The Histology-Driven Differential Diagnosis in Bowel Inflammatory Conditions Is Not All That Obvious: Evidence from a Survey Based on Digital Slides
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cerilli, L.A.; Greenson, J.K. The differential diagnosis of colitis in endoscopic biopsy specimens: A review article. Arch. Pathol. Lab. Med. 2012, 136, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Feakins, R.M.; Lauwers, G.Y. Non-neoplastic colorectal disease biopsies: Evaluation and differential diagnosis. J. Clin. Pathol. 2020, 73, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Seldenrijk, C.A.; Morson, B.C.; Meuwissen, S.G.; Schipper, N.W.; Lindeman, J.; Meijer, C.J. Histopathological evaluation of colonic mucosal biopsy specimens in chronic inflammatory bowel disease: Diagnostic implications. Gut 1991, 32, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Doherty, G.; Peyrin-Biroulet, L.; Svrcek, M.; Borralho, P.; Walsh, A.; Carneiro, F.; Rosini, F.; de Hertogh, G.; Biedermann, L.; et al. ECCO Position Paper: Harmonization of the Approach to Ulcerative Colitis Histopathology. J. Crohn’s Colitis 2020, 14, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Sabino, J.; Rosini, F.; Tripathi, M.; Borralho, P.; Baldin, P.; Danese, S.; Driessen, A.; O Gordon, I.; Iacucci, M.; et al. ECCO Position on Harmonisation of Crohn’s Disease Mucosal Histopathology. J. Crohn’s Colitis 2022, 16, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Feakins, R.; Torres, J.; Borralho-Nunes, P.; Burisch, J.; Gonçalves, T.C.; De Ridder, L.; Driessen, A.; Lobatón, T.; Menchén, L.; Mookhoek, A.; et al. ECCO Topical Review on Clinicopathological Spectrum and Differential Diagnosis of Inflammatory Bowel Disease. J. Crohn’s Colitis 2022, 16, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, L.R.; Leoncini, G.; Daperno, M.; Principi, M.B.; Baronchelli, C.; Manenti, S.; Caprioli, F.; Armuzzi, A.; Caputo, A.; Parente, P.; et al. Histopathology of non-IBD colitis practical recommendations from pathologists of IG-IBD Group. Dig. Liver Dis. 2021, 53, 950–957. [Google Scholar] [CrossRef]
- Yantiss, R.K.; Odze, R.D. Diagnostic difficulties in inflammatory bowel disease pathology. Histopathology 2006, 48, 116–132. [Google Scholar] [CrossRef]
- Canavese, G.; Villanacci, V.; Sapino, A.; Rocca, R.; Daperno, M.; Suriani, R.; Maletta, F.; Cassoni, P.; Members of the Piedmont IBD Group. The diagnosis of inflammatory bowel disease is often unsupported in clinical practice. Dig. Liver Dis. 2015, 47, 20–23. [Google Scholar] [CrossRef]
- Theodossi, A.; Spiegelhalter, D.J.; Jass, J.; Firth, J.; Dixon, M.; Leader, M.; A Levison, D.; Lindley, R.; Filipe, I.; Price, A. Observer variation and discriminatory value of biopsy features in inflammatory bowel disease. Gut 1994, 35, 961–968. [Google Scholar] [CrossRef]
