Characterization of Anesthesia in Rats from EEG in Terms of Long-Range Correlations
Abstract
:1. Introduction
2. Methods and Experiments
2.1. Subjects
2.2. EEG Recording
2.3. DFA
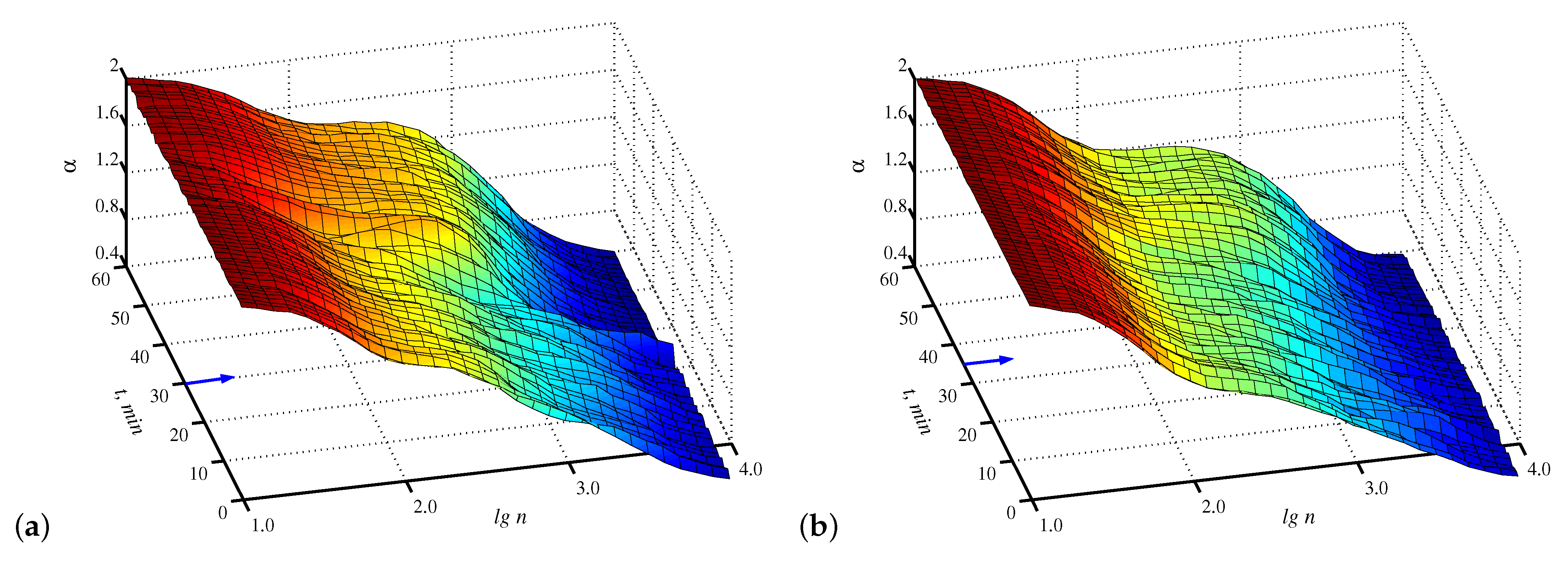
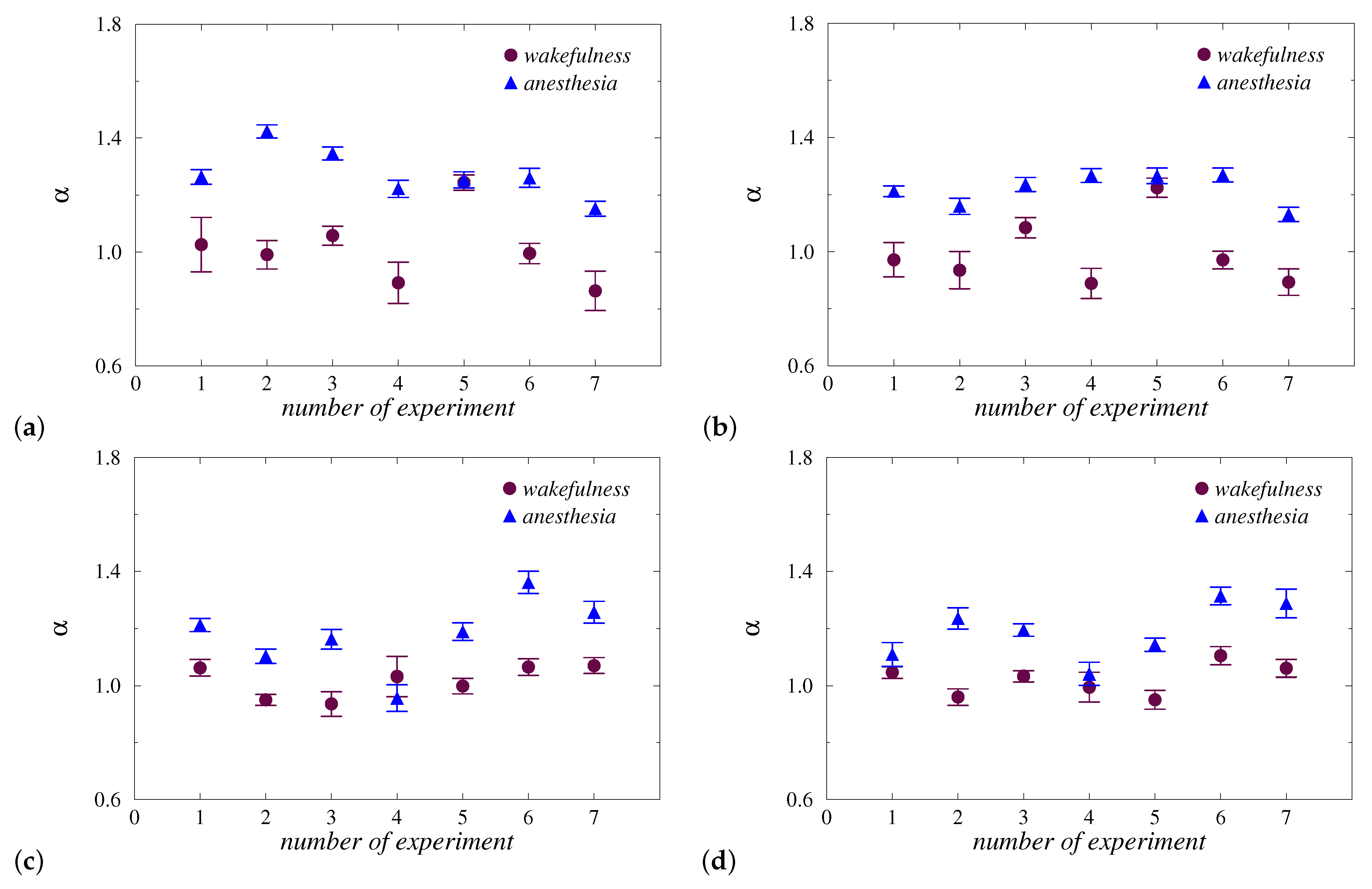
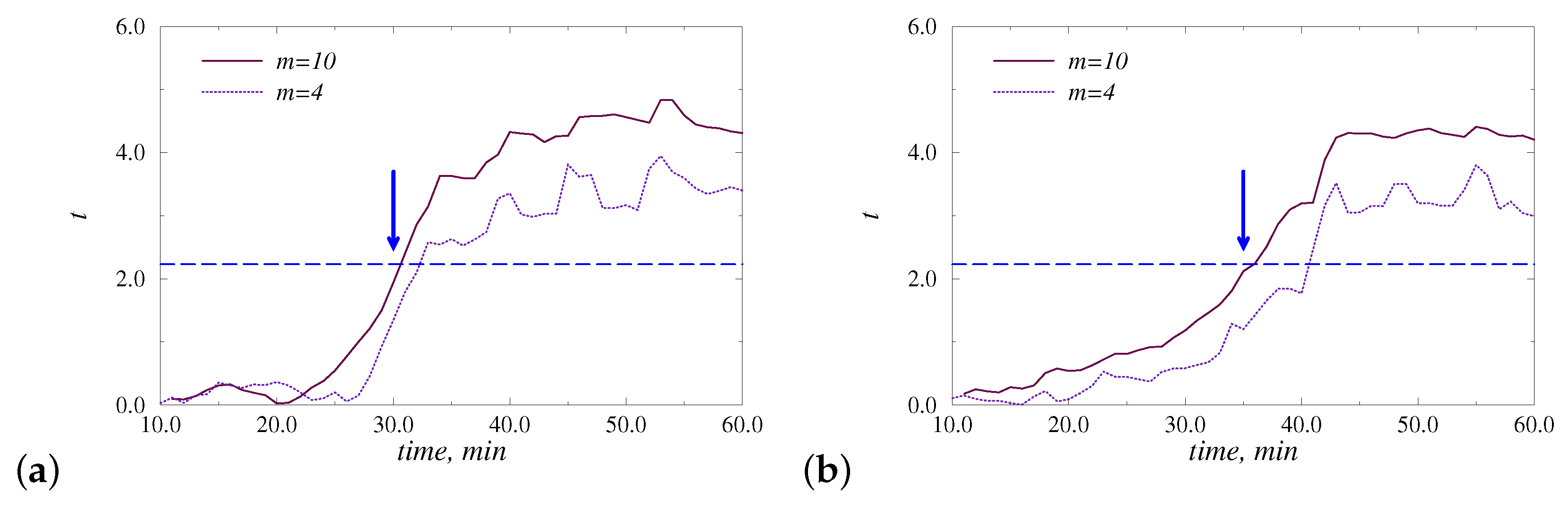
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | blood–brain barrier |
| DFA | detrended fluctuation analysis |
| EEG | electroencephalogram |
References
- Rangarajan, G.; Ding, M. (Eds.) Processes with Long-Range Correlations: Theory and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Pilgram, B.; Kaplan, D.T. Nonstationarity and 1/f noise characteristics in heart rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 276, R1–R9. [Google Scholar] [CrossRef]
- Ward, L.M. Dynamical Cognitive Science, 1st ed.; MIT Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Wen, H.; Liu, Z. Separating fractal and oscillatory components in the power spectrum of neurophysiological signal. Brain Topogr. 2016, 29, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Dave, S.; Brothers, T.A.; Swaab, T.Y. 1/f neural noise and electrophysiological indices of contextual prediction in aging. Brain Res. 2018, 1691, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Colley, I.D.; Dean, R.T. Origins of 1/f noise in human music performance from short-range autocorrelations related to rhythmic structures. PLoS ONE 2019, 14, e0216088. [Google Scholar] [CrossRef] [PubMed]
- Hurst, H.E. Long-term storage capacity of reservoirs. Trans. Am. Soc. Civil Eng. 1951, 116, 770–808. [Google Scholar] [CrossRef]
- Peng, C.-K.; Buldyrev, S.V.; Goldberger, A.L.; Havlin, S.; Sciortino, F.; Simons, M.; Stanley, H.E. Long-range correlations in nucleotide sequences. Nature 1992, 356, 168–170. [Google Scholar] [CrossRef]
