Blood Biomarkers in Patients with Parkinson’s Disease: A Review in Context of Anesthetic Care
Abstract
1. Introduction
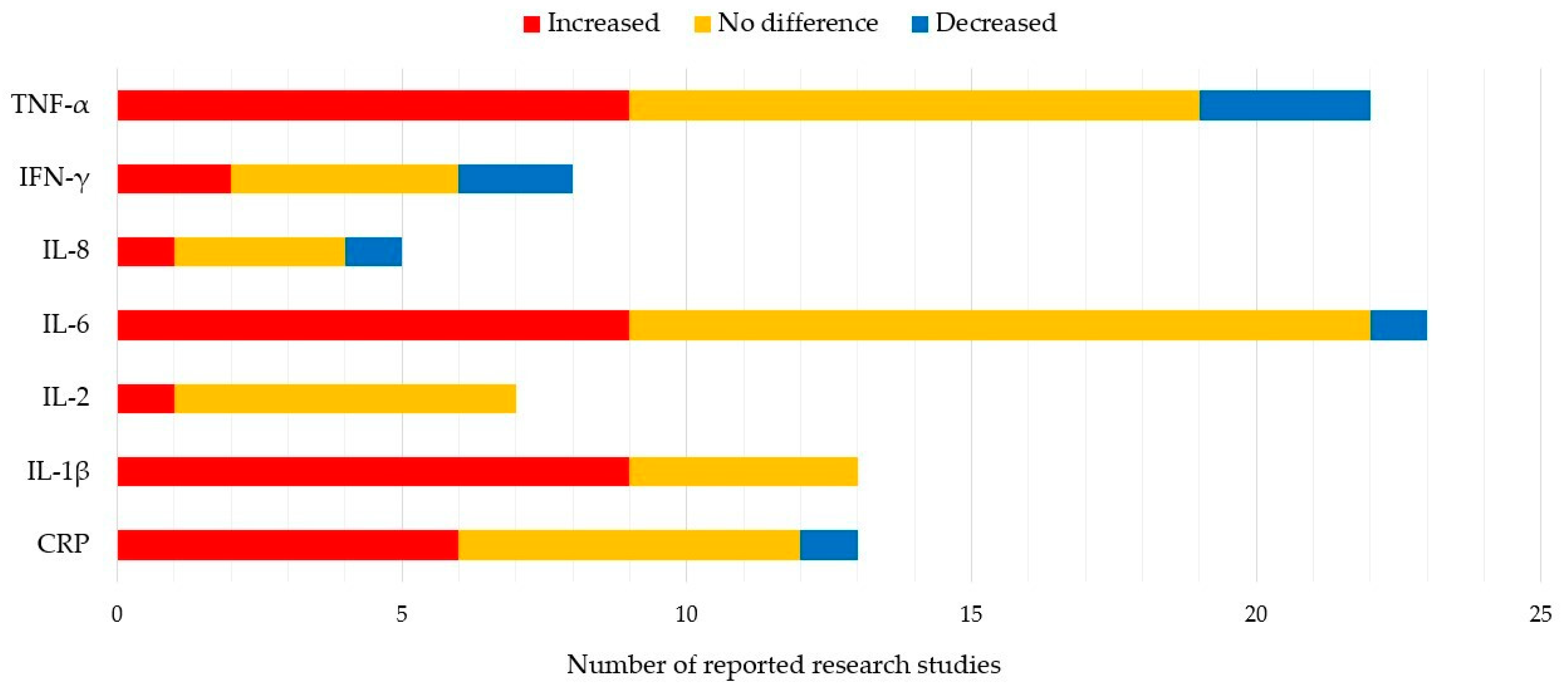
2. Blood Inflammatory Biomarkers in PD Patients
3. Effects of Blood Biomarkers on PD Symptoms
4. Influence of Peripheral Inflammation on the CNS
5. Impact of Surgery and Anesthesia on the Immune System
6. Influence of Surgery and Anesthesia on the Prognosis of Parkinson’s Disease
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delenclos, M.; Jones, D.R.; McLean, P.J.; Uitti, R.J. Biomarkers in Parkinson’s disease: Advances and strategies. Parkinsonism Relat. Disord. 2016, 22, S106–S110. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Odin, P.; Antonini, A.; Martinez-Martin, P. Parkinson’s disease: The non-motor issues. Parkinsonism Relat. Disord. 2011, 17, 717–723. [Google Scholar] [CrossRef]
- Corrado, L.; De Marchi, F.; Tunesi, S.; Oggioni, G.D.; Carecchio, M.; Magistrelli, L.; Tesei, S.; Riboldazzi, G.; Di Fonzo, A.; Locci, C.; et al. The Length of SNCA Rep1 Microsatellite May Influence Cognitive Evolution in Parkinson’s Disease. Front. Neurol. 2018, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, J.L.; Wszolek, Z.; Petrucelli, L. Molecular pathogenesis of Parkinson disease. Arch. Neurol. 2005, 62, 353–357. [Google Scholar] [CrossRef]
- Schapira, A.H.; Jenner, P. Etiology and pathogenesis of Parkinson’s disease. Mov. Disord. 2011, 26, 1049–1055. [Google Scholar] [CrossRef]
- More, S.V.; Kumar, H.; Kim, I.S.; Song, S.Y.; Choi, D.K. Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediators Inflamm. 2013, 2013, 952375. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat. Disord. 2004, 10, S3–S7. [Google Scholar] [CrossRef]
- González, H.; Elgueta, D.; Montoya, A.; Pacheco, R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J. Neuroimmunol. 2014, 274, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mosley, R.L.; Hutter-Saunders, J.A.; Stone, D.K.; Gendelman, H.E. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009381. [Google Scholar] [CrossRef]
- Lee, J.K.; Tran, T.; Tansey, M.G. Neuroinflammation in Parkinson’s disease. J. Neuroimmun. Pharmacol. 2009, 4, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Monahan, A.J.; Warren, M.; Carvey, P.M. Neuroinflammation and peripheral immune infiltration in Parkinson’s disease: An autoimmune hypothesis. Cell Transpl. 2008, 17, 363–372. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Akiyama, H.; McGeer, E.G. Rate of cell death in parkinsonism indicates active neuropathological process. Ann. Neurol. 1988, 24, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Mosley, R.L.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, Oxidative Stress and the Pathogenesis of Parkinson’s Disease. Clin. Neurosci. Res. 2006, 6, 261–281. [Google Scholar] [CrossRef]
- Church, F.C. Treatment Options for Motor and Non-Motor Symptoms of Parkinson’s Disease. Biomolecules 2021, 11, 612. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations (*). Ann. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Paul, W.E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 2010, 327, 1098–1102. [Google Scholar] [CrossRef]
- González, H.; Contreras, F.; Pacheco, R. Regulation of the Neurodegenerative Process Associated to Parkinson’s Disease by CD4+ T-cells. J. Neuroimmun. Pharmacol. 2015, 10, 561–575. [Google Scholar] [CrossRef]
- Tahmasebinia, F.; Pourgholaminejad, A. The role of Th17 cells in auto-inflammatory neurological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79 (Pt B), 408–416. [Google Scholar] [CrossRef]
- Jiang, S.; Gao, H.; Luo, Q.; Wang, P.; Yang, X. The correlation of lymphocyte subsets, natural killer cell, and Parkinson’s disease: A meta-analysis. Neurol. Sci. 2017, 38, 1373–1380. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Rasini, E.; Legnaro, M.; Bombelli, R.; Aleksic, I.; Blandini, F.; Comi, C.; Mauri, M.; Minafra, B.; Riboldazzi, G.; et al. Dopaminergic Receptors on CD4+ T Naive and Memory Lymphocytes Correlate with Motor Impairment in Patients with Parkinson’s Disease. Sci. Rep. 2016, 6, 33738. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Comi, C.; Magistrelli, L.; Rasini, E.; Legnaro, M.; Bombelli, R.; Aleksic, I.; Blandini, F.; Minafra, B.; Riboldazzi, G.; et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: Cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naïve and drug-treated patients. J. Neuroinflamm. 2018, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Wijeyekoon, R.; Yarnall, A.J.; Lawson, R.A.; Breen, D.P.; Evans, J.R.; Cummins, G.A.; Duncan, G.W.; Khoo, T.K.; Burn, D.J.; et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov. Disord. 2016, 31, 995–1003. [Google Scholar] [CrossRef]
