Association of Neutrophil-to-Lymphocyte Ratio and Bloodstream Infections with Survival after Curative-Intent Treatment in Elderly Patients with Oral Cavity Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Variables
2.3. Statistical Methods
3. Results
3.1. Patient Characteristics
3.2. Prognostic Utility of Inflammatory Biomarkers and Clinical Variables for BSI
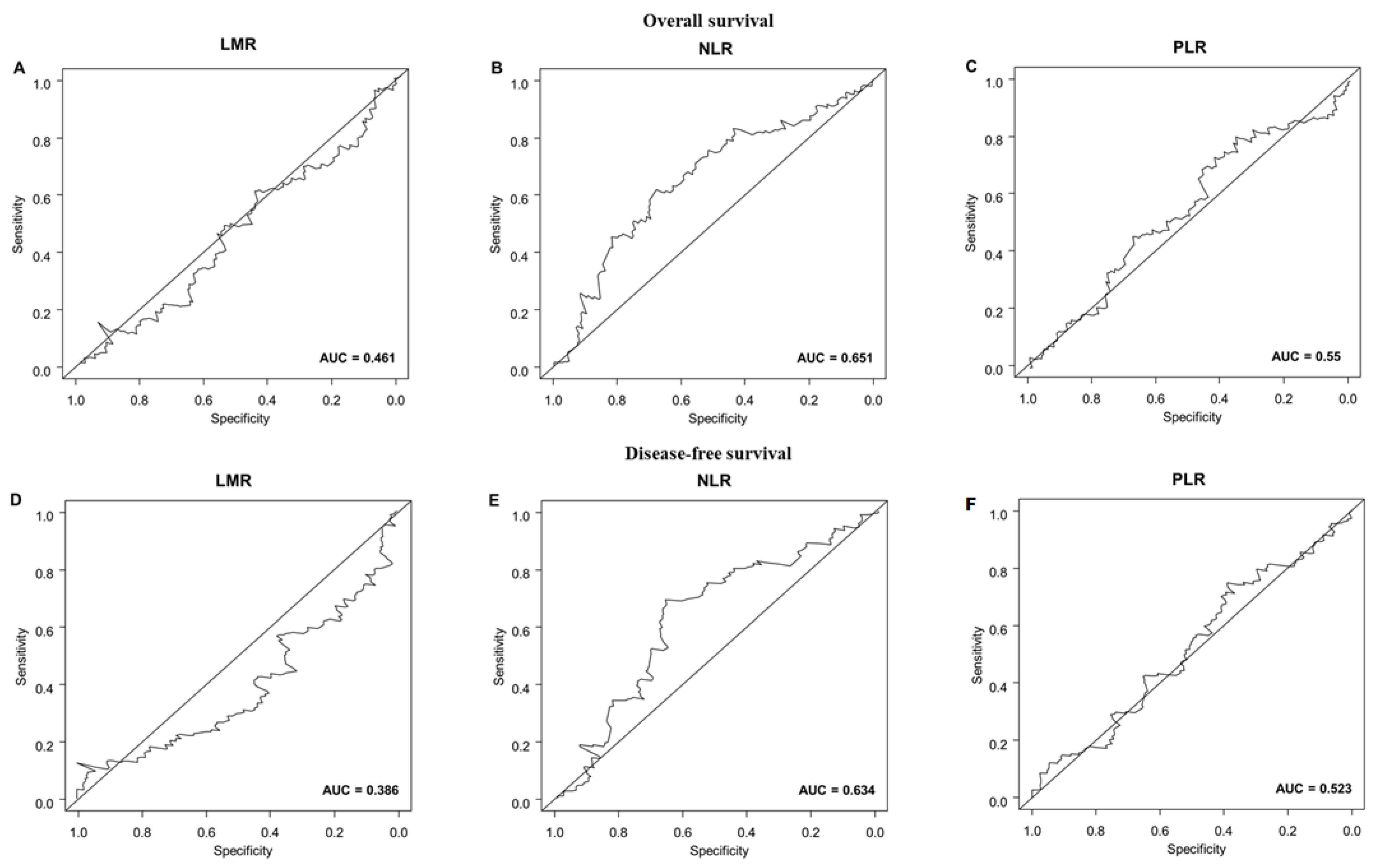
3.3. Survival Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar] [PubMed]
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J. NCCN guidelines insights: Head and neck cancers, version 1.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef]
- Kish, J.A.; Zhang, Q.; Langer, C.J.; Nguyen-Tân, P.F.; Rosenthal, D.I.; Weber, R.S.; List, M.A.; Wong, S.J.; Garden, A.S.; Hu, K.; et al. The impact of age on outcome in phase III NRG Oncology/RTOG trials of radiotherapy (XRT) +/- systemic therapy in locally advanced head and neck cancer. J. Geriatr. Oncol. 2021, 12, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Craigs, C.L.; Bennett, M.I.; Hurlow, A.; West, R.M.; Ziegler, L.E. Older age is associated with less cancer treatment: A longitudinal study of English cancer patients. Age Ageing 2018, 47, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Tjoa, T.; Rathi, V.K.; Goyal, N.; Yarlagadda, B.B.; Barshak, M.B.; Rich, D.L.; Emerick, K.S.; Lin, D.T.; Deschler, D.G.; Durand, M.L. Pneumonia, urinary tract infection, bacteremia, and Clostridioides difficile infection following major head and neck free and pedicled flap surgeries. Oral Oncol. 2021, 122, 105541. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Makiguchi, T.; Yamaguchi, T.; Suzuki, K.; Yokoo, S. Impact of sarcopenia on postoperative surgical site infections in patients undergoing flap reconstruction for oral cancer. Int. J. Oral Maxillofac. Surg. 2020, 49, 576–581. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.W.; Kwon, S.; Kim, H.J.; Cha, I.H.; Nam, W. Prognostic value of systemic inflammatory markers for oral cancer patients based on the 8th edition of AJCC staging system. Sci. Rep. 2020, 10, 12111. [Google Scholar] [CrossRef]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Rosset, A.; Spadola, L.; Ratib, O. OsiriX: An open-source software for navigating in multidimensional DICOM images. J. Digit. Imaging 2004, 17, 205–216. [Google Scholar] [CrossRef]
