Benign Intranodal Thyroid Tissue Similar to Nodal Metastasis of Thyroid Papillary Carcinoma: A Rare Case Report
Abstract
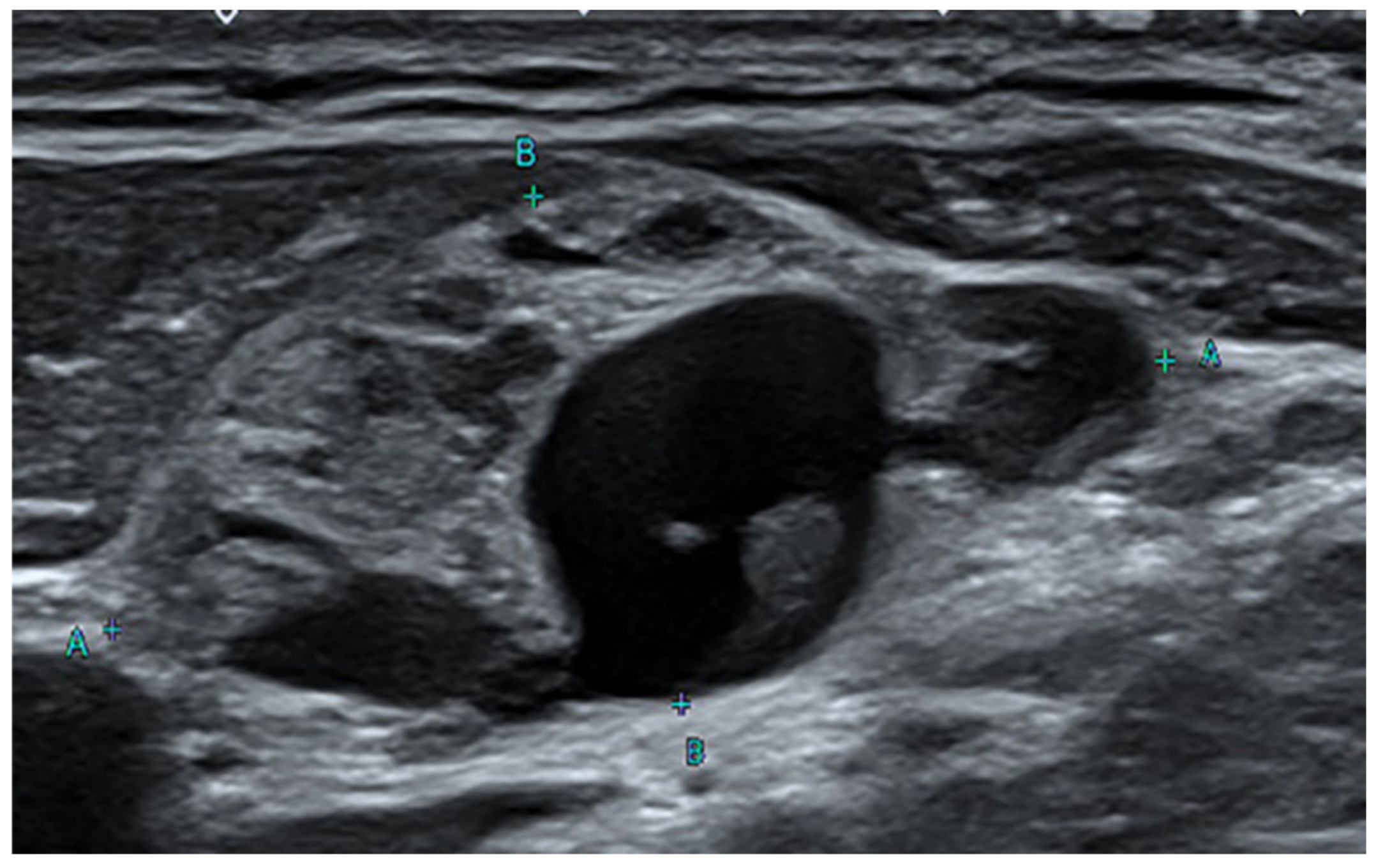
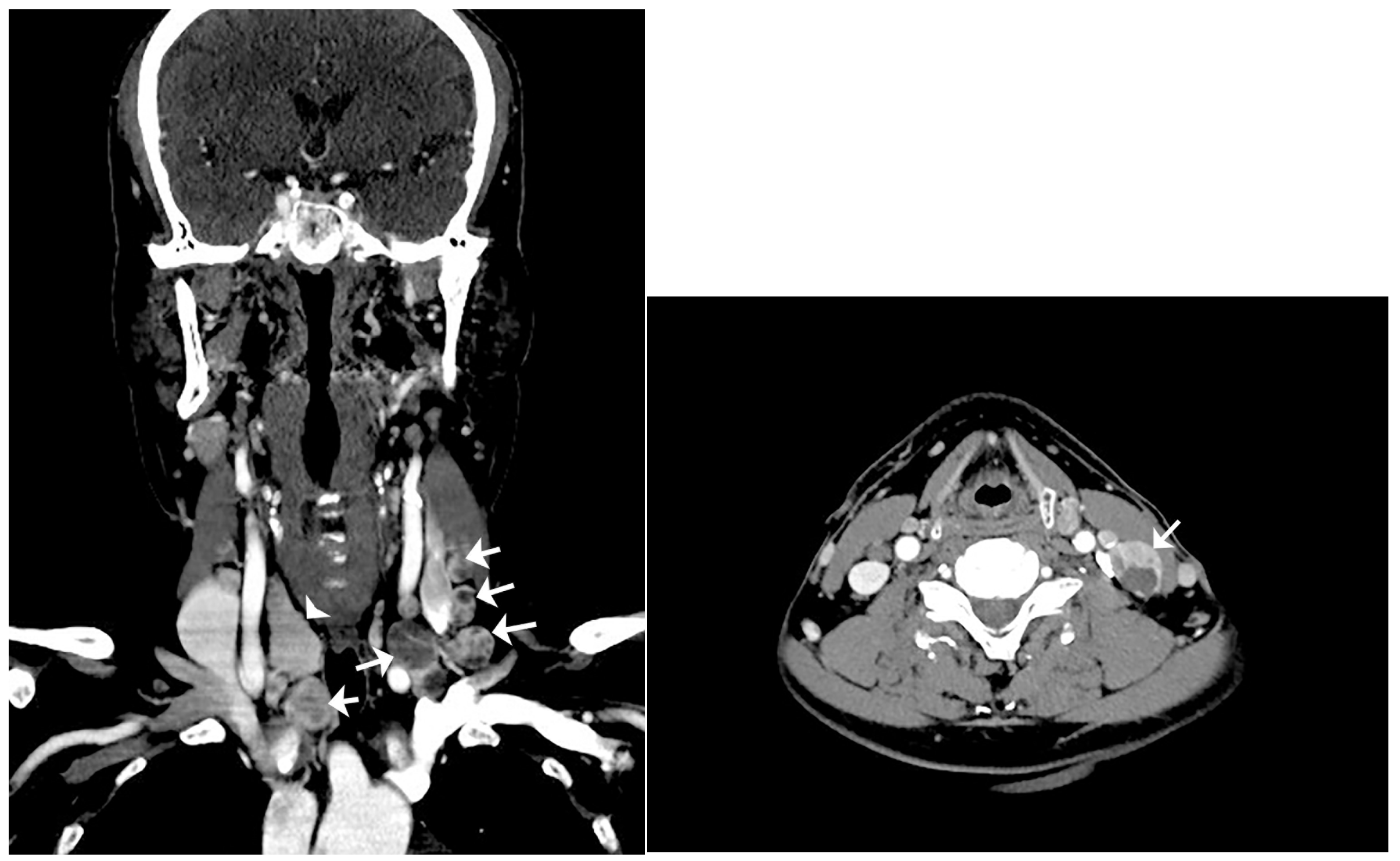
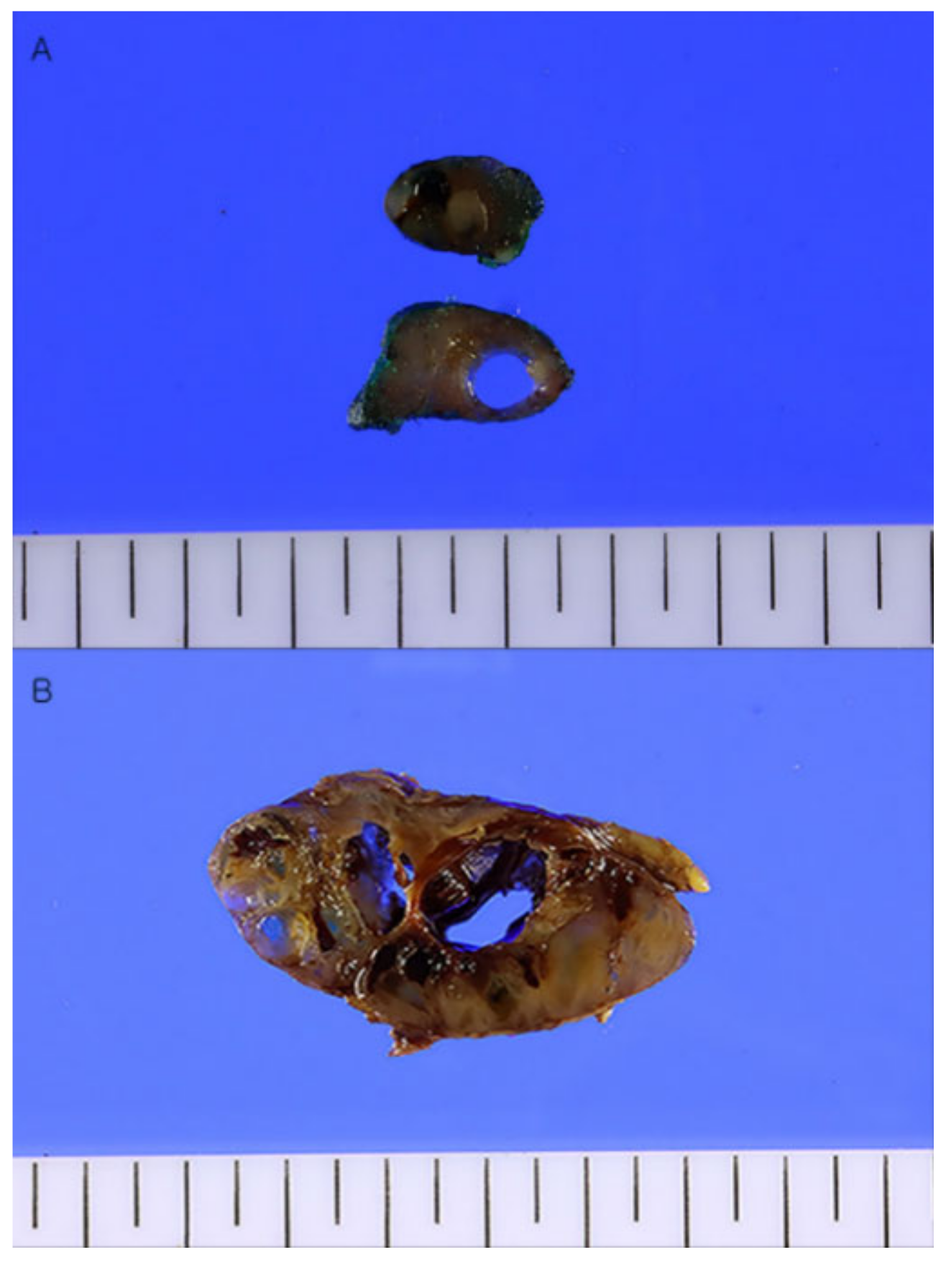
:

Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, A.L.; Truong, L.D.; Cagle, P.; Zhai, Q.J. Benign salivary gland tissue inclusion in a pulmonary hilar lymph node from a patient with invasive well-differentiated adenocarcinoma of the lung: A potential misinterpretation for the staging of carcinoma. Int. J. Surg. Pathol. 2011, 19, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.T.; Helwig, E.B. Benign nevus cells in the capsule of lymph nodes. Cancer 1969, 23, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.Y.; Ruffolo, P.R.; Srinivasan, K. Benign epithelial inclusion cyst in an axillary lymph node. N. Y. State J. Med. 1988, 88, 384–385. [Google Scholar] [PubMed]
- Reich, O.; Tamussino, K.; Haas, J.; Winter, R. Benign mullerian inclusions in pelvic and paraaortic lymph nodes. Gynecol. Oncol. 2000, 78, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.J.; Hill, S.; Millis, R.R. Benign lymph node inclusions mimicking metastatic carcinoma. J. Clin. Pathol. 1994, 47, 245–247. [Google Scholar] [CrossRef]