- Veselý, B.; Dufek, M.; Thon, V.; Brozman, M.; Királová, S.; Halászová, T.; Koriťáková, E.; Rektor, I. Interleukin 6 and complement serum level study in Parkinson’s disease. J. Neural. Transm (Vienna) 2018, 125, 875–881. [Google Scholar] [CrossRef]
- Schlachetzki, J.C.; Winkler, J. The innate immune system in Parkinson’s disease: A novel target promoting endogenous neuroregeneration. Neural Regen. Res. 2015, 10, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Storelli, E.; Cassina, N.; Rasini, E.; Marino, F.; Cosentino, M. Do Th17 Lymphocytes and IL-17 Contribute to Parkinson’s Disease? A Systematic Review of Available Evidence. Front. Neurol. 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Kaufman, E.; Brundin, L.; Hall, S.; Surova, Y.; Hansson, O. Non-motor symptoms in patients with Parkinson’s disease-correlations with inflammatory cytokines in serum. PLoS ONE 2012, 7, e47387. [Google Scholar] [CrossRef]
- Gupta, V.; Garg, R.K.; Khattri, S. Levels of IL-8 and TNF-α decrease in Parkinson’s disease. Neurol. Res. 2016, 38, 98–102. [Google Scholar] [CrossRef]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef]
- Pirbudak Cocelli, L.; Ugur, M.G.; Karadasli, H. Comparison of effects of low-flow sevoflurane and desflurane anesthesia on neutrophil and T-cell populations. Curr. Ther. Res. Clin. Exp. 2012, 73, 41–51. [Google Scholar] [CrossRef]
- Jiao, B.; Yang, C.; Huang, N.N.; Yang, N.; Wei, J.; Xu, H. Relationship between Volatile Anesthetics and Tumor Progression: Unveiling the Mystery. Curr. Med. Sci. 2018, 38, 962–967. [Google Scholar] [CrossRef]
- Marana, E.; Russo, A.; Colicci, S.; Polidori, L.; Bevilacqua, F.; Viviani, D.; Di Stasio, E. Desflurane versus sevoflurane: A comparison on stress response. Minerva Anestesiol. 2013, 79, 7–14. [Google Scholar]
- Allaouchiche, B.; Debon, R.; Goudable, J.; Chassard, D.; Duflo, F. Oxidative stress status during exposure to propofol, sevoflurane and desflurane. Anesth. Analg. 2001, 93, 981–985. [Google Scholar] [CrossRef]
- Shan, Z.; Cai, S.; Zhang, T.; Kuang, L.; Wang, Q.; Xiu, H.; Wen, J.; Gu, H.; Xu, K. Effects of sevoflurane on leucine-rich repeat kinase 2-associated Drosophila model of Parkinson’s disease. Mol. Med. Rep. 2015, 11, 2062–2070. [Google Scholar] [PubMed]
- Goh, G.S.; Zeng, G.J.; Tay, D.K.; Lo, N.N.; Yeo, S.J.; Liow, M.H.L. Patients With Parkinson’s Disease Have Poorer Function and More Flexion Contractures After Total Knee Arthroplasty. J. Arthropl. 2021, 36, 2325–2330. [Google Scholar] [CrossRef]
- Oğuz, E.; Öztürk, İ.; Özkan, D.; Ergil, J.; Aydın, G.B. Parkinson’s Disease and Spinal Anaesthesia. Turk. J. Anaesthesiol. Reanim. 2014, 42, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Merchán, E.C.; Kalbakdij-Sánchez, C. The impact of Parkinson’s disease on results of primary total knee arthroplasty. EFORT Open Rev. 2022, 7, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Rondon, A.J.; Tan, T.L.; Schlitt, P.K.; Greenky, M.R.; Phillips, J.L.; Purtill, J.J. Total Joint Arthroplasty in Patients With Parkinson’s Disease: Survivorship, Outcomes, and Reasons for Failure. J. Arthropl. 2018, 33, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Ton, T.G.; Jain, S.; Biggs, M.L.; Thacker, E.L.; Strotmeyer, E.S.; Boudreau, R.; Newman, A.B.; Longstreth, W.T., Jr.; Checkoway, H. Markers of inflammation in prevalent and incident Parkinson’s disease in the Cardiovascular Health Study. Parkinson. Relat. Disord. 2012, 18, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Andican, G.; Konukoglu, D.; Bozluolcay, M.; Bayülkem, K.; Firtiına, S.; Burcak, G. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson’s disease. Acta Neurol. Belg. 2012, 112, 155–159. [Google Scholar] [CrossRef]
- Sawada, H.; Oeda, T.; Umemura, A.; Tomita, S.; Hayashi, R.; Kohsaka, M.; Yamamoto, K.; Sudoh, S.; Sugiyama, H. Subclinical elevation of plasma C-reactive protein and illusions/hallucinations in subjects with Parkinson’s disease: Case-control study. PLoS ONE 2014, 9, e85886. [Google Scholar] [CrossRef]
- Qin, X.Y.; Zhang, S.P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, Y.G.; Li, A.L.; Long, Z.H.; Wang, D.; Li, X.X.; Xia, J.H.; Luo, S.Y.; Shan, Y.H. Relationship between levels of inflammatory cytokines in the peripheral blood and the severity of depression and anxiety in patients with Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3853–3856. [Google Scholar]
