Contrast-Enhanced Ultrasound in Distinguishing between Malignant and Benign Peripheral Pulmonary Consolidations: The Debated Utility of the Contrast Enhancement Arrival Time
Abstract
1. Introduction
2. Materials and Methods
2.1. US and CEUS Examination
2.2. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Comparison of CEUS Parameters between Benign and Malignant Lesions
3.3. CEUS Parameters between Lesions of Different Sizes
3.4. US and CEUS Parameters in Different Histopathology Subtypes of Malignant Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sperandeo, M.; Filabozzi, P.; Varriale, A.; Carnevale, V.; Piattelli, M.L.; Sperandeo, G.; Brunetti, E.; Decuzzi, M. Role of thoracic ultrasound in the assessment of pleural and pulmonary diseases. J. Ultrasound. 2008, 11, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, M.; Rotondo, A.; Guglielmi, G.; Catalano, D.; Feragalli, B.; Trovato, G.M. Transthoracic ultrasound in the assessment of pleural and pulmonary diseases: Use and limitations. Radiol. Med. 2014, 119, 729–740. [Google Scholar] [CrossRef]
- Sperandeo, M.; Maiello, E.; Graziano, P.; Simeone, A.; De Cosmo, S.; Dimitri, L.; Di Micco, C.; Perrone, E.; Taurchini, M.; Ferretti, G.; et al. Effectiveness and Safety of Transthoracic Ultrasound in Guiding Percutaneous Needle Biopsy in the Lung and Comparison vs. CT Scan in Assessing Morphology of Subpleural Consolidations. Diagnostics 2021, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Yusefi, H.; Helfield, B. Ultrasound Contrast Imaging: Fundamentals and Emerging Technology. Front. Phys. 2022, 10, 100. [Google Scholar] [CrossRef]