- Otondi, C.O.; Cason, F.D.; Kranc, M.; Waheed, A. Benign Ectopic Thyroid Tissue in the Neck: A Case Report of a Rare Finding. Cureus 2020, 12, e7172. [Google Scholar] [CrossRef] [PubMed]
- Moses, D.C.; Thompson, N.W.; Nishiyama, R.H.; Sisson, J.C. Ectopic thyroid tissue in the neck. Benign or malignant? Cancer 1976, 38, 361–365. [Google Scholar] [CrossRef]
- Meyer, J.S.; Steinberg, L.S. Microscopically benign thyroid follicles in cervical lymph nodes. Serial section study of lymph node inclusions and entire thyroid gland in 5 cases. Cancer 1969, 24, 302–311. [Google Scholar] [CrossRef]
- Hirokawa, M.; Horiguchi, H.; Wakatsuki, S.; Miki, H.; Sonoo, H.; Manabe, T.; Sano, T. Intranodal benign thyroid tissue: Significance of HBME-1 in differentiation from metastatic papillary thyroid carcinoma. APMIS 2001, 109, 875–880. [Google Scholar] [CrossRef]
- Block, M.A.; Wylie, J.H.; Patton, R.B.; Miller, J.M. Does benign thyroid tissue occur in the lateral part of the neck? Am. J. Surg. 1966, 112, 476–481. [Google Scholar] [CrossRef]