- Shao, Y.H.; Gu, G.F.; Jiang, Z.Q.; Zhou, W.X.; Sornette, D. Comparing the performance of FA, DFA and DMA using different synthetic long-range correlated time series. Sci. Rep. 2012, 2, 835. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.-K.; Buldyrev, S.V.; Havlin, S.; Simons, M.; Stanley, H.E.; Goldberger, A.L. Mosaic organization of DNA nucleotides. Phys. Rev. E 1994, 49, 1685–1689. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.-K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995, 5, 82–87. [Google Scholar] [CrossRef]
- Muzy, J.F.; Bacry, E.; Arneodo, A. Wavelets and multifractal formalism for singular signals: Applications to turbulence data. Phys. Rev. Lett. 1991, 67, 3515–3518. [Google Scholar] [CrossRef]
- Muzy, J.F.; Bacry, E.; Arneodo, A. The multifractal formalism revisited with wavelets. Int. J. Bifurc. Chaos 1994, 4, 245–302. [Google Scholar] [CrossRef]
- Hu, K.; Ivanov, P.C.; Chen, Z.; Carpena, P.; Stanley, H.E. Effect of trends on detrended fluctuation analysis. Phys. Rev. E 2001, 64, 011114. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Ivanov, P.C.; Hu, K.; Stanley, H.E. Effect of nonstationarities on detrended fluctuation analysis. Phys. Rev. E 2002, 65, 041107. [Google Scholar] [CrossRef] [Green Version]
- Bryce, R.M.; Sprague, K.B. Revisiting detrended fluctuation analysis. Sci. Rep. 2012, 2, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, P.C.; Amaral, L.A.N.; Goldberger, A.L.; Havlin, S.; Rosenblum, M.G.; Struzik, Z.; Stanley, H. Multifractality in human heartbeat dynamics. Nature 1999, 399, 461–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Gao, J.B.; Sanchez, J.C.; Principe, J.C.; Okun, M.S. Multiplicative multifractal modeling and discrimination of human neuronal activity. Phys. Lett. A 2005, 344, 253–264. [Google Scholar] [CrossRef]
- Hausdorff, J.M. Gait dynamics, fractals and falls: Finding meaning in the stride-to-stride fluctuations of human walking. Hum. Mov. Sci. 2007, 26, 555–589. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Huang, H.; Xie, H.; Wang, Z.; Hu, X. Multifractal analysis of ventricular fibrillation and ventricular tachycardia. Med. Eng. Phys. 2007, 29, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Kantelhardt, J.W.; Zschiegner, S.A.; Koscielny-Bunde, E.; Havlin, S.; Bunde, A.; Stanley, H.E. Multifractal detrended fluctuation analysis of nonstationary time series. Phys. A 2002, 316, 87–114. [Google Scholar] [CrossRef] [Green Version]