- Kim, R.; Kim, H.J.; Kim, A.; Jang, M.; Kim, A.; Kim, Y.; Yoo, D.; Im, J.H.; Choi, J.H.; Jeon, B. Peripheral blood inflammatory markers in early Parkinson’s disease. J. Clin. Neurosci. 2018, 58, 30–33. [Google Scholar] [CrossRef]
- King, E.; O’Brien, J.; Donaghy, P.; Williams-Gray, C.H.; Lawson, R.A.; Morris, C.M.; Barnett, N.; Olsen, K.; Martin-Ruiz, C.; Burn, D.; et al. Inflammation in mild cognitive impairment due to Parkinson’s disease, Lewy body disease, and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2019, 34, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Santos-García, D.; de Deus Fonticoba, T.; Suárez Castro, E.; Aneiros Díaz, A.; Paz González, J.M.; Feal Panceiras, M.J.; García Sancho, C.; Jesús, S.; Mir, P.; Aguilar, M.; et al. High ultrasensitive serum C-reactive protein may be related to freezing of gait in Parkinson’s disease patients. J. Neural. Transm. 2019, 126, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Bulut, M.; Kaya, M.C.; Demirpençe, Ö.; Sevim, B.; Akıl, E.; Varol, S. High-sensitivity C-reactive protein and high mobility group box-1 levels in Parkinson’s disease. Neurol. Sci. 2019, 40, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Gu, H.Y.; Mao, C.J.; Chen, J.; Liu, C.F. Association of inflammatory factors and aging in Parkinson’s disease. Neurosci. Lett. 2020, 736, 135259. [Google Scholar] [CrossRef] [PubMed]
- Dommershuijsen, L.J.; Ruiter, R.; Erler, N.S.; Rizopoulos, D.; Ikram, M.A.; Ikram, M.K. Peripheral Immune Cell Numbers and C-Reactive Protein in Parkinson’s Disease: Results from a Population-Based Study. J. Parkinsons Dis. 2022, 12, 667–678. [Google Scholar] [CrossRef]
- Koziorowski, D.; Tomasiuk, R.; Szlufik, S.; Friedman, A. Inflammatory cytokines and NT-proCNP in Parkinson’s disease patients. Cytokine 2012, 60, 762–766. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, S.Y.; Zuo, L.J.; Cao, C.J.; Wang, F.; Chen, Z.J.; Du, Y.; Lian, T.H.; Wang, Y.J.; Chan, P.; et al. Parkinson disease with REM sleep behavior disorder: Features, α-synuclein, and inflammation. Neurology 2015, 84, 888–894. [Google Scholar] [CrossRef]
- Milyukhina, I.V.; Karpenko, M.N.; Klimenko, V.M. Clinical parameters and the level of certain cytokines in blood and cerebrospinal fluid of patients with Parkinson’s disease. Klin. Med. 2015, 93, 51–55. [Google Scholar]
- Brockmann, K.; Schulte, C.; Schneiderhan-Marra, N.; Apel, A.; Pont-Sunyer, C.; Vilas, D.; Ruiz-Martinez, J.; Langkamp, M.; Corvol, J.C.; Cormier, F.; et al. Inflammatory profile in LRRK2-associated prodromal and clinical PD. Eur. J. Neurol. 2017, 24, 122. [Google Scholar] [CrossRef]
- Karpenko, M.N.; Vasilishina, A.A.; Gromova, E.A.; Muruzheva, Z.M.; Miliukhina, I.V.; Bernadotte, A. Interleukin-1β, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-α levels in CSF and serum in relation to the clinical diversity of Parkinson’s disease. Cell. Immunol. 2018, 327, 77–82. [Google Scholar] [CrossRef]
- Rocha, N.P.; Assis, F.; Scalzo, P.L.; Vieira, É.L.M.; Barbosa, I.G.; de Souza, M.S.; Christo, P.P.; Reis, H.J.; Teixeira, A.L. Reduced Activated T Lymphocytes (CD4+CD25+) and Plasma Levels of Cytokines in Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Alrafiah, A.; Al-Ofi, E.; Obaid, M.T.; Alsomali, N. Assessment of the Levels of Level of Biomarkers of Bone Matrix Glycoproteins and Inflammatory Cytokines from Saudi Parkinson Patients. Biomed. Res. Int. 2019, 2019, 2690205. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.H.; Guo, P.; Zuo, L.J.; Hu, Y.; Yu, S.Y.; Yu, Q.J.; Jin, Z.; Wang, R.D.; Li, L.X.; Zhang, W. Tremor-Dominant in Parkinson Disease: The Relevance to Iron Metabolism and Inflammation. Front. Neurosci. 2019, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Roy, A.; Banerjee, R.; Choudhury, S.; Mondal, B.; Halder, S.; Basu, P.; Shubham, S.; Dey, S.; Kumar, H. Inflammasome and α-synuclein in Parkinson’s disease: A cross-sectional study. J. Neuroimmunol. 2020, 338, 577089. [Google Scholar] [CrossRef]
- Fan, Z.; Pan, Y.T.; Zhang, Z.Y.; Yang, H.; Yu, S.Y.; Zheng, Y.; Ma, J.H.; Wang, X.M. Systemic activation of NLRP3 inflammasome and plasma α-synuclein levels are correlated with motor severity and progression in Parkinson’s disease. J. Neuroinflamm. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, P.; Stemberger, S.; Sprenger, F.; Rainer, J.; Hametner, E.; Kirchmair, R.; Grabmer, C.; Scherfler, C.; Wenning, G.K.; Seppi, K.; et al. An antibody microarray analysis of serum cytokines in neurodegenerative Parkinsonian syndromes. Proteome Sci. 2012, 10, 71. [Google Scholar] [CrossRef]
