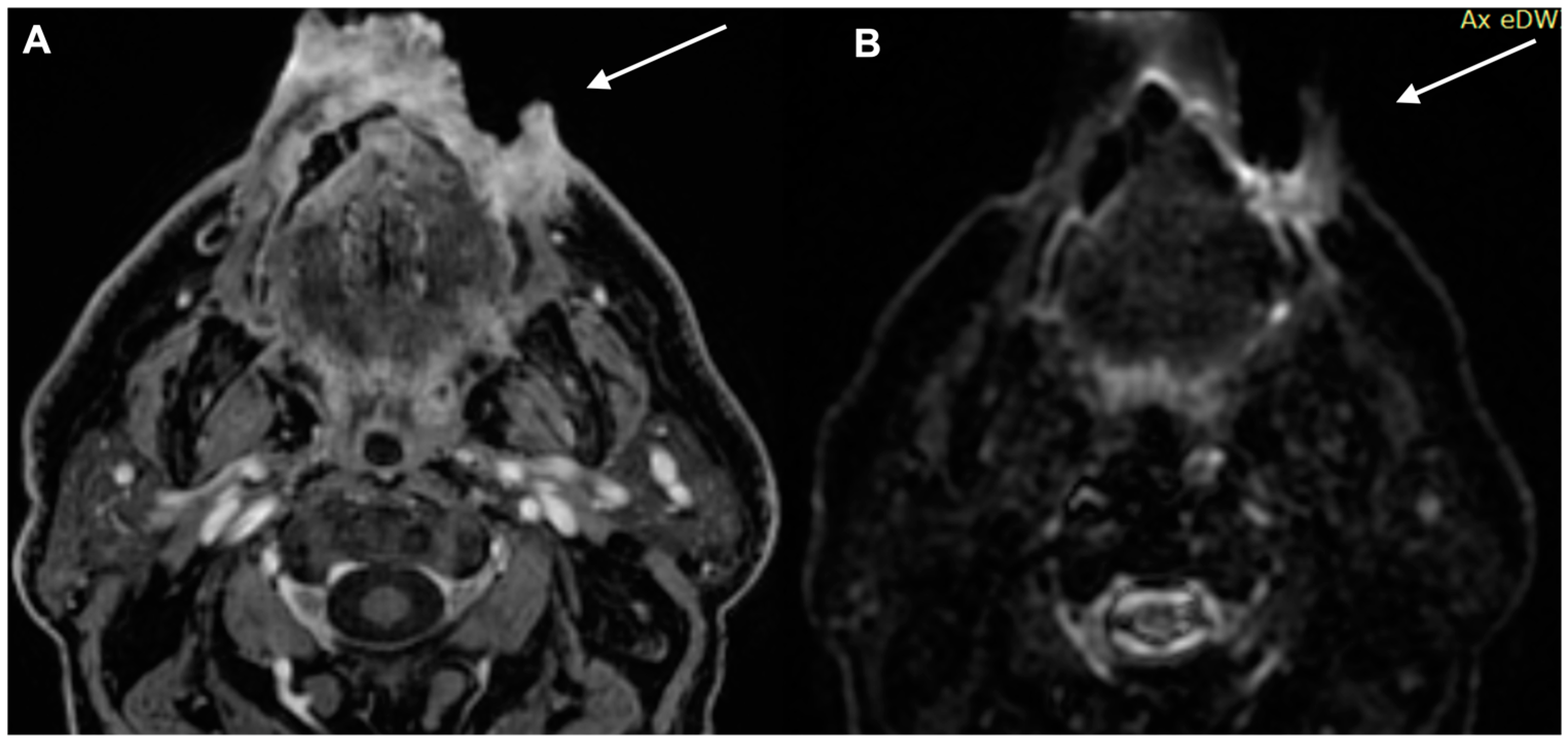
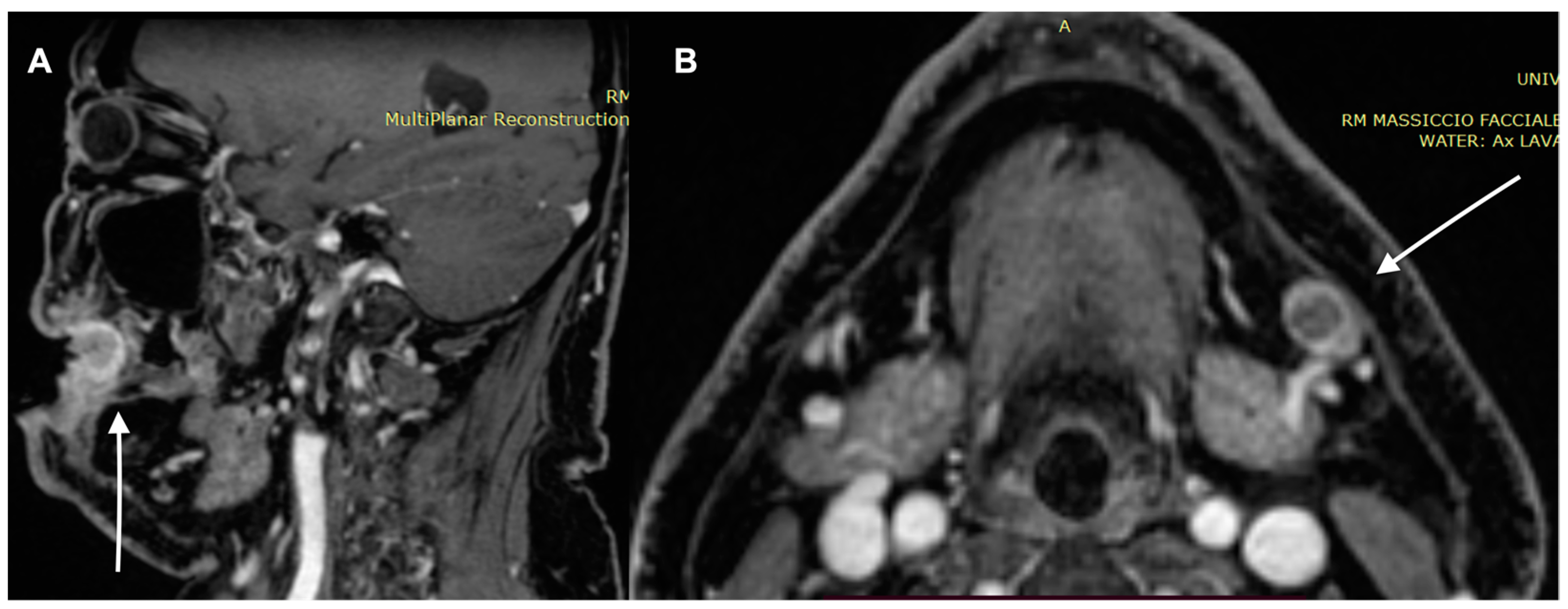
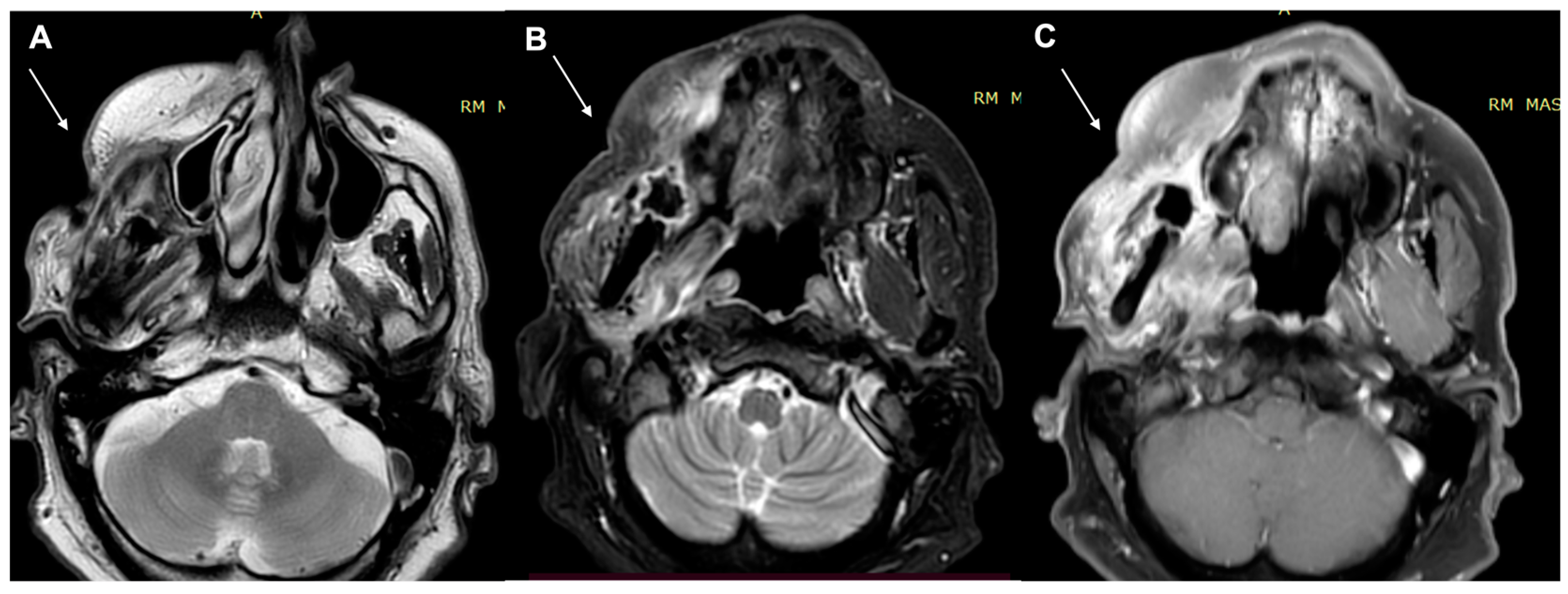
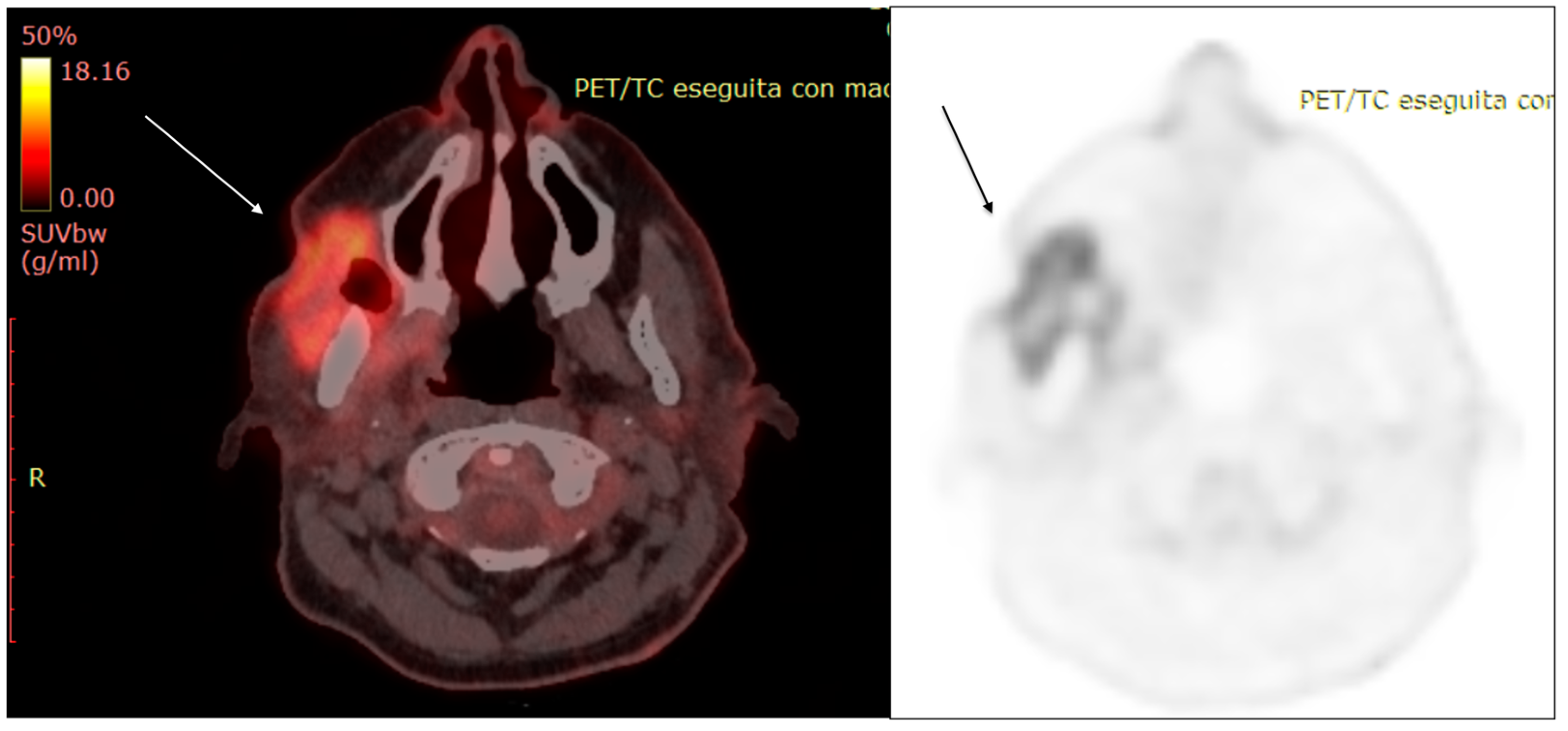
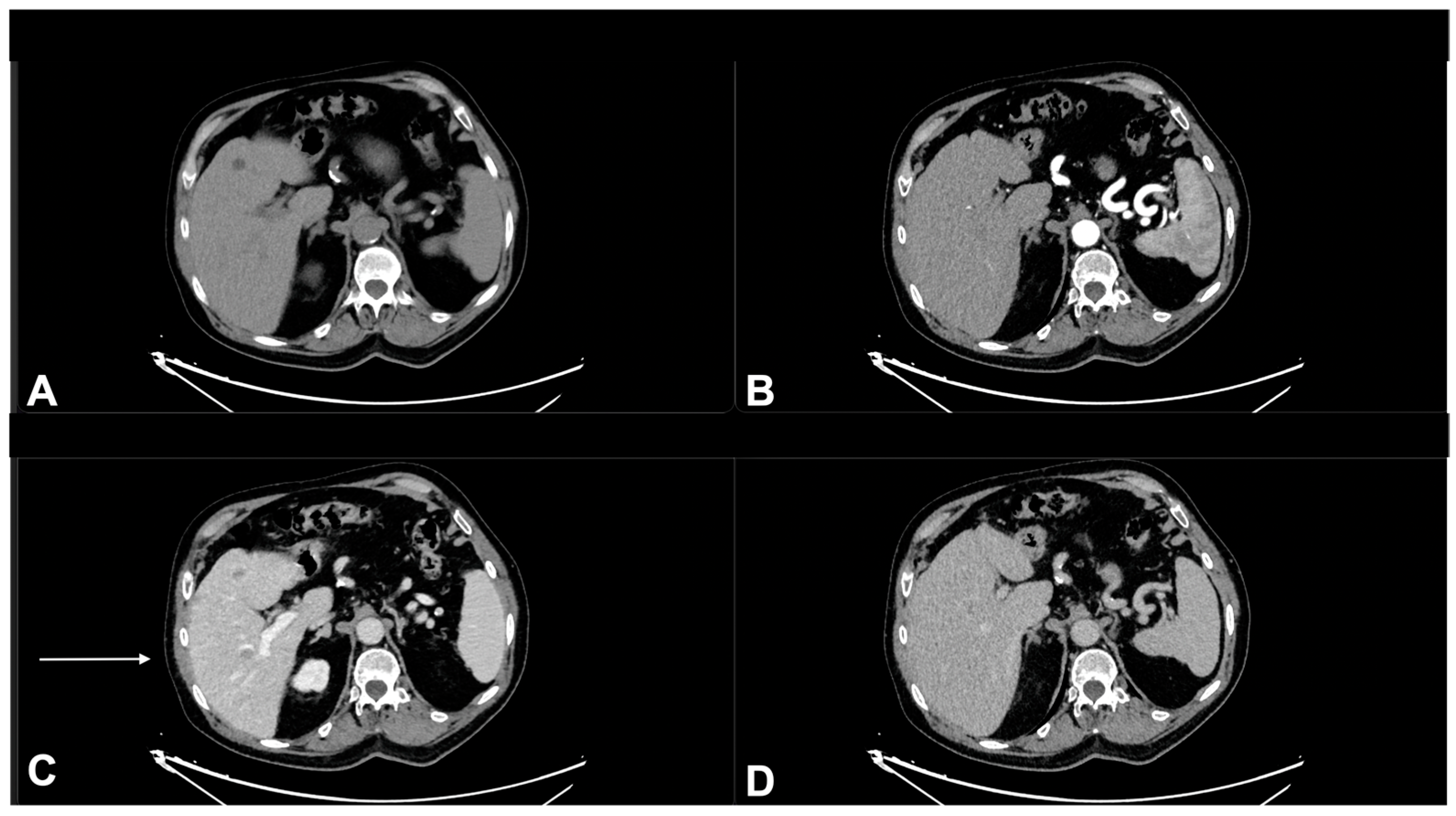


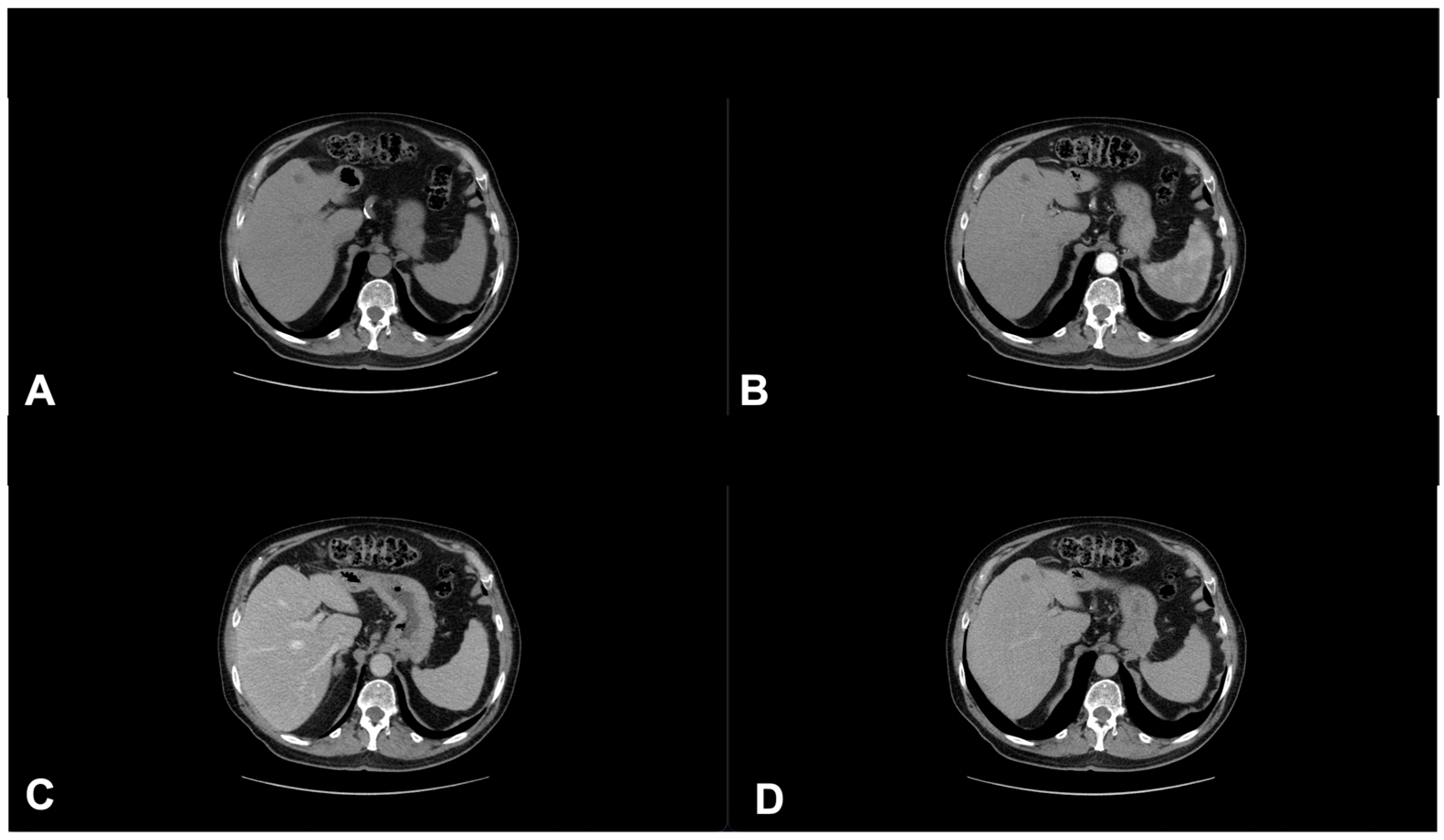
| Characteristics of Pseudo Progression and Hyper Progression | |
|---|---|
| Pseudo progression | Hyper-progression |
| Pseudo progression is an initial progression in which the tumor burden or the number of tumor lesions increase initially and then decreases over time. | Hyperprogression is a tumor response in which the existing underlying tumor grows rapidly after initiating treatment with an immune checkpoint inhibitor. |
