Abstract
Quantitative determination of anti-SARS-CoV2-S-RBD is necessary for the evaluation of vaccination effectiveness. The surrogate viral neutralization test (SVNT) is approved for measuring anti-SARS-CoV2-S-RBD, but a point-of-care platform is needed to simplify anti-SARS-CoV-2-S-RBD measurement. We aimed to evaluate the performance of a rapid fluorescent immunoassay-based kit, FastBio-RBDTM, compared to the SVNT. During April–September 2021, we enrolled two groups of subjects, convalescent subjects and subjects without a COVID-19 history. The subjects were tested for the anti-SARS-CoV2-S-RBD antibody using FastBio-RBDTM and the GenScript-cPASSTM SVNT. We measured the correlation coefficient and conducted an ROC analysis to determine the best cut-off value of anti-SARS-CoV2-S-RBD against the SVNT percent inhibition levels of 30% and 60%. We included 109 subjects. Anti-SARS-CoV-2-S-RBD strongly correlated to SVNT % inhibition with an R value of 0.866 (p < 0.0001). The ROC analysis showed that the anti-SARS-CoV-2-S-RBD of 6.71 AU/mL had 95.7% sensitivity and 87.5% specificity to detect a percentage inhibition of 30%. The anti-SARS-CoV-2-S-RBD of 59.76 AU/mL had a sensitivity of 88.1% and specificity of 97.0% to detect a percentage inhibition of 60%. FastBio-RBDTM could determine the presence and level of anti-SARS-CoV-2-S-RBD with good sensitivity and specificity. It has the potential to be deployed in health facilities with limited resources.
1. Introduction
Coronavirus disease-19 (COVID-19), the infection of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has become a pandemic [1]. COVID-19 vaccination and monoclonal antibodies have been one of the main strategies to face this problem. COVID-19 vaccination programs aim to reduce severe disease and death from COVID-19 [2]. A myriad of COVID-19 vaccines have been developed and used on different platforms, with different efficacy and immunogenicity [3]. Comorbidities in a person may impact the immunogenicity evoked by vaccines, and some comorbidities may impair the antibody response to vaccines [4]. An individual’s immune system response is the most important marker for determining mortality and morbidity [5]. In the early COVID-19 pandemic, there was limited scientific evidence on the development and duration of the immune response in individuals, both in cases of infection and post-vaccination against SARS-CoV-2 [6]. Antibody testing after vaccination can help identify the people who are low responders to vaccines and personalize the use of vaccines in these people. A person with a negative or low antibody level early in the disease can benefit from the provision of monoclonal antibodies.
Virus-neutralizing antibodies confer protection by blocking the interaction that mediates virus entry into susceptible host cells. For SARS-CoV-2, this interaction involves the binding of the receptor binding domain (RBD) of the SARS-CoV-2 spike glycoprotein with angiotensin-converting enzyme 2 (ACE2) [7]. The gold standard test of immune response and level of protection after SARS-CoV-2 vaccination is the plaque-reduction neutralization test (PRNT). However, the PRNT is tedious and can only be conducted in sophisticated laboratories with a biosafety level of (BSL)-3 [8]. A surrogate-viral neutralization test (SVNT) produced by GenScript was approved as sensitive and specific enough to detect neutralizing antibodies in 2021 [9]. The Genscript SARS-CoV-2 SVNT is a commercially available assay that detects the antibody that specifically inhibits the RBD-ACE2 interaction without using live SARS-CoV-2 [7]. However, this test is based on a competitive enzyme-linked immunoassay reaction, hence the need to properly handle equipment in a standardized immunological laboratory [8]. Given the width and breadth of the pandemic, it is imperative to have point-of-care testing, which can be implemented practically and safely in simple laboratories to identify people who are low-responders to vaccines.