- Unnikrishnan, S.; Klibanov, A.L. Microbubbles as Ultrasound Contrast Agents for Molecular Imaging: Preparation and Application. Am. J. Roentgenol. 2012, 199, 292–299. [Google Scholar] [CrossRef]
- Jakobsen, J.A.; Oyen, R.; Thomsen, H.S.; Morcos, S.K.; Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). Safety of ultrasound contrast agents. Eur. Radiol 2005, 15, 941–945. [Google Scholar] [CrossRef]
- Calliada, F.; Campani, R.; Bottinelli, O.; Bozzini, A.; Sommaruga, M.G. Ultrasound contrast agents: Basic principles. Eur. J. Radiol. 1998, 27 (Suppl. S2), S157–S160. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, 2–44. [Google Scholar] [CrossRef]
- Jacobsen, N.; Pietersen, P.I.; Nolsoe, C.; Konge, L.; Graumann, O.; Laursen, C.B. Clinical Applications of Contrast-Enhanced Thoracic Ultrasound (CETUS) Compared to Standard Reference Tests: A Systematic Review. Ultraschall Med. 2022, 43, 72–81. [Google Scholar] [CrossRef]
- Caremani, M.; Benci, A.; Lapini, L.; Tacconi, D.; Caremani, A.; Ciccotosto, C.; Magnolfi, A.L. Contrast enhanced ultrasonography (CEUS) in peripheral lung lesions: A study of 60 cases. J. Ultrasound. 2008, 11, 89. [Google Scholar] [CrossRef]
- Sartori, S.; Postorivo, S.; Vece FDi Ermili, F.; Tassinari, D.; Tombesi, P. Contrast-enhanced ultrasonography in peripheral lung consolidations: What’s its actual role? World J. Radiol. 2013, 5, 372. [Google Scholar] [CrossRef]