| Pseudo progression is not true tumour progression, which has been proven by histopathological biopsies that found infiltration and recruitment of various immune cells, such as T or B lymphocytes, in the tumor. | Tumor samples of people who experienced hyperprogression were found to have a greater number of tumor-associated macrophages (macrophages are cells that are part of the immune system that are present in the area surrounding tumors or “tumor microenvironment”). |
| The occurrence of pseudoprogression has led to the development of immune-related response-evaluation criteria. In this phenomenon, patients treated with immunotherapy experience an initial increase in tumor burden through enlargement of target lesions and/or development of new lesions, followed by a subsequent decrease in the tumor burden qualifying as a partial or complete response. | Hyper progression involves not only the more rapid growth of a tumor but a lower survival rate. In patients developing hyperprogression, immunotherapy treatment should be stopped and the patient should be managed appropriately. |
| Table of Definitions | |
|---|---|
| iCR | Immune control response is the disappearance of all lesions, measured or unmeasured, and no new lesions. |
| iSD | Immune stable disease is referred as cancer that is neither decreasing nor increasing in extent or severity. |
| iPr | unconfirmed progression disease |
| iUPD | increase of non-target lesions or appearance of new lesion called iUPD |
| iCPD | Development of another new lesion, increased size of the target or non-target lesions, and/or unequivocal progression of existing non-target lesions. |
| irAEs | ||
|---|---|---|
| Favourable | Neutral | Unfavourable |
| Developing an irAE | Pruritus | High grade irAEs |
| Certain irAEs: skin(vitiligo), endocrine, hepatic, gut hypophysitis, and colitis | Taking short term steroids for irAEs | Pre-existing autoimmune disease, i.e., earlier and high prevalence of irAEs |
| Pre-existing psoriasis | Radiotherapy after treatment | Steroid use for cancer related symptoms |
| Steroid-sparing therapy | Mucosal melanoma | |