- Patil, D.T.; Odze, R.D. Biopsy diagnosis of colitis: An algorithmic approach. Virchows Arch. 2018, 472, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Jessurun, J. The Differential Diagnosis of Acute Colitis: Clues to a Specific Diagnosis. Surg. Pathol. Clin. 2017, 10, 863–885. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, H.A.; Talley, N.J. The importance of clinicopathological correlation in the diagnosis of inflammatory conditions of the colon: Histological patterns with clinical implications. Am. J. Gastroenterol. 2000, 95, 878–896. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Balsitis, M.; Gallivan, S.; Dixon, M.F.; Gilmour, H.M.; A Shepherd, N.; Theodossi, A.; Williams, G.T. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J. Clin. Pathol. 1997, 50, 93–105. [Google Scholar] [CrossRef]
- Tanaka, M.; Riddell, R.H.; Saito, H.; Soma, Y.; Hidaka, H.; Kudo, H. Morphologic criteria applicable to biopsy specimens for effective distinction of inflammatory bowel disease from other forms of colitis and of Crohn’s disease from ulcerative colitis. Scand. J. Gastroenterol. 1999, 34, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Bentley, E.; Jenkins, D.; Campbell, F.; Warren, B. How could pathologists improve the initial diagnosis of colitis? Evidence from an international workshop. J. Clin. Pathol. 2002, 55, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, F.; Jansen, A.F.; Günther, M.; Pauli, R.; Lüth, S. Eosinophil Counts in Mucosal Biopsies of the Ileum and Colon: Interobserver Variance Affects Diagnostic Accuracy. Patholog Res. Int. 2018, 2018, 2638258. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.A.; Schmidt, P.T.; Lang-Schwarz, C.; Vieth, M. Branching crypts in inflammatory bowel disease revisited. J. Gastroenterol. Hepatol. 2022, 37, 440–445. [Google Scholar] [CrossRef]
- Rubio, C.A.; Schmidt, P.T. Preliminary Report: Asymmetric Crypt Fission in Biopsies from Patients with Ulcerative Colitis. In Vivo 2020, 34, 2693–2695. [Google Scholar] [CrossRef]
- Rubio, C.A.; Lang-Schwarz, C.; Matek, C.; Kamaradova, K.; Vieth, M. The Number of Colon Crypts in Digital Mucosal Samples: A New Independent Parameter for Diagnosing Ulcerative Colitis. Cancer Diagn. Progn. 2023, 3, 533–537. [Google Scholar] [CrossRef]
- Rubio, C.; Lang-Schwarz, C.; Vieth, M. Is the Routine Histopathologic Diagnosis of Ulcerative Colitis Based on Cross-cut Sections or on Well-oriented Sections? Cancer Diagn. Progn. 2023, 3, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.A.; Vieth, M.; Lang-Schwarz, C. Novel histological repertoire of crypt-associated anomalies in inflamed colon mucosa. J. Clin. Pathol. 2023, 76, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Feakins, R.; Borralho Nunes, P.; Driessen, A.; Gordon, I.O.; Zidar, N.; Baldin, P.; Christensen, B.; Danese, S.; Herlihy, N.; Iacucci, M.; et al. Definitions of histological abnormalities in inflammatory bowel disease: An ECCO position paper. J. Crohn’s Colitis 2023, jjad142. [Google Scholar] [CrossRef] [PubMed]