- Bi, K.; Zhou, R.R.; Zhang, Y.; Shen, M.J.; Chen, H.W.; Cong, Y.; Zhu, H.M.; Tang, C.H.; Yuan, J.; Wang, Y. US contrast agent arrival time difference ratio for benign versus malignant subpleural pulmonary lesions. Radiology 2021, 301, 200–210. [Google Scholar] [CrossRef]
- Görg, C.; Bert, T.; Kring, R.; Dempfle, A. Transcutaneous contrast enhanced sonography of the chest for evaluation of pleural based pulmonary lesions: Experience in 137 patients. Ultraschall Med. 2006, 27, 437–444. [Google Scholar] [CrossRef]
- Sperandeo, M.; Rea, G.; Grimaldi, M.A.; Trovato, F.; Dimitri, L.M.C.; Carnevale, V. Contrast-enhanced ultrasound does not discriminate between community acquired pneumonia and lung cancer. Thorax 2017, 72, 178–180. [Google Scholar] [CrossRef]
- Hong-Xia, Z.; Wen, H.; Ling-Gang, C.; Wen-Jia, C.; Shuo, L.; Li-Juan, D.; Hai-Man, S.; Yang, Z. A new method for discriminating between bronchial and pulmonary arterial phases using contrast-enhanced ultrasound. Ultrasound Med. Biol. 2016, 42, 1441–1449. [Google Scholar] [CrossRef]
- Bai, J.; Yang, W.; Wang, S.; Guan, R.H.; Zhang, H.; Fu, J.J.; Wu, W.; Yan, K. Role of Arrival Time Difference Between Lesions and Lung Tissue on Contrast-Enhanced Sonography in the Differential Diagnosis of Subpleural Pulmonary Lesions. J. Ultrasound Med. 2016, 35, 1523–1532. [Google Scholar] [CrossRef]
- West, J.B.; Luks, A. West’s Respiratory Physiology: The Essentials, 9th ed.; Kluwer, W., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, L.; Moldobaeva, A.; Zhong, Q.; Jenkins, J.; Snyder, M.; Brown, R.H.; Mitzner, W.; Wagner, E.M. Bronchial artery angiogenesis drives lung tumor growth. Cancer Res. 2016, 76, 5962. [Google Scholar] [CrossRef]