- Swartz, J.E.; Pothen, A.J.; Wegner, I.; Smid, E.J.; Swart, K.M.; de Bree, R.; Leenen, L.P.; Grolman, W. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016, 62, 28–33. [Google Scholar] [CrossRef]
- Orzell, S.; Verhaaren, B.F.J.; Grewal, R.; Sklar, M.; Irish, J.C.; Gilbert, R.; Brown, D.; Gullane, P.; de Almeida, J.R.; Yu, E.; et al. Evaluation of Sarcopenia in Older Patients Undergoing Head and Neck Cancer Surgery. Laryngoscope 2022, 132, 356–363. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Heagerty, P.J.; Lumley, T.; Pepe, M.S. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000, 56, 337–344. [Google Scholar] [CrossRef]
- Jensen, K.H.; Vogelius, I.; Moser, C.E.; Andersen, E.; Eriksen, J.G.; Johansen, J.; Farhadi, M.; Andersen, M.; Overgaard, J.; Friborg, J. Bloodstream infections in head and neck cancer patients after curative-intent radiotherapy: A population-based study from the Danish Head and Neck Cancer Group database. Br. J. Cancer 2021, 125, 458–464. [Google Scholar] [CrossRef]
- Penel, N.; Lefebvre, J.L.; Cazin, J.L.; Clisant, S.; Neu, J.C.; Dervaux, B.; Yazdanpanah, Y. Additional direct medical costs associated with nosocomial infections after head and neck cancer surgery: A hospital-perspective analysis. Int. J. Oral Maxillofac. Surg. 2008, 37, 135–139. [Google Scholar] [CrossRef]
- Haidar, Y.M.; Tripathi, P.B.; Tjoa, T.; Walia, S.; Zhang, L.; Chen, Y.; Nguyen, D.V.; Mahboubi, H.; Armstrong, W.B.; Goddard, J.A. Antibiotic prophylaxis in clean-contaminated head and neck cases with microvascular free flap reconstruction: A systematic review and meta-analysis. Head Neck 2018, 40, 417–427. [Google Scholar] [CrossRef]
- Veve, M.P.; Davis, S.L.; Williams, A.M.; McKinnon, J.E.; Ghanem, T.A. Considerations for antibiotic prophylaxis in head and neck cancer surgery. Oral Oncol. 2017, 74, 181–187. [Google Scholar] [CrossRef]