- Hahn, S.Y.; Shin, J.H.; Na, D.G.; Ha, E.J.; Ahn, H.S.; Lim, H.K.; Lee, J.H.; Park, J.S.; Kim, J.H.; Sung, J.Y.; et al. Ethanol Ablation of the Thyroid Nodules: 2018 Consensus Statement by the Korean Society of Thyroid Radiology. Korean J. Radiol. 2019, 20, 609–620. [Google Scholar] [CrossRef]
- Franssila, K.O.; Harach, H.R. Occult papillary carcinoma of the thyroid in children and young adults. A systemic autopsy study in Finland. Cancer 1986, 58, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Ansari-Lari, M.A.; Westra, W.H. The prevalence and significance of clinically unsuspected neoplasms in cervical lymph nodes. Head Neck 2003, 25, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, D.W.; Park, H.K.; Ha, T.K.; Kim, D.H.; Jung, S.J.; Bae, S.K. Benign intranodal thyroid tissue mimicking nodal metastasis in a patient with papillary thyroid carcinoma: A case report. Head Neck 2015, 37, E106–E108. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Choo, H.J.; Lee, Y.J.; Jung, S.J.; Eom, J.W.; Ha, T.K. Sonographic features of cervical lymph nodes after thyroidectomy for papillary thyroid carcinoma. J. Ultrasound Med. 2013, 32, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Park, J.S.; Son, K.R.; Kim, J.H.; Jeon, S.J.; Na, D.G. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: Comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid 2008, 18, 411–418. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.-N.; Cha, J.-G. Benign Intranodal Thyroid Tissue Similar to Nodal Metastasis of Thyroid Papillary Carcinoma: A Rare Case Report. Diagnostics 2023, 13, 577. https://doi.org/10.3390/diagnostics13030577
Kang Y-N, Cha J-G. Benign Intranodal Thyroid Tissue Similar to Nodal Metastasis of Thyroid Papillary Carcinoma: A Rare Case Report. Diagnostics. 2023; 13(3):577. https://doi.org/10.3390/diagnostics13030577
Chicago/Turabian StyleKang, Yoo-Na, and Jung-Guen Cha. 2023. "Benign Intranodal Thyroid Tissue Similar to Nodal Metastasis of Thyroid Papillary Carcinoma: A Rare Case Report" Diagnostics 13, no. 3: 577. https://doi.org/10.3390/diagnostics13030577
APA StyleKang, Y.-N., & Cha, J.-G. (2023). Benign Intranodal Thyroid Tissue Similar to Nodal Metastasis of Thyroid Papillary Carcinoma: A Rare Case Report. Diagnostics, 13(3), 577. https://doi.org/10.3390/diagnostics13030577