- Finn, J.P.; Baskaran, V.; Carr, J.C.; McCarthy, R.M.; Pereles, F.S.; Kroeker, R.; Laub, G.A. Thorax: Low-dose contrast-enhanced three-dimensional MR angiography with subsecond temporal resolution--initial results. Radiology 2002, 224, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Nael, K.; Michaely, H.J.; Kramer, U.; Lee, M.H.; Goldin, J.; Laub, G.; Finn, J.P. Pulmonary circulation: Contrast-enhanced 3.0-T MR angiography-initial results. Radiology 2006, 240, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Safai Zadeh, E.; Westhoff, C.C.; Keber, C.U.; Trenker, C.; Dietrich, C.F.; Alhyari, A.; Mohr, C.G.L.; Görg, C. Perfusion Patterns of Peripheral Organizing Pneumonia (POP) Using Contrast-Enhanced Ultrasound (CEUS) and Their Correlation with Immunohistochemically Detected Vascularization Patterns. Diagnostics 2021, 11, 1601. [Google Scholar] [CrossRef]
- Quarato, C.M.I.; Cipriani, C.; Dimitri, L.; Lacedonia, D.; Graziano, P.; Copetti, M.; De Cosmo, S.; Simeone, A.; Scioscia, G.; Foschino Barbaro, M.; et al. Assessing value of contrast-enhanced ultrasound vs. conventional transthoracic ultrasound in improving diagnostic yield of percutaneous needle biopsy of peripheral lung lesions. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5781–5789. [Google Scholar] [CrossRef] [PubMed]