- Balagopal, P.; Suresh, S.; Nath, S.R.; Sagila, S.; George, N.A. Pattern of Post-Operative Infections Among Oral Cavity Cancer Patients in a Tertiary Care Cancer Centre: A Prospective Study. Indian. J. Otolaryngol. Head Neck Surg. 2020, 74 (Suppl. 2), 2002–2007. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Andrukhov, O.; Wang, T.; Song, S.; Yan, C.; Zhang, F. Meta-analysis of the prognostic value of the neutrophil-to-lymphocyte ratio in oral squamous cell carcinoma. J. Oral Pathol. Med. 2018, 47, 353–358. [Google Scholar] [CrossRef]
- Valero, C.; Zanoni, D.K.; McGill, M.R.; Ganly, I.; Morris, L.G.T.; Quer, M.; Shah, J.P.; Wong, R.J.; León, X.; Patel, S.G. Pretreatment peripheral blood leukocytes are independent predictors of survival in oral cavity cancer. Cancer 2020, 126, 994–1003. [Google Scholar] [CrossRef]
- Shao, S.L.; Cong, H.Y.; Wang, M.Y.; Liu, P. The diagnostic roles of neutrophil in bloodstream infections. Immunobiology 2020, 225, 151858. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; Szekely, Y.; Burstein, B.; Ballal, Y.; Kim, E.Y.; van Diepen, S.; Tabi, M.; Wiley, B.; Kashani, K.B.; Lawler, P.R. Peripheral blood neutrophil-to-lymphocyte ratio is associated with mortality across the spectrum of cardiogenic shock severity. J. Crit. Care 2022, 68, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bassani, B.; Baci, D.; Gallazzi, M.; Poggi, A.; Bruno, A.; Mortara, L. Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities into Potent Anti-Tumor Effects. Cancers 2019, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Pillay, J.; Kamp, V.M.; van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.W.; Ulfman, L.H.; Leenen, L.P.; Pickkers, P.; Koenderman, L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef]
- Gürol, G.; Çiftci, İ.H.; Terizi, H.A.; Atasoy, A.R.; Ozbek, A.; Köroğlu, M. Are there standardized cutoff values for neutrophil-lymphocyte ratios in bacteremia or sepsis? J. Microbiol. Biotechnol. 2015, 25, 521–525. [Google Scholar] [CrossRef]
- Du, E.; Mazul, A.L.; Farquhar, D.; Brennan, P.; Anantharaman, D.; Abedi-Ardekani, B.; Weissler, M.C.; Hayes, D.N.; Olshan, A.F.; Zevallos, J.P. Long-term Survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and Human Papillomavirus Status. Laryngoscope 2019, 129, 2506–2513. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.Y.; Huang, L.; Yu, T.L.; Wan, S.Q.; Song, J.; Zhang, B.L.; Hu, M. Do betel quid and areca nut chewing deteriorate prognosis of oral cancer? A systematic review, meta-analysis, and research agenda. Oral Dis. 2021, 27, 1366–1375. [Google Scholar] [CrossRef]