- Schröder, J.B.; Pawlowski, M.; Meyer Zu Hörste, G.; Gross, C.C.; Wiendl, H.; Meuth, S.G.; Ruck, T.; Warnecke, T. Immune Cell Activation in the Cerebrospinal Fluid of Patients With Parkinson’s Disease. Front. Neurol. 2018, 9, 1081. [Google Scholar] [CrossRef]
- Tang, P.; Chong, L.; Li, X.; Liu, Y.; Liu, P.; Hou, C.; Li, R. Correlation between serum RANTES levels and the severity of Parkinson’s disease. Oxid. Med. Cell. Longev. 2014, 2014, 208408. [Google Scholar] [CrossRef]
- Delgado-Alvarado, M.; Gago, B.; Gorostidi, A.; Jiménez-Urbieta, H.; Dacosta-Aguayo, R.; Navalpotro-Gómez, I.; Ruiz-Martínez, J.; Bergareche, A.; Martí-Massó, J.F.; Martínez-Lage, P.; et al. Tau/α-synuclein ratio and inflammatory proteins in Parkinson’s disease: An exploratory study. Mov. Disord. 2017, 32, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek-Majkusiak, J.; Geremek, M.; Koziorowski, D.; Tomasiuk, R.; Szlufik, S.; Friedman, A. Serum levels of hepcidin and interleukin 6 in Parkinson’s disease. Acta Neurobiol. Exp. 2020, 80, 297–304. [Google Scholar] [CrossRef]
- Miliukhina, I.V.; Usenko, T.S.; Senkevich, K.A.; Nikolaev, M.A.; Timofeeva, A.A.; Agapova, E.A.; Semenov, A.V.; Lubimova, N.E.; Totolyan, A.A.; Pchelina, S.N. Plasma Cytokines Profile in Patients with Parkinson’s Disease Associated with Mutations in GBA Gene. Bull. Exp. Biol. Med. 2020, 168, 423–426. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Landi, G.; Marini, F.; Biancolillo, A.; Coelho-Junior, H.J.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Arcidiacono, A.; et al. A novel multi-marker discovery approach identifies new serum biomarkers for Parkinson’s disease in older people: An EXosomes in PArkiNson Disease (EXPAND) ancillary study. Geroscience 2020, 42, 1323–1334. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.C.; Chiang, H.L.; Liou, J.M.; Chang, C.M.; Lu, T.P.; Chuang, E.Y.; Tai, Y.C.; Cheng, C.; Lin, H.Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflamm. 2019, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, D.; Chang, T.; Udagama, P. Selected serum cytokines and nitric oxide as potential multi-marker biosignature panels for Parkinson disease of varying durations: A case-control study. BMC Neurol. 2019, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Usenko, T.S.; Nikolaev, M.A.; Miliukhina, I.V.; Bezrukova, A.I.; Senkevich, K.A.; Gomzyakova, N.A.; Beltceva, Y.A.; Zalutskaya, N.M.; Gracheva, E.V.; Timofeeva, A.A.; et al. Plasma cytokine profile in synucleinophaties with dementia. J. Clin. Neurosci. 2020, 78, 323–326. [Google Scholar] [CrossRef]
- Eidson, L.N.; Kannarkat, G.T.; Barnum, C.J.; Chang, J.; Chung, J.; Caspell-Garcia, C.; Taylor, P.; Mollenhauer, B.; Schlossmacher, M.G.; Ereshefsky, L.; et al. Candidate inflammatory biomarkers display unique relationships with alpha-synuclein and correlate with measures of disease severity in subjects with Parkinson’s disease. J. Neuroinflamm. 2017, 14, 164. [Google Scholar] [CrossRef]
- Martin-Ruiz, C.; Williams-Gray, C.H.; Yarnall, A.J.; Boucher, J.J.; Lawson, R.A.; Wijeyekoon, R.S.; Barker, R.A.; Kolenda, C.; Parker, C.; Burn, D.J.; et al. Senescence and Inflammatory Markers for Predicting Clinical Progression in Parkinson’s Disease: The ICICLE-PD Study. J. Parkinsons Dis. 2020, 10, 193–206. [Google Scholar] [CrossRef]
- Csencsits-Smith, K.; Suescun, J.; Li, K.; Luo, S.; Bick, D.L.; Schiess, M. Serum Lymphocyte-Associated Cytokine Concentrations Change More Rapidly over Time in Multiple System Atrophy Compared to Parkinson Disease. Neuroimmunomodulation 2016, 23, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Kouchaki, E.; Kakhaki, R.D.; Tamtaji, O.R.; Dadgostar, E.; Behnam, M.; Nikoueinejad, H.; Akbari, H. Increased serum levels of TNF-α and decreased serum levels of IL-27 in patients with Parkinson disease and their correlation with disease severity. Clin. Neurol. Neurosurg. 2018, 166, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, V.; Khorramdelazad, H.; Hassanshahi, G.; Moghadam-Ahmadi, A.; Vakilian, A. CXCL12 and CXCR4 in the Peripheral Blood of Patients with Parkinson’s Disease. Neuroimmunomodulation 2018, 25, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.Z.; Schönwald, S.V.; Schumacher-Schuh, A.F.; Braga, C.W.; Souza, D.O.; Oses, J.P.; Donis, K.C.; Rieder, C.R. Overnight S100B in Parkinson’s Disease: A glimpse into sleep-related neuroinflammation. Neurosci Lett. 2015, 608, 57–63. [Google Scholar] [CrossRef]