Lateral flow immunoassay is a simple technology that allows point-of-care testing to be conducted and has already been used in numerous settings (such as public health centres, hospitals, and home-care-based tests) for several biomarker targets, such as the detection of human chorionic gonadotropin (hCG) in pregnancy test kits, human immunodeficiency virus (HIV) tests, and others [10]. It is also affordable and can be implemented in the laboratory with limited resources, allowing for massive applications. However, they can only provide qualitative results, hence are unsuitable for antibody testing. Fluorescence immunoassay technology allows the application of lateral flow assays to be quantifiable with the simplicity of lateral flow assays [11]. Similarly, the latter technology has been used in various tests like the determination of inflammatory markers like CRP, PCT, and HBa1C for diabetes screening [12].
The objective of this study is to evaluate the performance of a point-of-care fluorescence immunoassay kit that measures the levels of antibodies to SARS-CoV-2: FastBio-RBD compared to a surrogate viral neutralization test.
2. Materials and Methods
2.1. Study Design
We conducted a cross-sectional survey enrolling subjects from April until September 2021. The subjects were invited based on their voluntary willingness and were stratified based on a sample block of convalescent subjects (with a history of COVID-19) and subjects without a history of COVID-19. The vaccinated subjects were examined around 2.5–4 months and between 4 and 6 months after full dose vaccination. We chose vaccinated subjects who had been vaccinated for two and a half months after vaccination because we wanted to ensure that the anti-S-RBD results measured were predominantly IgG, without much influence from IgA and IgM. This is known to occur within 2.5 months after the completion of vaccination [13]. All subjects consented to participate in the study and had no known uncontrolled comorbidities.
2.2. Data Collected
We collected data on demographics, such as age, sex, history of COVID-19 illness, vaccination status, type of vaccine, and vaccination interval. We collected data on comorbidities, such as diabetes, hypertension, chronic pulmonary diseases, and chronic kidney diseases.
2.3. Ethical Clearance
The study was approved by the Health Research Ethics Committee of Dr. Hasan Sadikin General Hospital, Universitas Padjadjaran with the ethics number 410/UN6.KEP/EC/2021 on 17 May 2021. The study was conducted in accordance with the Declaration of Helsinki and all data were kept anonymous.
2.4. Reference Test: GenScript cPass SARS-CoV-2 Neutralization Antibody Detection Kit
The GenScript cPass SARS-CoV-2 Neutralization Antibody Detection Kit (Genscript Biotech, Leiden, The Netherlands), SVNT, was used as the reference test. The test was performed according to the manufacturer’s instructions [14]. Briefly, serum samples and negative and positive controls were diluted at a ratio of 1:10 in sample dilution buffer, mixed at a ratio of 1:1 with a HRP-RBD working solution and incubated at 37 °C for 30 min. Subsequently, 100 µL of samples and controls were added into the wells of a 96-well plate, which was coated with the ACE2 receptor protein. The plate was incubated at 37 °C for 15 min and washed 4× with 300 µL of washing buffer. Next, 100 µL of substrate solution was added and the plate was incubated in the dark for 15 min at RT. Finally, 50 µL of stop solution was added per well, and the absorption at 450 nm was measured using an ELISA reader. The percentage of signal inhibition in relation to the negative control was calculated as follows:
Inhibition [%] = (1 − (Sample OD450/Average Negative Control OD450)) × 100
2.5. Test Being Validated: FastBio-RBD—SARS-CoV-2 Antibody Test
The FastBio-RBDTM test kits was produced by Wondfo (Wondfo Biotech Co., Ltd., Guangzhou, China) for distribution in Indonesia by PT Biofarma Indonesia (persero). The test was performed according to the manufacturer’s instructions [15]. It was based on a point-of-care SARS-CoV-2 RBD antibody test using fluorescence immunoassay technology. It was an immunochromatographic test using an upregulated phosphorylation indicator. The platform was based on a sandwich reaction where the test line contained the S-RBD antigen. The serum samples were added to the detection buffer, mixed, and added to the sample well. The patient’s anti-S-RBD antibodies in the serum would bind with the RBD antigen conjugated with a phosphorescent marker and form immune complexes. The immune complexes then migrate on the nitrocellulose membrane, which is then captured by the RBD antigen in the test line. The resulting complex would be detected using the related fluorescent immuno-assay (FIA) meter. Quantification was enabled wth the fluorescence intensity exited from the immunochromatographic test. The anti-S-RBD result was then displayed as the arbitrary unit (AU)/mL. The higher the antibody in the sample, the more fluorescent the complex, and, therefore, the higher the results of anti-S-RBD. According to the manufacturer, 20 times multiplication converts the FastBio-RBD AU result to the binding antibody unit (BAU), which the World Health Organization recommends. The BAU is the first WHO international standard for anti-SARS-CoV-2 immunoglobulin.