- Luan, C.W.; Tsai, Y.T.; Yang, H.Y.; Chen, K.Y.; Chen, P.H.; Chou, H.H. Pretreatment prognostic nutritional index as a prognostic marker in head and neck cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 17117. [Google Scholar] [CrossRef]
- Malik, A.; Mishra, A.; Chopda, P.; Singhvi, H.; Nair, S.; Nair, D.; Laskar, S.G.; Prabhash, K.; Agarwal, J.P.; Chaturvedi, P. Impact of age on elderly patients with oral cancer. Eur. Arch. Otorhinolaryngol. 2019, 276, 223–231. [Google Scholar] [CrossRef]
- Sommers, L.W.; Steenbakkers, R.; Bijl, H.P.; Vemer-van den Hoek, J.G.M.; Roodenburg, J.L.N.; Oosting, S.F.; Halmos, G.B.; de Rooij, S.E.; Langendijk, J.A. Survival Patterns in Elderly Head and Neck Squamous Cell Carcinoma Patients Treated With Definitive Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 793–801. [Google Scholar] [CrossRef]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar] [CrossRef]
- Singh, H.; Kanapuru, B.; Smith, C.; Fashoyin-Aje, L.A.; Myers, A.; Kim, G.; Pazdur, R. FDA analysis of enrollment of older adults in clinical trials for cancer drug registration: A 10-year experience by the US Food and Drug Administration. J. Clin. Oncol. 2017, 35, 10009. [Google Scholar] [CrossRef]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef]
- Reiss, J.; Iglseder, B.; Alzner, R.; Mayr-Pirker, B.; Pirich, C.; Kässmann, H.; Kreutzer, M.; Dovjak, P.; Reiter, R. Consequences of applying the new EWGSOP2 guideline instead of the former EWGSOP guideline for sarcopenia case finding in older patients. Age Ageing 2019, 48, 719–724. [Google Scholar] [CrossRef]
- Sierra, J.; Díaz, M.V.; de Jesús García, M.; Finello, M.; Suasnabar, D.F.; Richetta, L.; Toranzo, A.; Hernández, D.; Cometto, M.A.; Vázquez, S.M.; et al. Bloodstream infections in cancer patients. Medicina 2020, 80, 329–338. [Google Scholar]