- Dufek, M.; Rektorova, I.; Thon, V.; Lokaj, J.; Rektor, I. Interleukin-6 May Contribute to Mortality in Parkinson’s Disease Patients: A 4-Year Prospective Study. Parkinsons Dis. 2015, 2015, 898192. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Marta, D.; Dănău, A.; Lefter, A.; Tulbă, D.; Cozma, L.; Manole, E.; Gherghiceanu, M.; Ceafalan, L.C.; Popescu, B.O. Serum and Fecal Markers of Intestinal Inflammation and Intestinal Barrier Permeability Are Elevated in Parkinson’s Disease. Front. Neurosci. 2021, 15, 689723. [Google Scholar] [CrossRef]
- Green, H.F.; Khosousi, S.; Svenningsson, P. Plasma IL-6 and IL-17A Correlate with Severity of Motor and Non-Motor Symptoms in Parkinson’s Disease. J. Parkinsons Dis. 2019, 9, 705–709. [Google Scholar] [CrossRef]
- Gupta, M.; Paliwal, V.K.; Babu, G.N. Serum fractalkine and 3-nitrotyrosine levels correlate with disease severity in Parkinson’s disease: A pilot study. Metab. Brain Dis. 2022, 37, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Herlofson, K.; Heijnen, C.J.; Lange, J.; Alves, G.; Tysnes, O.B.; Friedman, J.H.; Fagundes, C.P. Inflammation and fatigue in early, untreated Parkinson’s Disease. Acta Neurol. Scand. 2018, 138, 394–399. [Google Scholar] [CrossRef]
- Lerche, S.; Zimmermann, M.; Wurster, I.; Roeben, B.; Fries, F.L.; Deuschle, C.; Waniek, K.; Lachmann, I.; Gasser, T.; Jakobi, M.; et al. CSF and Serum Levels of Inflammatory Markers in PD: Sparse Correlation, Sex Differences and Association With Neurodegenerative Biomarkers. Front. Neurol. 2022, 13, 834580. [Google Scholar] [CrossRef]
- Pereira, J.R.; Santos, L.V.D.; Santos, R.M.S.; Campos, A.L.F.; Pimenta, A.L.; de Oliveira, M.S.; Bacheti, G.G.; Rocha, N.P.; Teixeira, A.L.; Christo, P.P.; et al. IL-6 serum levels are elevated in Parkinson’s disease patients with fatigue compared to patients without fatigue. J. Neurol. Sci. 2016, 370, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Perner, C.; Perner, F.; Gaur, N.; Zimmermann, S.; Witte, O.W.; Heidel, F.H.; Grosskreutz, J.; Prell, T. Plasma VCAM1 levels correlate with disease severity in Parkinson’s disease. J. Neuroinflamm. 2019, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.P.; Teixeira, A.L.; Scalzo, P.L.; Barbosa, I.G.; de Sousa, M.S.; Morato, I.B.; Vieira, E.L.; Christo, P.P.; Palotás, A.; Reis, H.J. Plasma levels of soluble tumor necrosis factor receptors are associated with cognitive performance in Parkinson’s disease. Mov. Disord. 2014, 29, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Choudhury, S.; Banerjee, R.; Basu, P.; Kumar, H. Soluble LAG-3 and Toll-interacting protein: Novel upstream neuro-inflammatory markers in Parkinson’s disease. Parkinsonism Relat. Disord. 2021, 91, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Imamura, K.; Nagatsu, T. Role of cytokines in inflammatory processes in Parkinson’s disease. J. Neural Transm. Suppl. 2006, 70, 373–381. [Google Scholar]
- Sun, C.; Yu, W.; Zhao, Z.; Song, C.; Liu, Y.; Jia, G.; Wang, X.; Liu, Y. Peripheral humoral immune response is associated with the non-motor symptoms of Parkinson’s disease. Front. Neurosci. 2019, 13, 1057. [Google Scholar] [CrossRef] [PubMed]
- Umemura, A.; Oeda, T.; Yamamoto, K.; Tomita, S.; Kohsaka, M.; Park, K.; Sugiyama, H.; Sawada, H. Baseline Plasma C-Reactive Protein Concentrations and Motor Prognosis in Parkinson Disease. PLoS ONE 2015, 10, e0136722. [Google Scholar] [CrossRef]
- Veselý, B.; Koriťáková, E.; Bohnen, N.I.; Viszlayová, D.; Királová, S.; Valkovič, P.; Kurča, E.; Rektor, I. The contribution of cerebrovascular risk factors, metabolic and inflammatory changes to cognitive decline in Parkinson’s disease: Preliminary observations. J. Neural. Transm. 2019, 126, 1303–1312. [Google Scholar] [CrossRef]
- Yang, F.; Li, B.; Li, L.; Zhang, H. The clinical significance of the imbalance of Th17 and Treg cells and their related cytokines in peripheral blood of Parkinson’s disease patients. Int. J. Clin. Exp. Med. 2016, 9, 17946–17951. [Google Scholar]