- Ihlen, E.A.F. Introduction to multifractal detrended fluctuation analysis in Matlab. Front. Physiol. 2012, 3, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlov, A.N.; Khorovodov, A.P.; Mamedova, A.T.; Koronovskii, A.A., Jr.; Pavlova, O.N.; Semyachkina-Glushkovskaya, O.V.; Kurths, J. Changes in blood-brain barrier permeability characterized from electroencephalograms with a combined wavelet and fluctuation analysis. Eur. Phys. J. Plus 2021, 136, 577. [Google Scholar] [CrossRef]
- Pavlov, A.N.; Pavlova, O.N.; Semyachkina-Glushkovskaya, O.V.; Kurths, J. Extended detrended fluctuation analysis: Effects of nonstationarity and application to sleep data. Eur. Phys. J. Plus 2021, 136, 10. [Google Scholar] [CrossRef]
- Pavlov, A.N.; Dubrovskii, A.I.; Pavlova, O.N.; Semyachkina-Glushkovskaya, O.V. Effects of sleep deprivation on the brain electrical activity in mice. Appl. Sci. 2021, 11, 1182. [Google Scholar] [CrossRef]
- Runnova, A.; Zhuravlev, M.; Ukolov, R.; Blokhina, I.; Dubrovski, A.; Lezhnev, N.; Sitnikova, E.; Saranceva, E.; Kiselev, A.; Karavaev, A.; et al. Modified wavelet analysis of ECoG-pattern as promising tool for detection of the blood-brain barrier leakage. Sci. Rep. 2021, 11, 18505. [Google Scholar] [CrossRef] [PubMed]
- Semenova, N.; Sergeev, K.; Slepnev, A.; Runnova, A.; Zhuravlev, M.; Blokhina, I.; Dubrovsky, A.; Klimova, M.; Terskov, A.; Semyachkina-Glushkovskaya, O.; et al. Blood-brain barrier permeability changes: Nonlinear analysis of ECoG based on wavelet and machine learning approaches. Eur. Phys. J. Plus 2021, 136, 736. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Khorovodov, A.; Fedosov, I.; Pavlov, A.; Shirokov, A.; Sharif, A.E.; Dubrovsky, A.; Blokhina, I.; Terskov, A.; Navolokin, N.; et al. A novel method to stimulate lymphatic clearance of beta-amyloid from mouse brain using noninvasive music-induced opening of the blood-brain barrier with EEG markers. Appl. Sci. 2021, 11, 10287. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.V.; Karavaev, A.S.; Prokhorov, M.D.; Runnova, A.E.; Borovkova, E.I.; Ishbulatov, Y.M.; Hramkov, A.N.; Kulminskiy, D.D.; Semenova, N.I.; Sergeev, K.S.; et al. EEG biomarkers of activation of the lymphatic drainage system of the brain during sleep and opening of the blood-brain barrier. Comput. Struct. Biotechnol. J. 2023, 21, 758–768. [Google Scholar] [CrossRef]
- Spieth, L.; Berghoff, S.A.; Stumpf, S.K.; Winchenbach, J.; Michaelis, T.; Watanabe, T.; Gerndt, N.; Düking, T.; Hofer, S.; Ruhwedel, T.; et al. Anesthesia triggers drug delivery to experimental glioma in mice by hijacking caveolar transport. Neuro-Oncol. Adv. 2021, 3, vdab140. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X. The crosstalk between the blood-brain barrier dysfunction and neuroinflammation after general anaesthesia. Curr. Issues Mol. Biol. 2022, 44, 5700–5717. [Google Scholar] [CrossRef]
- Yang, S.; Gu, C.; Mandeville, E.T.; Dong, Y.; Esposito, E.; Zhang, Y.; Yang, G.; Shen, Y.; Fu, X.; Lo, E.H.; et al. Anesthesia and surgery impair blood-brain barrier and cognitive function in mice. Front. Immunol. 2017, 8, 902. [Google Scholar] [CrossRef] [Green Version]
- Tétrault, S.; Chever, O.; Sik, A.; Amzica, F. Opening of the blood-brain barrier during isoflurane anaesthesia. Eur. J. Neurosci. 2008, 28, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Hedenstierna, G.; Edmark, L. Effects of anesthesia on the respiratory system. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 273–284. [Google Scholar] [CrossRef]