2.6. Statistical Analysis
The demographic and baseline characteristics are presented in a frequency tabulation. The numeric values of the test results are presented with a median and IQR. The anti-S-RBD results measured with FastBio-RBD are reported in AU/mL. The percentage inhibition measured with SVNT is reported in %.
The correlation between the FastBio-RBD result and the % inhibition result of the SVNT test was determined using the Spearman’s ranked test after the log transformation of the values. Based on the value of both tests, we also determined the ROC for the detection of the specific levels of inhibition. Next, based on the ROC, we determined the sensitivity and specificity of FastBio-RBD at a % inhibition cut-off of 30%, which was the positive cut-off provided in the SVNT kit [14] and a cut-off of 60%, which was determined to correlate with a vaccine efficacy of 70–90% [16]. All calculations were determined using IBM SPSS version 25 (IBM Corporation, New York, NY, USA). The graphics were further refined using GraphPad Prism version 8.0. (Graphpad Software, LLC, San Diego, CA, USA).
3. Results
3.1. Study Sample
We enrolled 109 subjects in this study, 58 of which were post-COVID-19 subjects in the convalescent phase and 51 were without a COVID-19 history. Their baseline characteristics are shown in Table 1. Thirty (51.7%) subjects of the convalescent group had received two vaccination doses. Fifteen (29.4%) subjects among those without a history of COVID-19 were never vaccinated with any COVID-19 vaccines, while 36 (70.6%) had completed two doses of vaccinations. Besides the vaccination status proportion, there are no statistically significant characteristic differences between the two groups. All subjects in both groups who had been vaccinated received CoronaVac, an inactivated virus vaccine.

Table 1.
Baseline characteristics of the participating subjects.
3.2. Anti-S-RBD Titer of Convalescent Subjects and Vaccinees without a COVID-19 History Measured Using FastBio-RBD
The distribution of the anti-S-RBD antibody levels of all subjects is shown in Figure 1a. Among the unvaccinated subjects, we saw four people who already had high antibody titers. Two people were also shown to have high antibody titers in the group who had been vaccinated. We observed slightly higher anti-S-RBD titers in the subjects without a COVID-19 history at 2.5–4 months compared to the group > 4 months after vaccination. However, this increase was not statistically significant. The anti-S-RBD titers of the convalescent subjects were significantly higher than the rest of the groups.
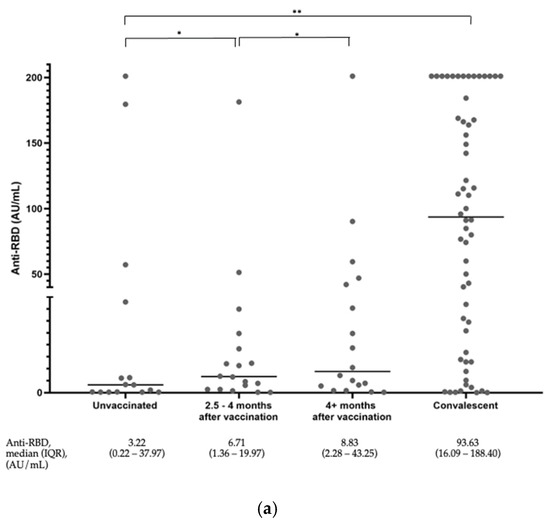
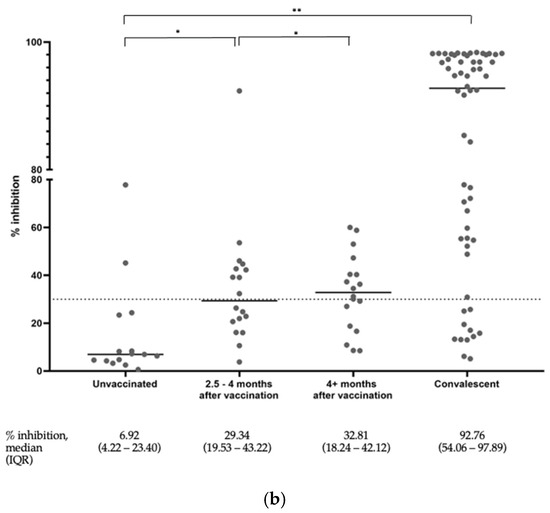
Figure 1.