- Yilmaz, R.; Strafella, A.P.; Bernard, A.; Schulte, C.; van den Heuvel, L.; Schneiderhan-Marra, N.; Knorpp, T.; Joos, T.O.; Leypoldt, F.; Geritz, J.; et al. Serum Inflammatory Profile for the Discrimination of Clinical Subtypes in Parkinson’s Disease. Front. Neurol. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Dantzer, R. Cytokine-induced sickness behaviour: A neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 2004, 500, 399–411. [Google Scholar] [CrossRef]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Steinman, L. Inflammatory cytokines at the summits of pathological signal cascades in brain diseases. Sci. Signal. 2013, 6, pe3. [Google Scholar] [CrossRef] [PubMed]
- Mrak, R.E.; Griffin, W.S. Glia and their cytokines in progression of neurodegeneration. Neurobiol. Aging 2005, 26, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef]
- Villeda, S.A.; Plambeck, K.E.; Middeldorp, J.; Castellano, J.M.; Mosher, K.I.; Luo, J.; Smith, L.K.; Bieri, G.; Lin, K.; Berdnik, D.; et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014, 20, 659–663. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Daneman, R. The blood-brain barrier in health and disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef]
- Bi, J.; Shan, W.; Luo, A.; Zuo, Z. Critical role of matrix metallopeptidase 9 in postoperative cognitive dysfunction and age-dependent cognitive decline. Oncotarget. 2017, 8, 51817–51829. [Google Scholar] [CrossRef] [PubMed]
- He, H.J.; Wang, Y.; Le, Y.; Duan, K.M.; Yan, X.B.; Liao, Q.; Liao, Y.; Tong, J.B.; Terrando, N.; Ouyang, W. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci. Ther. 2012, 18, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Feng, X.; Valdearcos, M.; Lutrin, D.; Uchida, Y.; Koliwad, S.K.; Maze, M. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br. J. Anaesth. 2018, 120, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gu, C.; Mandeville, E.T.; Dong, Y.; Esposito, E.; Zhang, Y.; Yang, G.; Shen, Y.; Fu, X.; Lo, E.H.; et al. Anesthesia and Surgery Impair Blood-Brain Barrier and Cognitive Function in Mice. Front. Immunol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Terrando, N.; Eriksson, L.I.; Ryu, J.K.; Yang, T.; Monaco, C.; Feldmann, M.; Jonsson Fagerlund, M.; Charo, I.F.; Akassoglou, K.; Maze, M. Resolving postoperative neuroinflammation and cognitive decline. Ann. Neurol. 2011, 70, 986–995. [Google Scholar] [CrossRef]
- Li, Z.; Mo, N.; Li, L.; Cao, Y.; Wang, W.; Liang, Y.; Deng, H.; Xing, R.; Yang, L.; Ni, C.; et al. Surgery-Induced Hippocampal Angiotensin II Elevation Causes Blood-Brain Barrier Disruption via MMP/TIMP in Aged Rats. Front. Cell. Neurosci. 2016, 10, 105. [Google Scholar] [CrossRef]
- Terrando, N.; Monaco, C.; Ma, D.; Foxwell, B.M.; Feldmann, M.; Maze, M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl. Acad. Sci. USA 2010, 107, 20518–20522. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Guo, D.; Wang, H.; Xie, K.; Wang, C.; Li, Y.; Wang, C.; Wang, C.; Yu, Y.; Wang, G. Involvement of the blood-brain barrier opening in cognitive decline in aged rats following orthopedic surgery and high concentration of sevoflurane inhalation. Brain Res. 2014, 1551, 13–24. [Google Scholar] [CrossRef]
- Phillipson, M.; Kubes, P. The neutrophil in vascular inflammation. Nature Med. 2011, 17, 1381–1390. [Google Scholar] [CrossRef]
- Rossaint, J.; Zarbock, A. Pathogenesis of multiple organ failure in sepsis. Crit. Rev. Immunol. 2015, 35, 277–291. [Google Scholar] [CrossRef]
- Fudulu, D.; Angelini, G. Oxidative stress after surgery on the immature heart. Oxid. Med. Cell Longev. 2016, 2016, 1971452. [Google Scholar] [CrossRef] [PubMed]
- Mokart, D.; Leone, M.; Sannini, A.; Brun, J.P.; Tison, A.; Delpero, J.R.; Houvenaeghel, G.; Blache, J.L.; Martin, C. Predictive perioperative factors for developing severe sepsis after major surgery. Br. J. Anaesth. 2005, 95, 776–781. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Smith, R.M.; Perry, S.L.; Windsor, A.J.; Dickson, R.A.; Bellamy, M.C. Immediate IL-10 expression following major orthopaedic trauma: Relationship to anti-inflammatory response and subsequent development of sepsis. Int. Care Med. 2000, 26, 1076–1081. [Google Scholar] [CrossRef]
- Baigrie, R.J.; Lamont, P.M.; Kwiatkowski, D.; Dallman, M.J.; Morris, P.J. Systemic cytokine response after major surgery. Br. J. Surg. 1992, 79, 757–760. [Google Scholar] [CrossRef]
- Diehl, S.; Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- Welden, B.; Gates, G.; Mallari, R.; Garrett, N. Effects of anesthetics and analgesics on natural killer cell activity. AANA J. 2009, 77, 287–292. [Google Scholar] [PubMed]