- Watabe, Y.; Aoki, K.; Ichikawa, H.; Matsuzaki, H.; Ito, A.; Tanaka, J.I.; Kamiyama, I.; Shigematsu, S. A preoperative prognostic nutritional index is a prognostic indicator in oral squamous cell carcinoma patients undergoing radical surgery. Int. J. Oral Maxillofac. Surg. 2021, 50, 1413–1421. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, R.; Ren, F.; Guo, R.; Zhang, P. Prognostic and clinicopathological significance of neutrophil-to-lymphocyte ratio in patients with oral cancer. Biosci. Rep. 2018, 38, 20181550. [Google Scholar] [CrossRef]
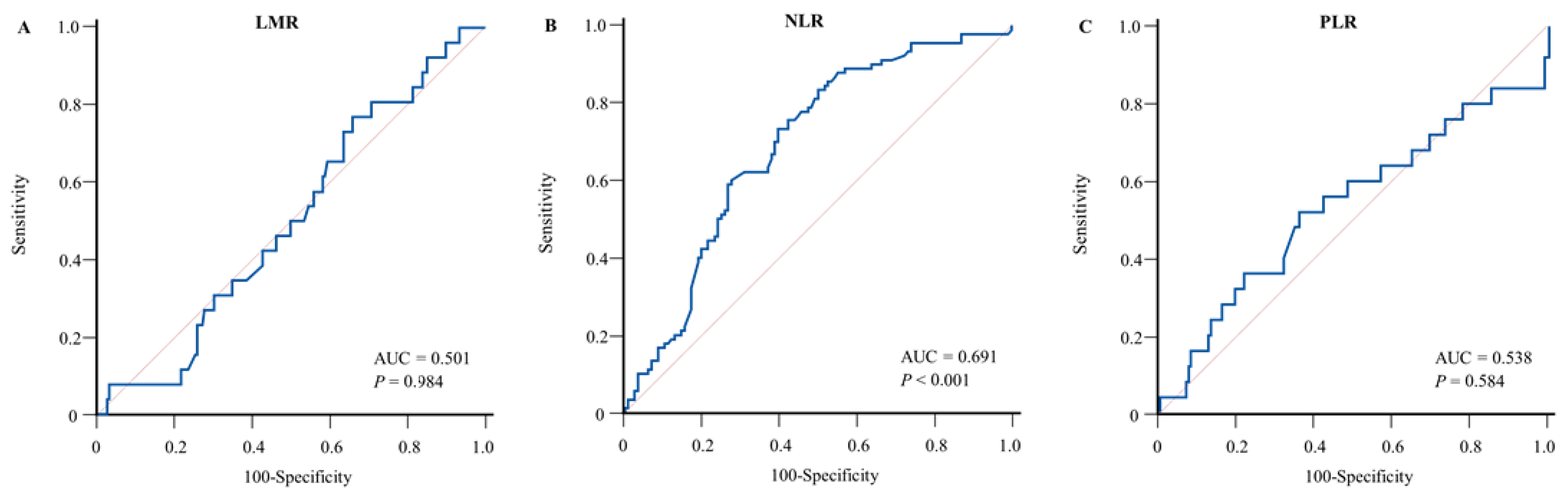

| BSI | ||||
|---|---|---|---|---|
| Characteristics, n (%) | Total | Yes | No | p |
| (n = 235) | (n = 27) | (n = 208) | ||
| Age in years, median (IQR) | 71.0 (67.0–75.0) | 69.5 (67.7–73.0) | 71.0 (67.0–76.0) | 0.400 |
| 65–69 | 107 (45.6) | 13 (48.1) | 94 (45.2) | 0.264 |
| 70–75 | 56 (23.8) | 9 (33.3) | 47 (22.6) | |
| ≥76 | 72 (30.6) | 5 (18.6) | 67 (32.2) | |
| Sex | ||||
| Male | 177 (75.3) | 21 (77.8) | 156 (75) | 0.753 |
| Female | 58 (24.7) | 6 (22.2) | 52 (25) | |
| Alcohol | ||||
| Never | 116 (49.4) | 11 (40.7) | 105 (50.5) | 0.341 |
| Ever | 119 (50.6) | 16 (59.3) | 103 (49.5) | |
| Smoking | ||||
| Never | 75 (31.9) | 10 (37) | 65 (31.2) | 0.544 |
| Ever | 160 (68.1) | 17 (63) | 143 (68.8) | |
| Betel nut | ||||
| Never | 95 (40.4) | 7 (25.9) | 88 (42.3) | 0.103 |
| Ever | 140 (59.6) | 20 (74.1) | 120 (57.7) | |
| CCI, median (IQR) | 6.0 (2.0–7.0) | 6.0 (1.0–8.0) | 6.0 (3.0–7.0) | 0.702 |
| <5 | 86 (36.6) | 10 (37) | 76 (36.5) | 0.960 |
| ≥5 | 149 (63.4) | 17 (63) | 132 (63.5) | |
| Albumin | ||||
| <3.5 | 112 (47.7) | 14 (51.9) | 98 (52.9) | 0.643 |
| ≥3.5 | 123 (52.3) | 13 (48.1) | 110 (47.1) | |
| SMI in cm2/m2, M (SD) | 45.9 (8.4) | 45.9 (8.4) | 45.8 (8.4) | 0.786 |
| Sarcopenia | ||||
| No | 70 (29.8) | 6 (22.2) | 64 (30.8) | 0.361 |
| Yes | 165 (70.2) | 21 (77.8) | 144 (69.2) | |
| Cancer site | ||||
| Buccal mucosa | 66 (28.1) | 9 (33.3) | 57 (27.4) | 0.423 |
| Tongue | 50 (21.3) | 4 (14.8) | 46 (22.1) | |
| Lower gum | 33 (14) | 3 (11.1) | 30 (14.4) | |
| Lower lip | 20 (8.5) | 2 (7.4) | 18 (8.7) | |
| Retromolar trigone | 15 (6.4) | 1 (3.7) | 14 (6.7) | |
| Hard palate | 12 (5.1) | 0 (0) | 12 (5.8) | |
| Other sites | 39 (16.6) | 8 (29.7) | 31 (14.9) | |
| Pathological stage | ||||
| I | 74 (31.5) | 4 (14.8) | 70 (33.6) | 0.212 |
| II | 59 (25.1) | 7 (25.9) | 52 (25) | |
| III | 14 (6) | 2 (7.4) | 12 (5.8) | |