(a) Distribution of anti-S-RBD antibody levels among all subjects. The graph represents a plot of the results, and the horizontal line depicts the median values. * p value ≥ 0.05, ** p-value < 0.05. (b) Distribution of the surrogate viral neutralization percent inhibition among all subjects. The graph represents a plot of the results. The horizontal lines depict the median values. * p value ≥ 0.05, ** p-value < 0.05.
3.3. Percentage Inhibition of the Convalescent Subjects and Vaccinees without a COVID-19 History Measured Using the SVNT
The distribution of the SVNT % inhibition of all subjects measured using the SVNT test is shown in Figure 1b. Similarly, the % inhibition in the subjects without a COVID-19 history at 2.5–4 months and >4 months after vaccination were shown to be statistically higher compared to the unvaccinated group. The % inhibition among the convalescent subjects also showed statistically significantly higher values than the rest of the group. The SVNT test was conducted to be more sensitive than anti-S-RBD to detect the presence of inhibition. From the same subject serum, we observed that 51 subjects (46.7%) had reached higher than mid- to high-range values of the SVNT, whereas this was only 33 subjects (30.2%) in the anti-S-RBD tests.
3.4. Correlation between the Anti-S-RBD Titer and Percentage Inhibition
The Spearman’s ranked correlation between the anti-S-RBD titer and the % inhibition also had very strong correlation, with a R of 0.866 (95% CI 0.808, 0.908), p < 0.0001 (Figure 2).
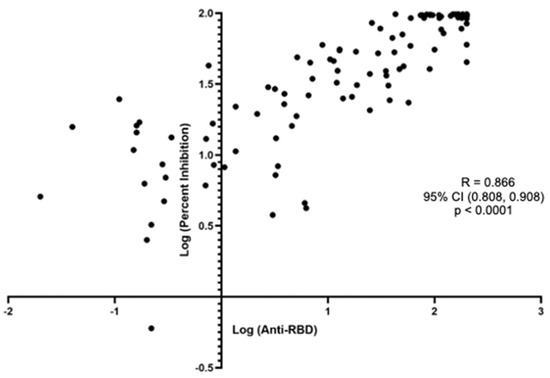
Figure 2.
Spearman’s correlation between the anti-S-RBD antibody level to the surrogate viral neutralization test % inhibition among all subjects.
3.5. Accuracy of the FastBio-RBD vs. SVNT
We performed three ROC analyses to determine the best cut-off of the FastBio-RBD kit using the 30% and 60% inhibition levels with the SVNT as the standard. The first ROC curve corresponded to 30% inhibition, showing an excellent AUC of 0.957, 95% CI (0.922, 0.992), p < 0.0001. Using this curve, we obtained an anti-S-RBD value of 6.71 AU/mL (134.2 BAU/mL) as the best cut-off value. With this cut-off value, the accuracy of FastBio-RBD to detect 30% inhibition was very good (Table 2).

Table 2.
Accuracy of the anti-S-RBD at the best cut-off point for the 30% and 60% inhibition level of surrogate viral neutralization.
Next, we plotted the ROC curve using the cut-off inhibition percentage of 60%, which also showed an excellent AUC of 0.956, 95% CI (0.917, 0.995), p < 0.0001. Using this curve, we obtained the best cut-off point for an anti-S-RBD value of 59.76 AU/mL (1.195.2 BAU/mL). With this value, the FastBio-RBD detected 60% inhibition with a positive predictive value and a negative predictive value of 94.9% and 92.9%, respectively (Table 2).