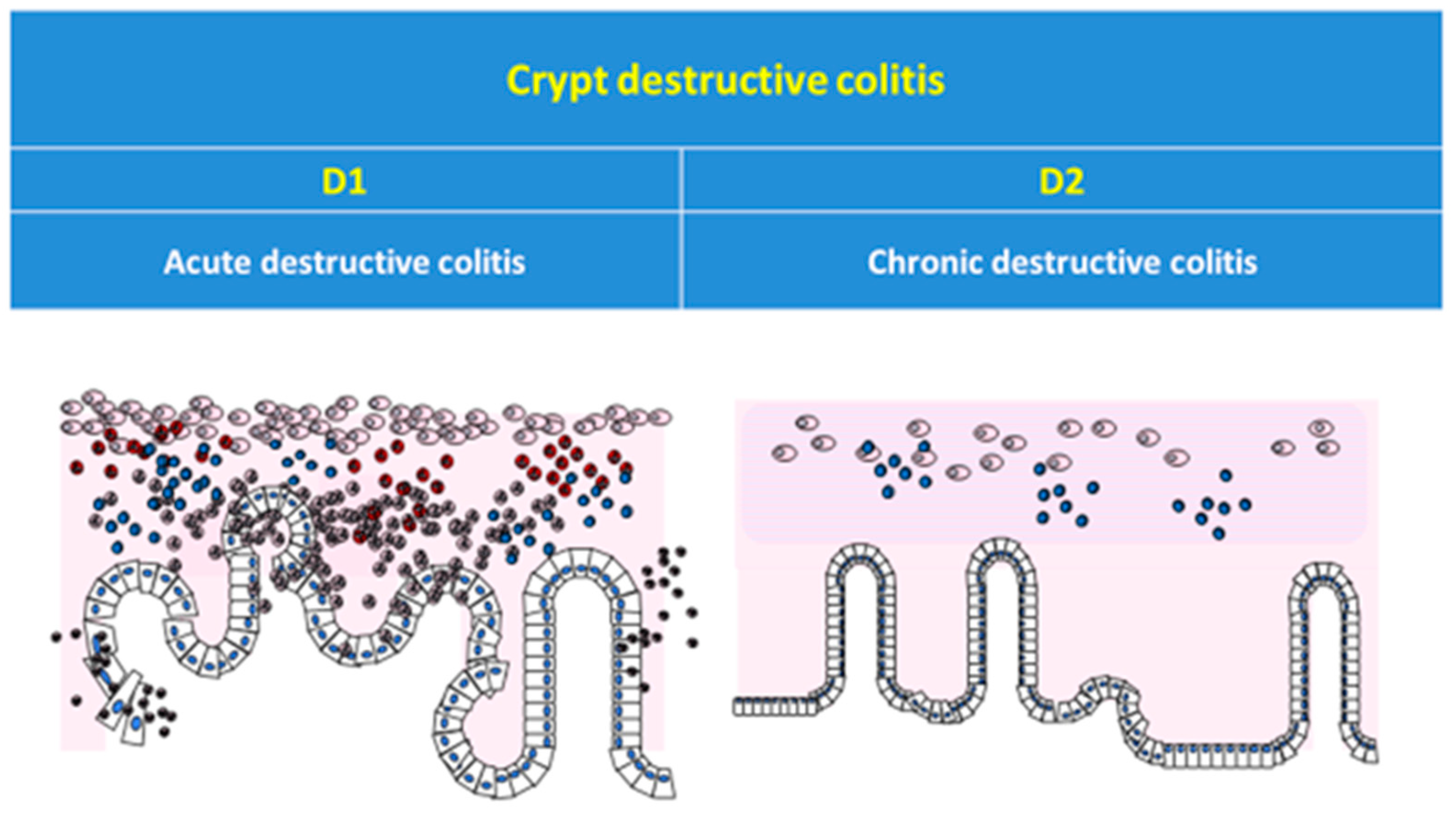
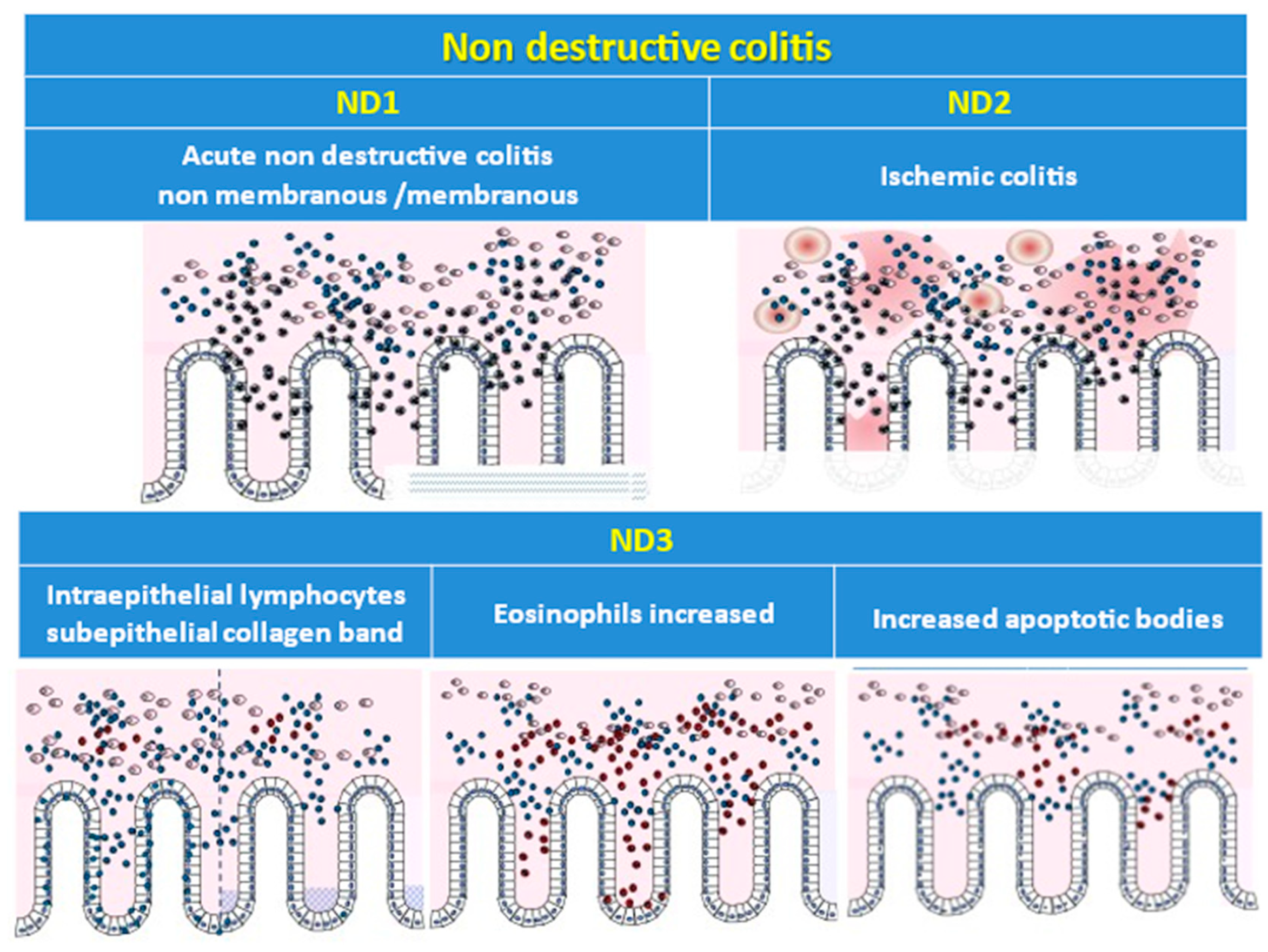
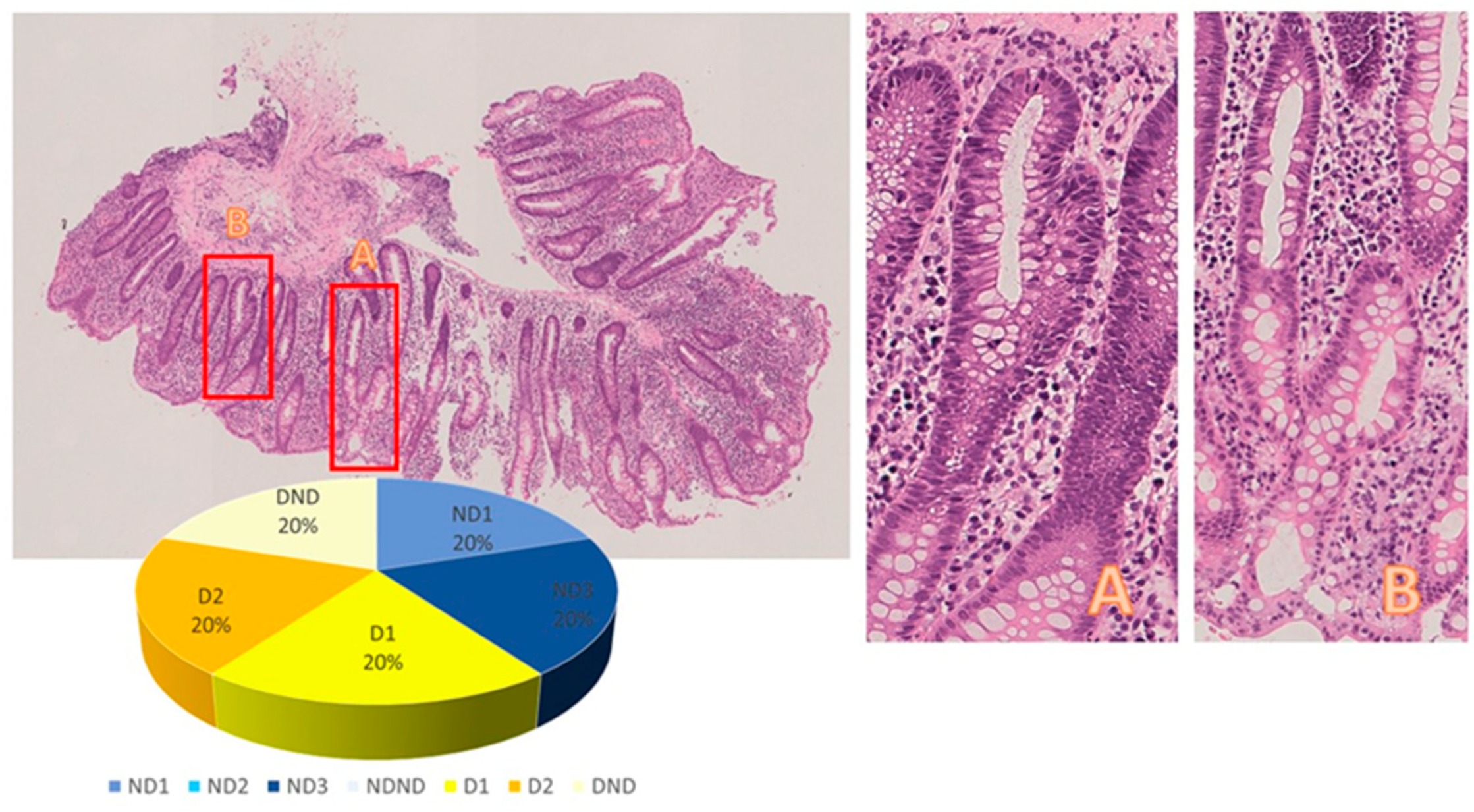
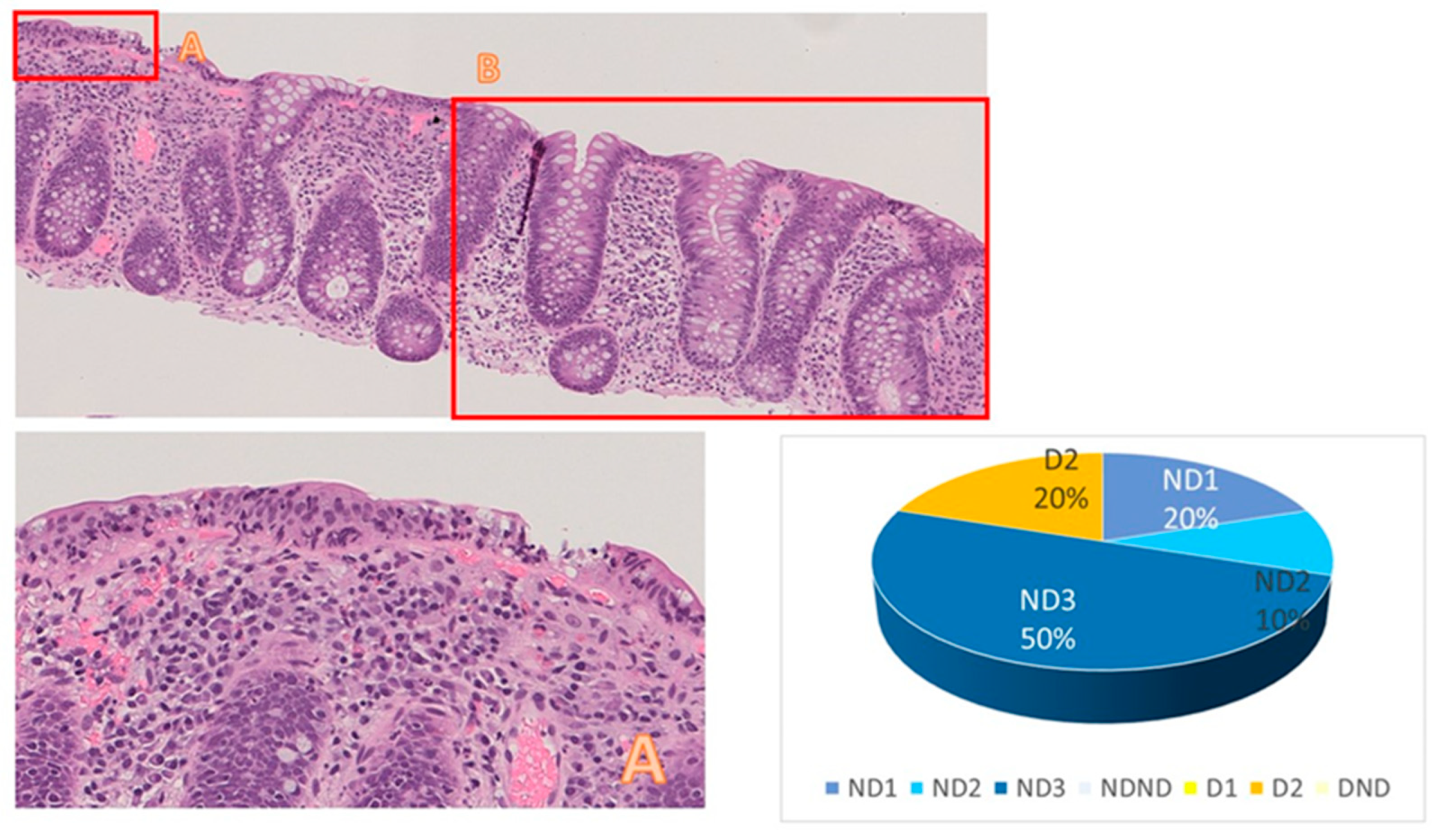
| Patient ID | Category | Subcategory | Category | Subcategory | |||||
|---|---|---|---|---|---|---|---|---|---|
| ND | ND1 | ND2 | ND3 | Undefined | D | D1 | D2 | Undefined | |
| 1 | 11 | 3 | 1 | 7 | 0 | 0 | 0 | 0 | 0 |
| 2 | 9 | 0 | 9 | 0 | 0 | 2 | 1 | 1 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 7 | 2 | 4 | 1 |
| 4 | 9 | 2 | 0 | 7 | 0 | 0 | 0 | 0 | 0 |
| 5 | 8 | 2 | 1 | 5 | 0 | 2 | 0 | 1 | 0 |
| 6 | 1 | 1 | 0 | 0 | 0 | 7 | 3 | 4 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 6 | 5 | 1 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 7 | 5 | 1 | 1 |
| 9 | 2 | 1 | 0 | 1 | 0 | 3 | 1 | 1 | 1 |
| 10 | 4 | 1 | 0 | 2 | 1 | 3 | 0 | 3 | 0 |
| 11 | 2 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 |
| 12 | 1 | 1 | 0 | 0 | 0 | 5 | 5 | 0 | 0 |
| 13 | 2 | 1 | 0 | 1 | 0 | 3 | 1 | 2 | 0 |
| 14 | 4 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 15 | 3 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 16 | 1 | 0 | 0 | 1 | 0 | 3 | 0 | 3 | 0 |
| 17 | 4 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 |
| 18 | 2 | 2 | 0 | 0 | 0 | 3 | 1 | 2 | 0 |