- Schneemilch, C.E.; Schilling, T.; Bank, U. Effects of general anaesthesia on inflammation. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Eckenhoff, R.G. Mechanisms of the immunological effects of volatile anesthetics: A review. Anesth. Analg. 2016, 123, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Kurosawa, S.; Horinouchi, T.; Kato, M.; Hashimoto, Y. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology 2001, 95, 1467–1472. [Google Scholar] [CrossRef]
- Picq, C.A.; Clarençon, D.; Sinniger, V.E.; Bonaz, B.L.; Mayol, J.F. Impact of anesthetics on immune functions in a rat model of vagus nerve stimulation. PLoS ONE 2013, 8, e67086. [Google Scholar] [CrossRef] [PubMed]
- Schneemilch, C.E.; Ittenson, A.; Ansorge, S.; Hachenberg, T.; Bank, U. Effect of 2 anesthetic techniques on the postoperative proinflammatory and anti-inflammatory cytokine response and cellular immune function to minor surgery. J. Clin. Anesth. 2005, 17, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Kushida, A.; Inada, T.; Shingu, K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol. Immunotoxicol. 2007, 29, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.F.; Li, W.Z.; Meng, F.Y.; Lin, C.F. Differential effects of propofol and isoflurane on the activation of T-helper cells in lung cancer patients. Anaesthesia 2010, 65, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhata, H.; Shimizu, R.; Yokoyama, M.M. Suppressive effects of volatile anesthetics on cytokine release in human peripheral blood mononuclear cells. Int. J. Immunopharmacol. 1995, 17, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Bani Hani, D.A.; Aleshawi, A.J.; Al Shalakhti, M.H.; Alhowary, A.; Al-Jararahih, O.; Al-Mistarehi, A.H.; Yassin, A. Spinal versus general anesthesia for patients with Parkinson’s disease. Int. J. Gen. Med. 2020, 13, 9–15. [Google Scholar] [CrossRef]
- Milosavljevic, S.B.; Pavlovic, A.P.; Trpkovic, S.V.; Ilić, A.N.; Sekulic, A.D. Influence of spinal and general anesthesia on the metabolic, hormonal, and hemodynamic response in elective surgical patients. Med. Sci. Monit. 2014, 20, 1833–1840. [Google Scholar] [PubMed]
- Liu, J.; Ma, C.; Elkassabany, N.; Fleisher, L.A.; Neuman, M.D. Neuraxial anesthesia decreases postoperative systemic infection risk compared with general anesthesia in knee arthroplasty. Anesth. Analg. 2013, 117, 1010–1016. [Google Scholar] [CrossRef]
- Yeager, M.P.; Lunt, P.; Arruda, J.; Whalen, K.; Rose, R.; DeLeo, J.A. Cerebrospinal fluid cytokine levels after surgery with spinal or general anesthesia. Reg. Anesth. Pain Med. 1999, 24, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.F.; Cata, J.P. Anesthetic care influences long-term outcomes: What is the evidence? Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, T.; Wang, P.; Chen, Y.; Ji, F.; Hernanz, F.; Zucca-Matthes, G.; Youssif, S.; Peng, S.; Xu, D. Can anesthetic effects and pain treatment influence the long-term prognosis of early-stage lymph node-negative breast cancer after breast-conserving surgery? Ann. Transl. Med. 2021, 9, 1467. [Google Scholar] [CrossRef]
- Hu, C.; Iwasaki, M.; Liu, Z.; Wang, B.; Li, X.; Lin, H.; Li, J.; Li, J.V.; Lian, Q.; Ma, D. Lung but not brain cancer cell malignancy inhibited by commonly used anesthetic propofol during surgery: Implication of reducing cancer recurrence risk. J. Adv. Res. 2021, 31, 1–12. [Google Scholar] [CrossRef]
- Jackson, A.; Forsyth, C.B.; Shaikh, M.; Voigt, R.M.; Engen, P.A.; Ramirez, V.; Keshavarzian, A. Diet in Parkinson’s Disease: Critical Role for the Microbiome. Front. Neurol. 2019, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef] [PubMed]
- Martin-Jiménez, C.A.; Gaitán-Vaca, D.M.; Echeverria, V.; González, J.; Barreto, G.E. Relationship Between Obesity, Alzheimer’s Disease, and Parkinson’s Disease: An Astrocentric View. Mol. Neurobiol. 2017, 54, 7096–7115. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, A.; Montinaro, E.; Fatati, G.; Plebani, M.; Colosimo, C. Diabetes mellitus and Parkinson’s disease: Dangerous liaisons between insulin and dopamine. Neural. Regen. Res. 2022, 17, 523–533. [Google Scholar]
- Malek, N.; Lawton, M.A.; Swallow, D.M.A.; Grosset, K.A.; Marrinan, S.L.; Bajaj, N.; Barker, R.A.; Burn, D.J.; Hardy, J.; Morris, H.R.; et al. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson’s disease. Mov. Disord. 2016, 87, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Tuon, T.; Souza, P.S.; Santos, M.F.; Pereira, F.T.; Pedroso, G.S.; Luciano, T.F.; De Souza, C.T.; Dutra, R.C.; Silveira, P.C.; Pinho, R.A. Physical training regulates mitochondrial parameters and neuroinflammatory mechanisms in an experimental model of Parkinson’s disease. Oxid. Med. Cell Longev. 2015, 2015, 261809. [Google Scholar] [CrossRef]