4. Discussion
This study shows that FastBio-RBD shows adequate accuracy in detecting the presence of antibodies to SARS-CoV-2 with good agreement with the GenScript SVNT. We have shown that the parameters properly show the increasing antibody levels among the vaccinated individuals. The results correlate well with the GenScript SVNT results across a wide range of values. With the proper cut-offs, we could show good test accuracy to detect the protection levels of 30% and 60%. The quantitative detection of SARS-CoV-2 NAb titres is crucial to screen therapeutic antibodies from convalescent patients, predict humoral protection, evaluate vaccine efficacy, and optimize immunization strategies.
GenScript SVNT is a good surrogate test to PRNT and can be applied as a screening test to detect the presence of neutralization antibodies against SARS-CoV-2 if a test is needed [9]. Due to the lack of availability of PRNT in our country, we used the Genscript SVNT as the gold standard for evaluating the FastBio-RBD.
There are many commercial serological assays based on the principles of enzyme immunoassays (EIAs), fluorescence immunoassays (FIAs), and chemiluminescent immunoassays (CLIAs), and have been developed to improve large-scale immunity tests [17]. FastBio-RBD, with its chromatographic fluorescence immunoassay technology, has the potential to allow a faster detection and measurement of anti-S-RBD antibodies in serum samples and can be used in point-of-care-testing settings. This practicality aspect is important in Indonesia, where a significant amount of the population lives in hard-to-reach areas where technically demanding laboratory procedures cannot be performed. In addition, as a standardized commercial test, the test results can be compared across multiple settings and easily converted to the standard unit of BAU/mL. This contrasts with tests like PRNT, where the antibody measured depends on the experimental setup (such as the type of virus particles used, dilution, and type of cell culture) and hence can only be compared with caution.
Anti-S-RBD and percentage inhibition measurements in our subjects showed that the anti-S-RBD is higher in the convalescent subjects than in the vaccinated subjects. This validation study was conducted in the second year of the pandemic, before the delta virus outbreak in Indonesia. Therefore, the overall neutralizing antibody level in the population was still low [18]. This is similar to the studies conducted in this period by Gluck et al. in Germany [19]. In contrast to the mRNA vaccine, the inactivated virus vaccine does confer a reduced antibody response than the convalescent person [20]. The FastBio-RBD is sensitive enough to detect seropositivity, which can be shown to have positive values (≥1 AU, or ≥20 BAU) among the vaccinated person. However, the SVNT seemed to be more sensitive to detecting the presence of neutralization antibodies than FastBio-RBD.
The correlation analysis showed that FastBio-RBD showed a very strong positive correlation to the Genscript SVNT, comparable with more sophisticated tests like the ECLIA and ELISA-based tests [21]. This result is also better in comparison with other immunochromatographic FIA tests [21]. Hence, the correlation coefficient provided in this paper could be considered to detect antibody responses in SARS-CoV2.
The detection of the protection level is the main objective of the parameter. Using the suggested cut-offs at a 30% and 60% inhibition level, the FastbioRBD shows good results. The strength of the FastbioRBD is its high specificity or high positive predictive value (94.5%) to detect 60%. With this result, we can interpret that if a person has reached 59.8 AU/mL, there is a 97% probability that they have already reached a 60% protection level. A high negative predictive value to detect 60% inhibition means that if they have not reached 59.8% AU/mL, there is a 98% probability that this level has not been reached. This interpretation is necessary for deciding if a person needs an additional booster. SVNT levels of 60% have been shown to correspond with 70% protection efficacy [16].
Therefore, the measurement of anti-S-RBD using FastBio-RBD in both convalescent and vaccinated subjects can accurately detect whether the measured anti-S-RBD means sufficient protection against COVID-19. Individuals with adequate protection could be offered additional vaccinations, especially if the individuals have known immunocompromised states. Even more widely, the FastBio-RBD could also determine whether herd immunity has been achieved in a specific area or community [18]. Here, we resume the advantages and disadvantages of FastBio-RBDTM and GenScript-cPASSTM in Table 3 [22,23].