- Barker, S.J.; Gamel, D.M.; Tremper, K.K. Cardiovascular effects of anesthesia and operation. Crit. Care Clin. 1987, 3, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Ou, M.; Zhang, D.; Zhao, W.; Yang, Y.; Liu, J.; Yang, H.; Zhu, T.; Li, Y.; Zhou, C. The effects of general anesthetics on synaptic transmission. Curr. Neuropharmacol. 2020, 18, 936–965. [Google Scholar] [CrossRef] [PubMed]
- Riehl, J.R.; Palanca, B.J.; Ching, S. High-energy brain dynamics during anesthesia-induced unconsciousness. Netw. Neurosci. 2017, 1, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.X. Analyzing Neural Time Series Data: Theory and Practice, 1st ed.; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Schomer, D.L.; Lopes da Silva, F.H. (Eds.) Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, 7th ed.; Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Stanley, H.E.; Amaral, L.A.N.; Goldberger, A.L.; Havlin, S.; Ivanov, P.C.; Peng, C.-K. Statistical physics and physiology: Monofractal and multifractal approaches. Phys. A 1999, 270, 309–324. [Google Scholar] [CrossRef]
- Heneghan, C.; McDarby, G. Establishing the relation between detrended fluctuation analysis and power spectral density analysis for stochastic processes. Phys. Rev. E 2000, 62, 6103–6110. [Google Scholar] [CrossRef] [Green Version]
- Talkner, P.; Weber, R.O. Power spectrum and detrended fluctuation analysis: Application to daily temperatures. Phys. Rev. E 2000, 62, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Frolov, N.S.; Grubov, V.V.; Maksimenko, V.A.; Lüttjohann, A.; Makarov, V.V.; Pavlov, A.N.; Sitnikova, E.; Pisarchik, A.N.; Kurths, J.; Hramov, A.E. Statistical properties and predictability of extreme epileptic events. Sci. Rep. 2019, 9, 7243. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, A.N.; Pitsik, E.N.; Frolov, N.S.; Badarin, A.; Pavlova, O.N.; Hramov, A.E. Age-related distinctions in EEG signals during execution of motor tasks characterized in terms of long-range correlations. Sensors 2020, 20, 5843. [Google Scholar] [CrossRef]


| Rat Number | EEG-Channel | Maximum t-Value | Optimal |
|---|---|---|---|
| Injection anesthesia | |||
| 1 | 1 | 3.41 | 3.4 |