| 19 | 4 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| 20 | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canavese, G.; Falco, E.C.; Perez-Diaz-del-Campo, N.; Caviglia, G.P.; Di Giovanni, F.; Ribaldone, D.G. The Histology-Driven Differential Diagnosis in Bowel Inflammatory Conditions Is Not All That Obvious: Evidence from a Survey Based on Digital Slides. Diagnostics 2023, 13, 3684. https://doi.org/10.3390/diagnostics13243684
Canavese G, Falco EC, Perez-Diaz-del-Campo N, Caviglia GP, Di Giovanni F, Ribaldone DG. The Histology-Driven Differential Diagnosis in Bowel Inflammatory Conditions Is Not All That Obvious: Evidence from a Survey Based on Digital Slides. Diagnostics. 2023; 13(24):3684. https://doi.org/10.3390/diagnostics13243684
Chicago/Turabian StyleCanavese, Gabriella, Enrico Costantino Falco, Nuria Perez-Diaz-del-Campo, Gian Paolo Caviglia, Fabrizia Di Giovanni, and Davide Giuseppe Ribaldone. 2023. "The Histology-Driven Differential Diagnosis in Bowel Inflammatory Conditions Is Not All That Obvious: Evidence from a Survey Based on Digital Slides" Diagnostics 13, no. 24: 3684. https://doi.org/10.3390/diagnostics13243684
APA StyleCanavese, G., Falco, E. C., Perez-Diaz-del-Campo, N., Caviglia, G. P., Di Giovanni, F., & Ribaldone, D. G. (2023). The Histology-Driven Differential Diagnosis in Bowel Inflammatory Conditions Is Not All That Obvious: Evidence from a Survey Based on Digital Slides. Diagnostics, 13(24), 3684. https://doi.org/10.3390/diagnostics13243684