| Combined with radiotherapy | Low PD L1 expression | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, G.M.; Russo, A.; Urraro, F.; Cioce, F.; Gallo, L.; Belfiore, M.P.; Sangiovanni, A.; Napolitano, S.; Troiani, T.; Verolino, P.; et al. Management of Non-Melanoma Skin Cancer: Radiologists Challenging and Risk Assessment. Diagnostics 2023, 13, 793. https://doi.org/10.3390/diagnostics13040793
Russo GM, Russo A, Urraro F, Cioce F, Gallo L, Belfiore MP, Sangiovanni A, Napolitano S, Troiani T, Verolino P, et al. Management of Non-Melanoma Skin Cancer: Radiologists Challenging and Risk Assessment. Diagnostics. 2023; 13(4):793. https://doi.org/10.3390/diagnostics13040793
Chicago/Turabian StyleRusso, Gaetano Maria, Anna Russo, Fabrizio Urraro, Fabrizio Cioce, Luigi Gallo, Maria Paola Belfiore, Angelo Sangiovanni, Stefania Napolitano, Teresa Troiani, Pasquale Verolino, and et al. 2023. "Management of Non-Melanoma Skin Cancer: Radiologists Challenging and Risk Assessment" Diagnostics 13, no. 4: 793. https://doi.org/10.3390/diagnostics13040793
APA StyleRusso, G. M., Russo, A., Urraro, F., Cioce, F., Gallo, L., Belfiore, M. P., Sangiovanni, A., Napolitano, S., Troiani, T., Verolino, P., Sica, A., Brancaccio, G., Briatico, G., Nardone, V., & Reginelli, A. (2023). Management of Non-Melanoma Skin Cancer: Radiologists Challenging and Risk Assessment. Diagnostics, 13(4), 793. https://doi.org/10.3390/diagnostics13040793