| IV | 88 (37.4) | 14 (51.9) | 74 (35.6) | |
| Treatment type | ||||
| Surgery alone | 109 (46.4) | 10 (37) | 99 (47.6) | 0.100 |
| Adjuvant Chemo | 28 (11.9) | 2 (7.4) | 26 (12.5) | |
| Adjuvant RT | 14 (6) | 0 (0) | 14 (6.7) | |
| Adjuvant CRT | 84 (35.7) | 15 (55.6) | 69 (33.2) | |
| LMR, median (IQR) | 4.0 (2.6–5.1) | 3.6 (2.8–5.4) | 4.0 (2.6–5.1) | 0.292 |
| NLR, median (IQR) | 3.8 (1.7–4.8) | 4.6 (2.0–5.8) | 3.4 (1.7–4.5) | 0.047 |
| PLR, median (IQR) | 135.6 (104.1–177.0) | 143.1 (99.3–222.5) | 135.5 (104.6–174.0) | 0.892 |
| Variable, n (%) | Total | Months 1–3 | Months 4–6 |
|---|---|---|---|
| All pathogens | 50 (100) | 40 (80) | 10 (20) |
| Gram-positive bacteria | 19 (38) | 15 (37.5) | 4 (40) |
| Enterococcus faecium | 1 (2) | 1 (2.5) | 0 (0) |
| VRE | 1 (2) | 1 (2.5) | 0 (0) |
| Bacillus cereus | 2 (4) | 1 (2.5) | 1 (10) |
| Staphylococcus aureus | 6 (12) | 5 (12.5) | 1 (10) |
| ORSA | 1 (2) | 1 (2.5) | 0 (0) |
| CoNS | 2 (4) | 1 (2.5) | 1 (10) |
| Peptostreptococcus species | 2 (4) | 2 (5) | 0 (0) |
| Viridans streptococci | 4 (8) | 3 (7.5) | 1 (10) |
| Gram-negative bacteria | 27 (54) | 22 (55) | 5 (50) |
| Pseudomonas aeruginosa | 6 (12) | 6 (15) | 0 (0) |
| Acinetobacter baumannii | 6 (12) | 5 (12.5) | 1 (10) |
| CRAB | 2 (4) | 2 (5) | 0 (0) |
| Prevotella buccae | 2 (4) | 2 (5) | 0 (0) |
| Enterobacter cloacae | 4 (8) | 1 (2.5) | 3 (30) |
| Stenotrophomonas maltophilia | 1 (2) | 1 (2.5) | 0 (0) |
| Klebsiella pneumoniae | 4 (8) | 3 (7.5) | 1 (10) |
| Eikenella corrodens | 1 (2) | 1 (2.5) | 0 (0) |
| Salmonella | 1 (2) | 1 (2.5) | 0 (0) |
| Fungi | 4 (8) | 3 (7.5) | 1 (10) |
| Candida albicans | 4 (8) | 3 (7.5) | 1 (10) |
| Polymicrobial BSI | 22 (44) | 22 (44) | 0 (0) |
| MDR organisms | 4 (8) | 4 (8) | 0 (0) |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age ≥ 65 | 0.97 (0.48–1.28) | 0.346 | ||
| Alcohol | ||||
| Never | 1 [reference] | |||
| Ever | 1.48 (0.65–3.34) | 0.343 | ||
| Smoking | ||||
| Never | 1 [reference] | |||
| Ever | 0.77 (0.33–1.78) | 0.545 | ||
| Betel nut | ||||
| Never | 1 [reference] | |||
| Ever | 2.09 (0.84–5.17) | 0.109 | ||
| CCI | ||||
| <5 | 1 [reference] | |||
| ≥5 | 0.97 (0.42–2.24) | 0.960 | ||
| Albumin | ||||
| ≥3.5 | 1 [reference] | |||