Table 3.
Advantages and disadvantages of FastBio-RBDTM (fluorescence immunoassay) and GenScript-cPASSTM (SVNT).
The limitation of our study was that we compared our anti-S-RBD results with GenScript SVNT only. Ideally, we should have compared the anti-S-RBD against PRNT. However, PRNT is currently unable to be performed in Indonesia. By comparing the anti-S-RBD to GenScript SVNT, which was a surrogate of PRNT, we can provide an approximation that the FastBio-RBD is indeed accurate.
5. Conclusions
FastBio-RBD can detect the immune response to COVID-19 or vaccination as a proxy of the neutralization antibody level they have acquired. It is a specific test that demonstrates if we have reached an anti-RBD of >59.76 AU/mL, we can be certain at 94.9% that the person already has achieved a 60% inhibition level, corresponding to 70% vaccine efficacy. On the other hand, if the patient has not reached this level of anti-RBD, we can be 92.9% certain that they have not reached this level of inhibition. FastBio-RBD is based on the immunochromatographic FIA test and can be used at the point-of-care test, hence, it can be widely implemented even in the primary care setting.
Author Contributions
Conceptualization, M.R.T., H.D., B.A., R.W. and A.R.I.; data curation, M.R.T., H.D., F.R.R., A.C.R., D.U., F.C.S.P., B.A., R.W. and A.R.I.; investigation, M.R.T., H.D., F.R.R., A.C.R., D.U., F.C.S.P., B.A., R.W. and A.R.I.; writing—original draft preparation, M.R.T., B.A., F.R.R., R.W. and A.R.I.; review, M.R.T., B.A., F.R.R., R.W., H.P. and A.R.I.; editing, M.R.T., B.A., F.R.R., R.W. and A.R.I.; supervision, B.A., R.W. and A.R.I. All authors have read and agreed to the published version of the manuscript.
Funding
We received funding from the Indonesian National Research and Innovation Agency (BRIN) to conduct the study. Support for FastBio-RBDTM test kits was kindly provided by PT Biofarma Indonesia (Persero) and Wondfo, Guangzhou Biotech, Co., Ltd., China.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Committee of Universitas Padjadjaran (Ethical Approval No. 410/UN6.KEP/EC/2021), the approval date was 17 May 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to support the findings of this study are included in the article.
Acknowledgments
We are grateful for the support of the Indonesian National Research and Innovation Agency (BRIN) for providing the operational support for conducting the study. We are also thankful for the contributions from Wondfo, Guangzhou Biotech, Co., Ltd., China, and PT. Biofarma Indonesia (Persero) for providing the FastBioRBD™ fluorescent immunoassay reader and the rapid test reagents for our study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 13 September 2023).