| 2 | 3.85 | 2.5 | |
| 2 | 1 | 7.14 | 2.6 |
| 2 | 4.27 | 2.0 | |
| 3 | 1 | 6.47 | 2.8 |
| 2 | 3.61 | 2.0 | |
| 4 | 1 | 4.76 | 2.7 |
| 2 | 6.49 | 2.4 | |
| 5 | 1 | 2.24 | 3.1 |
| 2 | 2.31 | 3.1 | |
| 6 | 1 | 4.67 | 2.7 |
| 2 | 6.86 | 2.3 | |
| 7 | 1 | 5.29 | 2.9 |
| 2 | 4.72 | 2.9 | |
| Inhalation anesthesia | |||
| 1 | 1 | 4.16 | 2.1 |
| 2 | 3.78 | 2.0 | |
| 2 | 1 | 3.22 | 2.6 |
| 2 | 5.56 | 2.5 | |
| 3 | 1 | 3.52 | 2.8 |
| 2 | 2.54 | 2.6 | |
| 4 | 1 | 2.68 | 2.3 |
| 2 | 1.46 | 2.9 | |
| 5 | 1 | 6.94 | 3.0 |
| 2 | 5.58 | 2.9 | |
| 6 | 1 | 5.24 | 2.7 |
| 2 | 4.04 | 2.7 | |
| 7 | 1 | 5.40 | 2.8 |
| 2 | 6.27 | 2.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blokhina, I.A.; Koronovskii, A.A., Jr.; Dmitrenko, A.V.; Elizarova, I.V.; Moiseikina, T.V.; Tuzhilkin, M.A.; Semyachkina-Glushkovskaya, O.V.; Pavlov, A.N. Characterization of Anesthesia in Rats from EEG in Terms of Long-Range Correlations. Diagnostics 2023, 13, 426. https://doi.org/10.3390/diagnostics13030426
Blokhina IA, Koronovskii AA Jr., Dmitrenko AV, Elizarova IV, Moiseikina TV, Tuzhilkin MA, Semyachkina-Glushkovskaya OV, Pavlov AN. Characterization of Anesthesia in Rats from EEG in Terms of Long-Range Correlations. Diagnostics. 2023; 13(3):426. https://doi.org/10.3390/diagnostics13030426
Chicago/Turabian StyleBlokhina, Inna A., Alexander A. Koronovskii, Jr., Alexander V. Dmitrenko, Inna V. Elizarova, Tatyana V. Moiseikina, Matvey A. Tuzhilkin, Oxana V. Semyachkina-Glushkovskaya, and Alexey N. Pavlov. 2023. "Characterization of Anesthesia in Rats from EEG in Terms of Long-Range Correlations" Diagnostics 13, no. 3: 426. https://doi.org/10.3390/diagnostics13030426
APA StyleBlokhina, I. A., Koronovskii, A. A., Jr., Dmitrenko, A. V., Elizarova, I. V., Moiseikina, T. V., Tuzhilkin, M. A., Semyachkina-Glushkovskaya, O. V., & Pavlov, A. N. (2023). Characterization of Anesthesia in Rats from EEG in Terms of Long-Range Correlations. Diagnostics, 13(3), 426. https://doi.org/10.3390/diagnostics13030426