| <3.5 | 2.02 (0.83–4.91) | 0.118 | ||
| Sarcopenia | ||||
| No | 1 [reference] | |||
| Yes | 1.55 (0.59–4.03) | 0.364 | ||
| Pathological stage | ||||
| I-II | 1 [reference] | |||
| III- IV | 2.06 (0.91–4.66) | 0.082 | ||
| Treatment type | ||||
| Surgery alone | 1 [reference] | 1 [reference] | ||
| Adjuvant Chemo | 0.52 (0.12–2.79) | 0.499 | 0.49 (0.09–2.57) | 0.404 |
| Adjuvant CRT | 2.43 (1.02–5.76) | 0.043 | 2.00 (0.80–5.03) | 0.137 |
| LMR | ||||
| <4.0 | 1 [reference] | |||
| ≥4.0 | 1.40 (0.58–3.35) | 0.448 | ||
| NLR | ||||
| <5 | 1 [reference] | 1 [reference] | ||
| ≥5 | 9.17 (2.37–9.86) | <0.001 | 9.78 (4.14–9.99) | <0.001 |
| PLR | ||||
| <135.6 | 1 [reference] | |||
| ≥135.6 | 1.31 (0.56–3.06) | 0.522 | ||
| Variable | Overall Survival | Disease-Free Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 65 | 1.03 (0.98–1.08) | 0.237 | 1.01 (0.97–1.04) | 0.455 |
| Alcohol | ||||
| Never | 1 [reference] | 1 [reference] | ||
| Ever | 1.31 (0.78–2.19) | 0.294 | 1.17 (0.82–1.67) | 0.359 |
| Smoking | ||||
| Never | 1 [reference] | 1 [reference] | ||
| Ever | 1.02 (0.59–1.76) | 0.928 | 1.05 (0.71–1.54) | 0.798 |
| Betel nut | ||||
| Never | 1 [reference] | 1 [reference] | ||
| Ever | 2.03 (1.14–3.59) | 0.015 | 1.46 (1.01–2.12) | 0.044 |
| CCI | ||||
| <5 | 1 [reference] | 1 [reference] | ||
| ≥5 | 1.64 (0.85–3.18) | 0.136 | 0.89 (0.60–1.31) | 0.566 |
| Albumin | ||||
| ≥3.5 | 1 [reference] | 1 [reference] | ||
| <3.5 | 2.66 (1.31–5.40) | 0.006 | 1.56 (0.92–2.64) | 0.096 |
| Sarcopenia | ||||
| No | 1 [reference] | 1 [reference] | ||
| Yes | 1.51 (0.91–2.51) | 0.107 | 1.33 (0.93–1.90) | 0.109 |
| Pathological stage | ||||
| I-II | 1 [reference] | 1 [reference] | ||
| III-IV | 2.18 (1.31–3.62) | 0.003 | 1.45 (1.02–2.07) | 0.038 |
| Treatment type | ||||
| Surgery alone | 1 [reference] | 1 [reference] | ||
| Adjuvant Chemo | 1.68 (0.82–3.45) | 0.151 | 1.53 (0.90–2.60) | 0.108 |
| Adjuvant RT | 1.32 (0.39–4.42) | 0.646 | 1.49 (0.67–3.30) | 0.324 |
| Adjuvant CRT | 1.68 (0.94–2.99) | 0.077 | 1.41 (0.94–2.10) | 0.090 |
| BSI | ||||
| No | 1 [reference] | 1 [reference] | ||
| Yes | 2.03 (1.05–3.92) | 0.033 | 1.32 (0.78–2.20) | 0.290 |
| LMR | ||||
| <4.0 | 1 [reference] | 1 [reference] | ||