- De Gier, B.; van Asten, L.; Boere, T.M.; van Roon, A.; van Roekel, C.; Pijpers, J.; van Werkhoven, C.H.H.; van den Ende, C.; Hahne, S.J.M.; de Melker, H.E.; et al. Effect of COVID-19 vaccination on mortality by COVID-19 and on mortality by other causes, the Netherlands, January 2021–January 2022. Vaccine 2023, 41, 4488–4496. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, immunogenicity and safety of COVID-19 vaccines: A systematic review and meta-analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Uysal, E.B.; Gumus, S.; Bektore, B.; Bozkurt, H.; Gozalan, A. Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. 2022, 94, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Azam Khan, M.K.; Alam Khan, M.Q.; Rathore, M.A.; Parveen, B.; Noor, M.; Ghani, E.; Tahir, A.B.; Tipu, H.N.; Lin, B. Comparison of immune response to SARS-CoV-2 vaccine in COVID-recovered versus non-infected Individuals. Clin. Exp. Med. 2023, 23, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, E.J.; Manguiat, K.; Robinson, A.; Chen, J.C.; Dimitrova, K.; Philipson, C.; Lamoureux, L.; McLachlan, E.; Schiffman, Z.; Drebot, M.A.; et al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Diagn. Microbiol. Infect. Dis. 2021, 99, 115294. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.; Li, Q.; Hu, H.; Lu, J.; Che, Z. Advances in Neutralization Assays for SARS-CoV-2. Scand. J. Immunol. 2021, 94, e13088. [Google Scholar] [CrossRef]
- Hofmann, N.; Grossegesse, M.; Neumann, M.; Schaade, L.; Nitsche, A. Evaluation of a commercial ELISA as alternative to plaque reduction neutralization test to detect neutralizing antibodies against SARS-CoV-2. Sci. Rep. 2022, 12, 3549. [Google Scholar] [CrossRef] [PubMed]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ozinskas, A.J. Principles of Fluorescence Immunoassay. In Topics in Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2002; Volume 4, pp. 449–496. [Google Scholar]
- Wondfo. Finecare—FIA Meter Plus (FS-113); Wondfo: Guangzhou, China, 2023. [Google Scholar]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Millsa, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020, 5, eabe0367. [Google Scholar] [CrossRef] [PubMed]
- GenScript. cPass SARS-CoV-2 Neutralization Antibody Detection Kit Instruction for Use; GenScript: Piscataway, NJ, USA, 2022. [Google Scholar]
- Wondfo Biotech Co., Ltd. The Finecare 2019-nCoV RBD Antibody Test; Wondfo: Guangzhou, China, 2021. [Google Scholar]
- Taylor, S.C. A practical approach to SARS-CoV-2 testing in a pre and post-vaccination era. J. Clin. Virol. Plus 2021, 1, 100044. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jung, J.; Lee, J.H.; Lee, D.G.; Kim, Y.B.; Oh, E.J. Comparison of Six Serological Immunoassays for the Detection of SARS-CoV-2 Neutralizing Antibody Levels in the Vaccinated Population. Viruses 2022, 14, 946. [Google Scholar] [CrossRef] [PubMed]
- Muslimah, A.H.; Tiara, M.R.; Djauhari, H.; Dewantara, M.H.; Susandi, E.; Indrati, A.R.; Alisjahbana, B.; Soeroto, A.Y.; Wisaksana, R. High Levels of Anti-SARS-CoV-2 Receptor-Binding Domain (RBD) Antibodies One Year Post Booster Vaccinations among Hospital Workers in Indonesia: Was the Second Booster Needed? Vaccines 2023, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Glück, V.; Tydykov, L.; Mader, A.L.; Warda, A.S.; Bertok, M.; Weidlich, T.; Gottwald, C.; Köstler, J.; Salzberger, B.; Wagner, R.; et al. Humoral immunity in dually vaccinated SARS-CoV-2-naïve individuals and in booster-vaccinated COVID-19-convalescent subjects. Infection 2022, 50, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.H.G.; Souza, T.d.F.G.d.; Araújo, F.M.d.C.; Andrade, L.O.M.d. Dynamics of antibody response to CoronaVac vaccine. J. Med. Virol. 2022, 94, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Ryu, J.H.; Jang, J.H.; Bae, H.; Yoo, S.-H.; Choi, A.-R.; Jo, S.J.; Lim, J.; Lee, J.; Ryu, H.; et al. Comparison of SARS-CoV-2 antibody responses and seroconversion in COVID-19 patients using twelve commercial immunoassays. Ann. Lab. Med. 2021, 41, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Hasugian, A.R.; Khariri. Laboratory examination to measure antibodies formed after vaccination of COVID-19. IOP Conf. Ser. Earth Environ. Sci. 2021, 824, 012073. [Google Scholar] [CrossRef]
- Zedan, H.T.; Yassine, H.M.; Al-Sadeq, D.W.; Liu, N.; Qotba, H.; Nicolai, E.; Pieri, M.; Bernardini, S.; Abu-Raddad, L.J.; Nasrallah, G.K. Evaluation of commercially available fully automated and ELISA-based assays for detecting anti-SARS-CoV-2 neutralizing antibodies. Sci. Rep. 2022, 12, 19020. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).