- Hwang, W.J.; Joo, M.A.; Joo, J. Effects of anesthetic method on inflammatory response in patients with Parkinson’s disease: A randomized controlled study. BMC Anesthesiol. 2020, 20, 187. [Google Scholar] [CrossRef]
| Clinical Trials | Blood Markers | Related Symptoms |
|---|---|---|
| Alrafiah et al. [55] | IL-1β, IL-6, TNF-α | |
| Andican et al. [39] | CRP, ICAM-1 | |
| Bagheri et al. [73] | CXCL 12, CXCR4 | |
| Baran et al. [46] | CRP, HMGB1 | |
| Brockmann et al. [52] | FABP, IL-10, IL-12p40, SCF, BDNF | |
| Calvani et al. [65] | MIP-1α, MIP-1β, IL-8, IL-9 | |
| Carvalho et al. [74] | S100B * | nonmotor |
| Chatterjee et al. [57] | IL-1β, NLRP3 | |
| Csencsits-Smith et al. [71] | MCP-1, IP-10, TNF-α | |
| Delgado-Alvarado et al. [62] | IL-6 * | motor, nonmotor |
| Dommershuijsen et al. [48] | CRP | |
| Dufek et al. [75] | IL-6 * | mortality |
| Dumitrescu et al. [76] | calprotectin | |
| Eidson et al. [69] | IL-8 *, IFN-γ, NGAL, TNF-α | motor, nonmotor |
| Fan et al. [58] | IL-1β *, NLRP3 | motor, nonmotor |
| Green et al. [77] | IL-6 *, IL-17A *, TNF-α, TGF-β | motor, nonmotor |
| Gupta et al. [78] | Fractakine *, 3-NT * | motor |
| Gupta et al. [27] | IL-8, TNF- α | |
| Herlofson et al. [79] | IL1-Ra *, VCAM-1 * | nonmotor |
| Hu et al. [50] | IL-1β, TNF-α | |
| Jin et al. [47] | CRP | |
| Karpenko et al. [53] | IL-1β, IL-1Ra, IL-6, IL-10 *, TNF-α * | nonmotor |
| Kim et al. [43] | CRP, IL-1β, IL-2, IL-6, IL-10 *, TNF-α | nonmotor |
| King et al. [44] | CRP, IL-2 *, IL-4, IL-6, IL-8, IL-10, IFN-γ, TNF-α | motor |
| Kouchaki et al. [72] | IL-27 *, TNF-α * | motor |
| Koziorowski et al. [49] | IL-1β, IL-6 *, IL-10, IL-12, TNF-α, NT-proCNP | motor |
| Kwiatek-Majkusiak et al. [63] | IL-6 | |
| Lerche et al. [80] | FABP *, TNF-α *, CA-125 *, BDNF* | motor, nonmotor |
| Lian et al. [56] | IL-1β, IL-6 * | motor |
| Lin et al. [66] | IL-1β, IL-2, IL-4, IL-6, IL-13, IL-18, IFN-γ, TNF-α | |
| Lindqvist et al. [26] | CRP, IL-6 *, sIL-2R, TNF-a | nonmotor |
| Mahlknecht et al. [59] | MCP-4, ICAM-1, IL-2, IL-6, Leptin, PDGF-BB, prolactin, RANTES | |
| Martin-Ruiz et al. [70] | CRP *, IL-6 *, IL-10, TNF-α | nonmotor |
| Miliukhina et al. [64] | MCP-1, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, IL-13, IL-21, IL-23, INF-γ, TNF-α | |
| Milyukhina et al. [51] | IL-1β *, IL-6, IL-10 *, TNF-α * | nonmotor |
| Pereira et al. [81] | IL-6 * | nonmotor |
| Perner et al. [82] | VCAM-1 * | motor |
| Rathnayake et al. [67] | IL-10, IFN-γ, TNF-α | |
| Rocha et al. [54] | IL-1β, IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ, TNF-α | |
| Rocha et al. [83] | sTNFR1 *, sTNFR2 * | nonmotor |
| Roy et al. [84] | NLRP3 | |
| Santos-Garcia et al. [45] | CRP* | motor |
| Sawada et al. [85] | CRP* | nonmotor |
| Schroder et al. [60] | IL-2, IL-4, IL-5, IL-6, IL-9, IL10, IL-13, IL-17A, IL-17F, IL-21, IL-22, IFN-γ, TNF-α, 1111, CCL17, CCL20, CXCL1, CXCL5, CXCL9, CXCL11, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES | |
| Sun et al. [86] | C3 *, C4 | nonmotor |
| Tang et al. [61] | RANTES *, IL-6 * | motor |
| Ton et al. [38] | CRP, IL-6 | |
| Umemura et al. [87] | CRP * | motor |
| Usenko et al. [68] | MCP-1, IL-4, IL-6, IL-10*, INF-γ, TNF-α * | nonmotor |
| Vesely et al. [23] | C3 *, C4*, IL-6 * | nonmotor |
| Vesely et al. [88] | C3 * | nonmotor |
| Wang et al. [42] | CRP *, IL-1β, sIL-2R *, IL-6, IFN-γ, TNF-α* | nonmotor |
| Yang et al. [89] | IL-6, IL-10, IL-17, IL-23, TGF-β | |
| Yilmaz et al. [90] | IL-12p40 * | motor, nonmotor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, J.; Jeong, J.; Park, H.J. Blood Biomarkers in Patients with Parkinson’s Disease: A Review in Context of Anesthetic Care. Diagnostics 2023, 13, 693. https://doi.org/10.3390/diagnostics13040693
Joo J, Jeong J, Park HJ. Blood Biomarkers in Patients with Parkinson’s Disease: A Review in Context of Anesthetic Care. Diagnostics. 2023; 13(4):693. https://doi.org/10.3390/diagnostics13040693
Chicago/Turabian StyleJoo, Jin, Jongmin Jeong, and Hue Jung Park. 2023. "Blood Biomarkers in Patients with Parkinson’s Disease: A Review in Context of Anesthetic Care" Diagnostics 13, no. 4: 693. https://doi.org/10.3390/diagnostics13040693
APA StyleJoo, J., Jeong, J., & Park, H. J. (2023). Blood Biomarkers in Patients with Parkinson’s Disease: A Review in Context of Anesthetic Care. Diagnostics, 13(4), 693. https://doi.org/10.3390/diagnostics13040693