| ≥4.0 | 1.22 (0.73–2.05) | 0.433 | 1.18 (0.82–1.71) | 0.357 |
| NLR | ||||
| <2.9 | 1 [reference] | 1 [reference] | ||
| ≥2.9 | 1.14 (1.04–1.24) | 0.004 | 1.63 (1.11–2.39) | 0.012 |
| PLR | ||||
| <135.6 | 1 [reference] | 1 [reference] | ||
| ≥135.6 | 1.31 (0.76–2.23) | 0.323 | 1.00 (0.68–1.45) | 0.997 |
| Variable | Overall Survival | Disease-Free Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Betel nut | ||||
| Never | 1 [reference] | 1 [reference] | ||
| Ever | 2.99 (1.11–8.05) | 0.016 | 0.69 (0.47–1.03) | 0.071 |
| Albumin | ||||
| ≥3.5 | 1 [reference] | |||
| <3.5 | 2.32 (1.11–4.83) | 0.024 | ||
| Pathological stage | ||||
| I-II | 1 [reference] | 1 [reference] | ||
| III-IV | 2.32 (1.08–4.99) | 0.031 | 1.25 (0.84–1.84) | 0.262 |
| BSI | ||||
| No | 1 [reference] | |||
| Yes | 1.12 (0.41–3.06) | 0.818 | ||
| NLR | ||||
| <2.9 | 1 [reference] | 1 [reference] | ||
| ≥2.9 | 1.39 (0.64–3.01) | 0.404 | 1.55 (1.060–2.29) | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Chou, Y.-F.; Hsieh, T.-C.; Chen, P.-R. Association of Neutrophil-to-Lymphocyte Ratio and Bloodstream Infections with Survival after Curative-Intent Treatment in Elderly Patients with Oral Cavity Squamous Cell Carcinoma. Diagnostics 2023, 13, 493. https://doi.org/10.3390/diagnostics13030493
Huang C-H, Chou Y-F, Hsieh T-C, Chen P-R. Association of Neutrophil-to-Lymphocyte Ratio and Bloodstream Infections with Survival after Curative-Intent Treatment in Elderly Patients with Oral Cavity Squamous Cell Carcinoma. Diagnostics. 2023; 13(3):493. https://doi.org/10.3390/diagnostics13030493
Chicago/Turabian StyleHuang, Chun-Hou, Yu-Fu Chou, Tsung-Cheng Hsieh, and Peir-Rong Chen. 2023. "Association of Neutrophil-to-Lymphocyte Ratio and Bloodstream Infections with Survival after Curative-Intent Treatment in Elderly Patients with Oral Cavity Squamous Cell Carcinoma" Diagnostics 13, no. 3: 493. https://doi.org/10.3390/diagnostics13030493
APA StyleHuang, C.-H., Chou, Y.-F., Hsieh, T.-C., & Chen, P.-R. (2023). Association of Neutrophil-to-Lymphocyte Ratio and Bloodstream Infections with Survival after Curative-Intent Treatment in Elderly Patients with Oral Cavity Squamous Cell Carcinoma. Diagnostics, 13(3), 493. https://doi.org/10.3390/diagnostics13030493