- Quarato, C.M.I.; De Cosmo, S.; D’Agostino, F.; Gaudiuso, G.; Sperandeo, M. Commentary: Ultrasound-Guided Biopsy of Pleural-Based Pulmonary Lesions by Injection of Contrast-Enhancing Drugs. Front. Pharmacol. 2020, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, D.T.; Rafailidis, V.; Peterson, C.; Grant, E.G.; Sidhu, P.; Barr, R.G. Artifacts in contrast-enhanced ultrasound: A pictorial essay. Abdom. Radiol. 2018, 43, 977–997. [Google Scholar] [CrossRef] [PubMed]
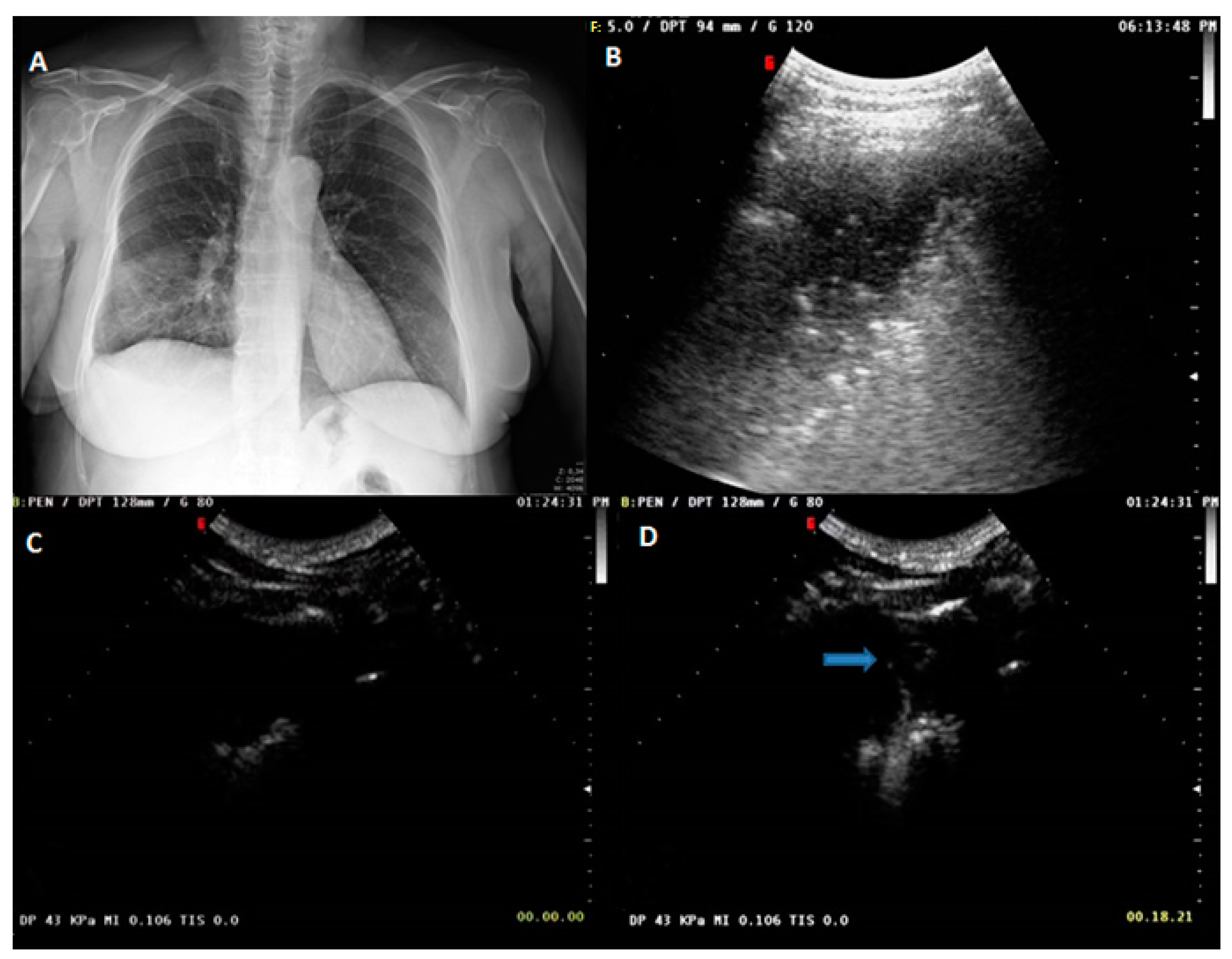
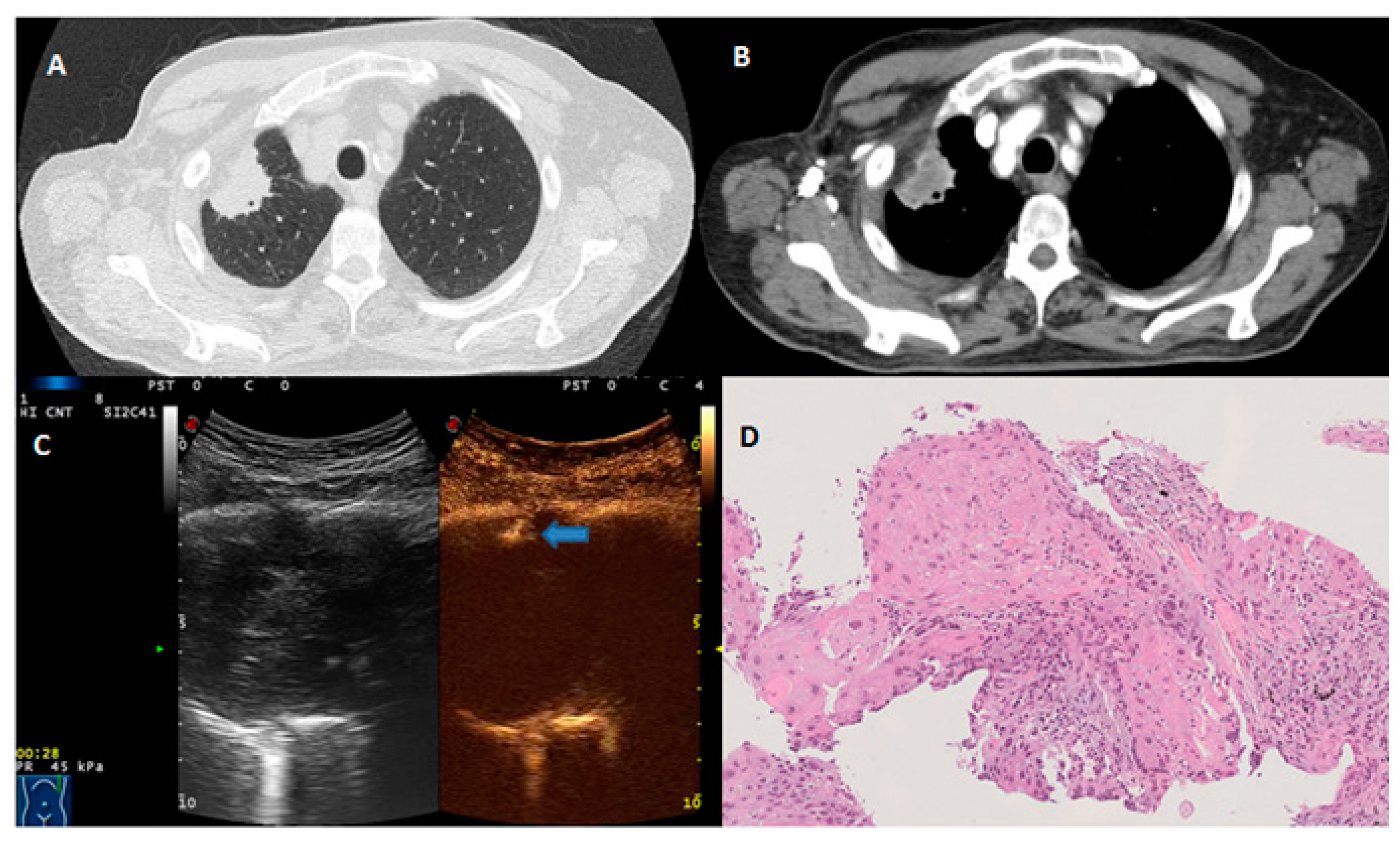
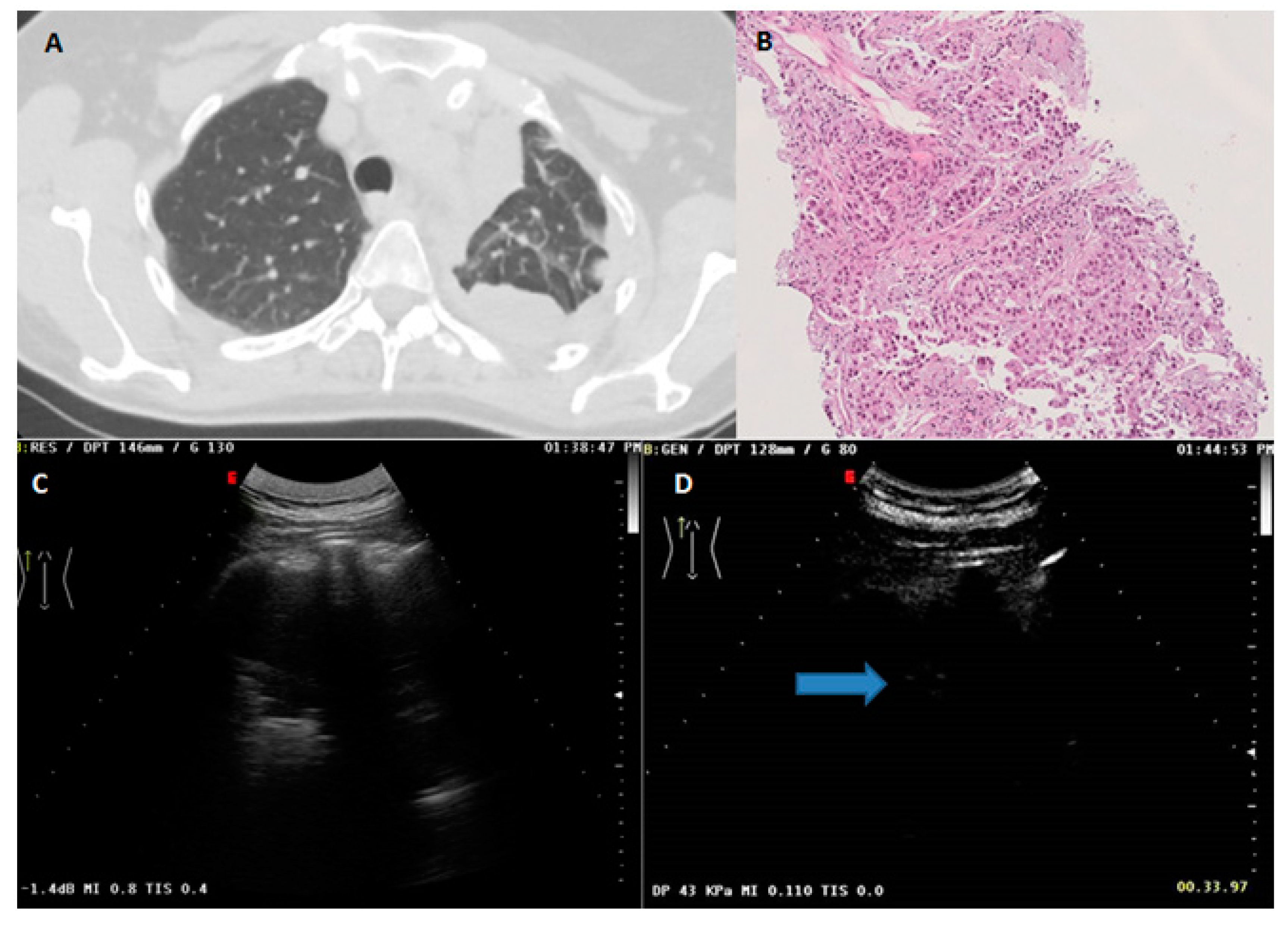

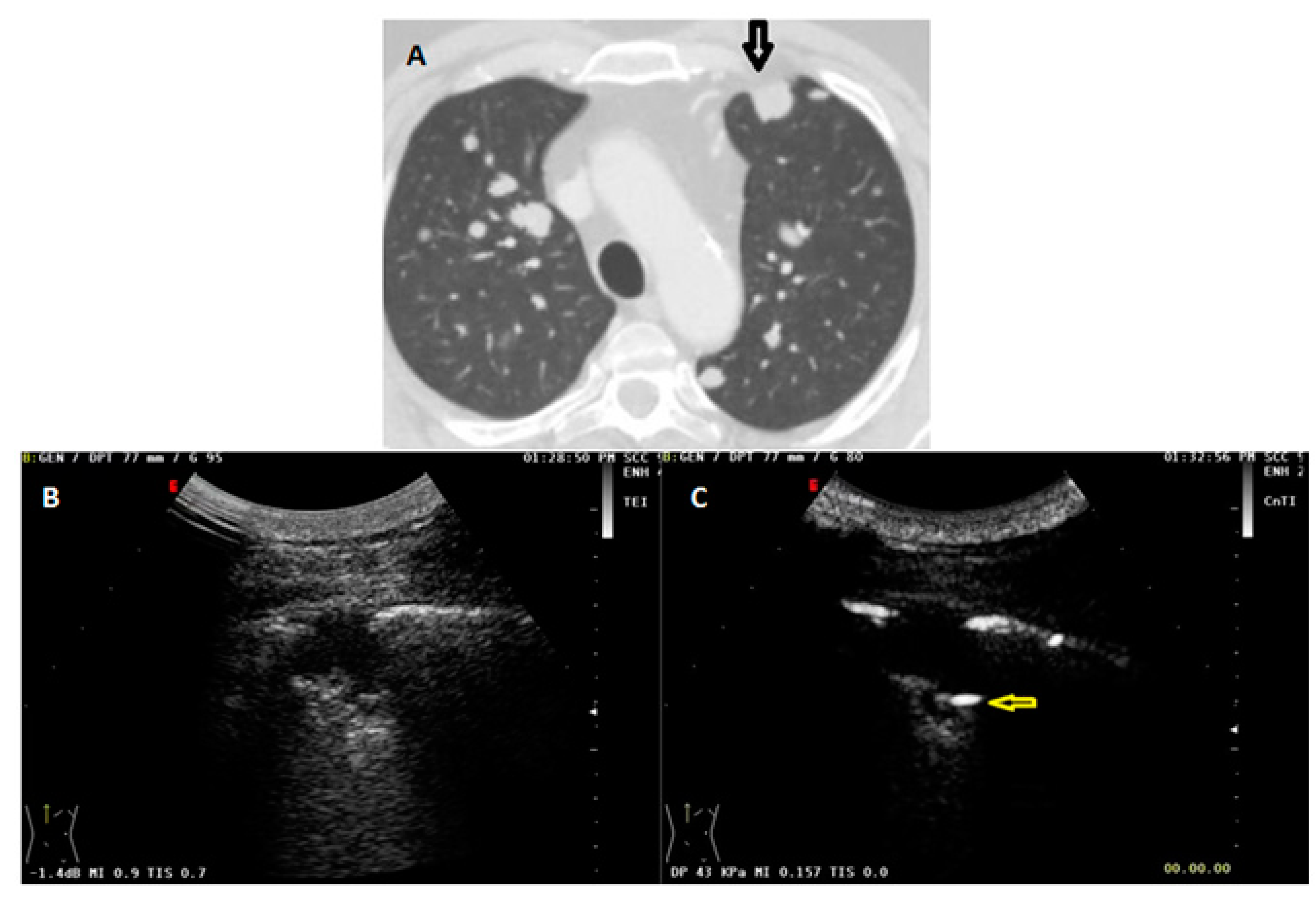

| Variable | Benign (n = 170) | Malignant (n = 147) | p-Value |
|---|---|---|---|
| Age, years; mean ± SD (min-max) | 40.4 ± 9.1 (19–81) | 65.7 ± 5.7 (50–88) | <0.0001 |
| Sex: | |||
| Male; n, % | 102 (60.0%) | 86 (58.5%) | 0.91 |
| Female; n, % | 68 (40.0%) | 61 (41.5%) | |
| Smokers; n, % | 104 (61.2%) | 85 (57.8%) | 0.57 |
| Lesion diameter, cm | 3.2 ± 0.9 (1.5–8.0) | 2.7 ± 0.5 (1.25–5.75) | 0.0004 |
| Pleural effusion; n, % | 84 (49.4%) | 81 (55.1%) | 0.31 |
| Histopathology Subtype | Malignancies (n = 147) |
|---|---|
| Lung metastasis | 12 (8.2%) |
| Small cell lung cancer | 7 (4.8%) |
| Squamous cell lung carcinoma | 35 (23.8%) |
| Adenocarcinoma | 60 (40.8%) |
| Undifferentiated lung carcinoma | 33 (22.4%) |
| Overall Lesions | Benign (n = 170) | Malign (n = 147) | p-Value |
|---|---|---|---|
| CE Arrival Time (AT) | 26.8 ± 7.4 | 27.0 ± 6.5 | 0.39 |
| Early CE AT ≤ 10 s | 9 (5.29%) | 5 (3.40%) | 0.67 |
| Homogeneous CE | 130 (76.5%) | 107 (72.8%) | 0.52 |
| Non-homogeneous CE | 40 (23.5%) | 40 (27.2%) | |
| Wash-out Time (WOT) | 281.4 ± 22.7 | 249.7 ± 18.1 | <0.0001 |
| Delayed CE WOT > 300 s | 28 (16.47%) | 5 (3.40%) | 0.0001 |
| CEUS Parameters | <3 cm (n = 176) | ≥3 cm (n = 141) | ||||
|---|---|---|---|---|---|---|
| Benign (n = 80) | Malign (n = 96) | p-Value | Benign (n = 90) | Malign (n = 51) | p-Value | |
| Arrival Time (AT) | 25.0 ± 6.6 | 25.7 ± 6.3 | 0.46 | 28.4 ± 7.9 | 27.7 ± 6.5 | 0.92 |
| Early CE AT ≤ 10 s | 5 (6.3%) | 3 (3.1%) | 0.32 | 4 (4.4%) | 2 (3.9%) | 0.88 |
| Homogeneous CE | 63 (78.8%) | 67 (69.8%) | 0.23 | 67 (74.4%) | 40 (78.4%) | 0.68 |
| Non-homogeneous CE | 17 (21.2%) | 29 (30.2%) | 23 (25.6%) | 11 (21.6%) | ||
| Wash-out Time (WOT) | 282.1 ± 25.4 | 250.8 ± 15.7 | <0.0001 | 280.7 ± 20.1 | 247.5 ± 22.3 | <0.0001 |
| Delayed CE WOT > 300 s | 14 (17.5%) | 3 (3.1%) | 0.001 | 14 (15.6%) | 2 (3.9%) | 0.08 |
| US/CEUS Parameters | LM (n = 12) | SCLC (n = 7) | SCC (n = 35) | ADLC (n = 60) | UDLC (n = 33) | p-Value |
|---|---|---|---|---|---|---|
| Lesion diameter, cm | 2.9 ± 0.6 | 2.7 ± 0.6 | 2.6 ± 0.5 | 2.8 ± 0.5 | 2.7 ± 0.4 | 0.35 |
| Arrival Time | 24.1 ± 4.2 | 25.4 ± 3.6 | 30.7 ± 8.1 | 27.0 ± 6.4 | 24.4 ± 5.7 | 0.03 |
| Homogeneous CE | 7 (58.3%) | 6 (85.7%) | 26 (74.3%) | 44 (73.3%) | 24 (72.7%) | 0.75 |
| Non-homogeneous CE | 5 (41.7%) | 1 (14.3%) | 9 (25.7%) | 16 (26.7%) | 9 (27.3%) | |
| Wash-out Time | 242.1 ± 14.9 | 251.6 ± 12.4 | 253.6 ± 16.7 | 250.0 ± 17.2 | 247.2 ± 22.3 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quarato, C.M.I.; Feragalli, B.; Lacedonia, D.; Rea, G.; Scioscia, G.; Maiello, E.; Di Micco, C.; Borelli, C.; Mirijello, A.; Graziano, P.; et al. Contrast-Enhanced Ultrasound in Distinguishing between Malignant and Benign Peripheral Pulmonary Consolidations: The Debated Utility of the Contrast Enhancement Arrival Time. Diagnostics 2023, 13, 666. https://doi.org/10.3390/diagnostics13040666
Quarato CMI, Feragalli B, Lacedonia D, Rea G, Scioscia G, Maiello E, Di Micco C, Borelli C, Mirijello A, Graziano P, et al. Contrast-Enhanced Ultrasound in Distinguishing between Malignant and Benign Peripheral Pulmonary Consolidations: The Debated Utility of the Contrast Enhancement Arrival Time. Diagnostics. 2023; 13(4):666. https://doi.org/10.3390/diagnostics13040666
Chicago/Turabian StyleQuarato, Carla Maria Irene, Beatrice Feragalli, Donato Lacedonia, Gaetano Rea, Giulia Scioscia, Evaristo Maiello, Concetta Di Micco, Cristina Borelli, Antonio Mirijello, Paolo Graziano, and et al. 2023. "Contrast-Enhanced Ultrasound in Distinguishing between Malignant and Benign Peripheral Pulmonary Consolidations: The Debated Utility of the Contrast Enhancement Arrival Time" Diagnostics 13, no. 4: 666. https://doi.org/10.3390/diagnostics13040666
APA StyleQuarato, C. M. I., Feragalli, B., Lacedonia, D., Rea, G., Scioscia, G., Maiello, E., Di Micco, C., Borelli, C., Mirijello, A., Graziano, P., Dimitri, L., Villani, R., & Sperandeo, M. (2023). Contrast-Enhanced Ultrasound in Distinguishing between Malignant and Benign Peripheral Pulmonary Consolidations: The Debated Utility of the Contrast Enhancement Arrival Time. Diagnostics, 13(4), 666. https://doi.org/10.3390/diagnostics13040666









