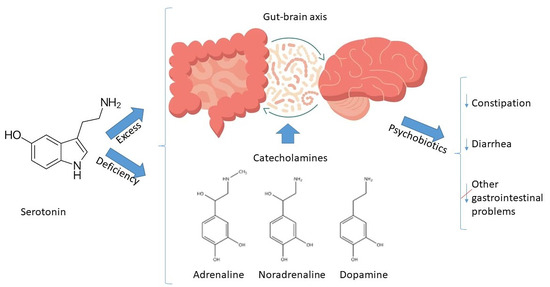
Variety of Serotonin Levels in Pediatric Gastrointestinal Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Selection
- Control group (I): 37 patients (27.4%).
- Group with psychoanxiety disorders (II): 65 patients (48.1%).
- Group with psychiatric disorders (III): 33 people (24.4%).
2.2. Clinical Assessment
2.3. Paraclinical Assessment
2.4. Statistical Analysis
2.5. Ethics Committee Approval
3. Results
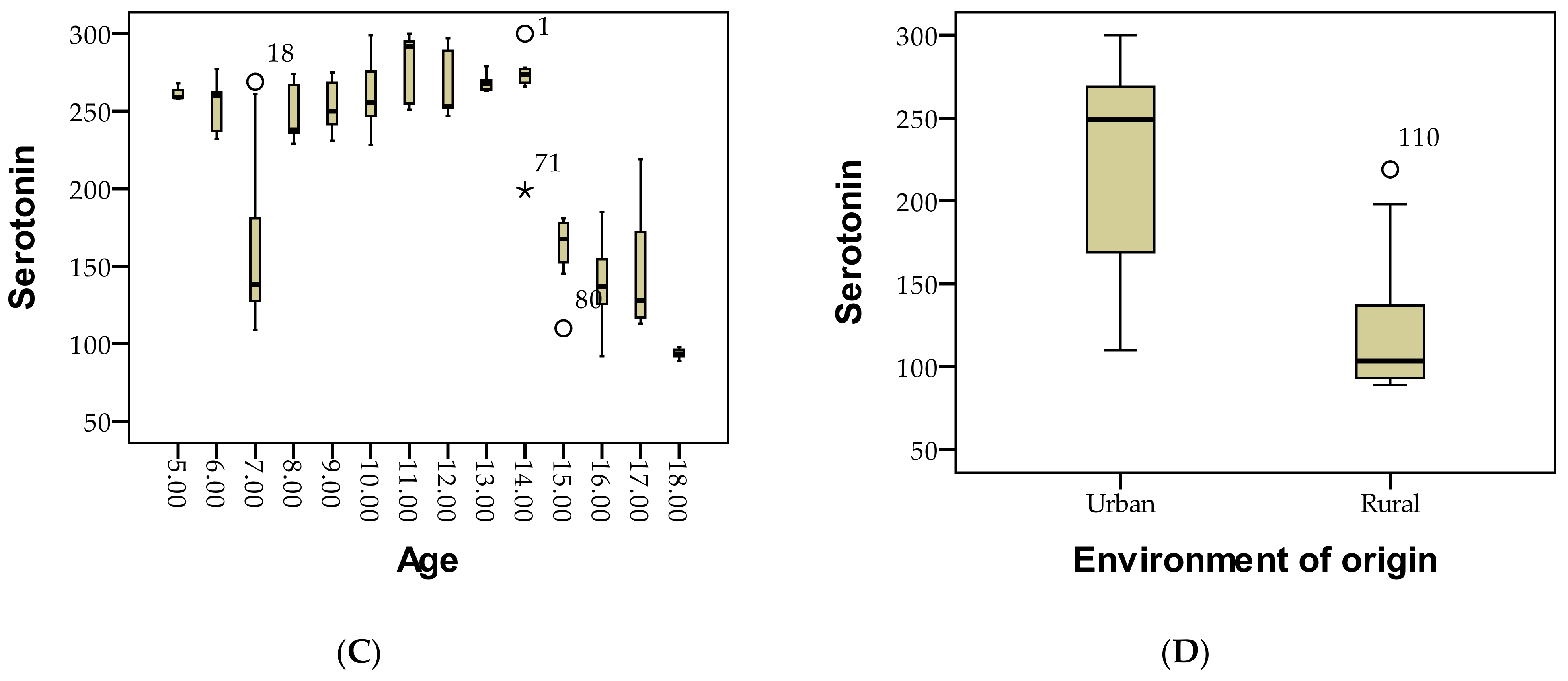
3.1. Demographic Description of Patients Based on Serotonin Level Assessment Is as Follows
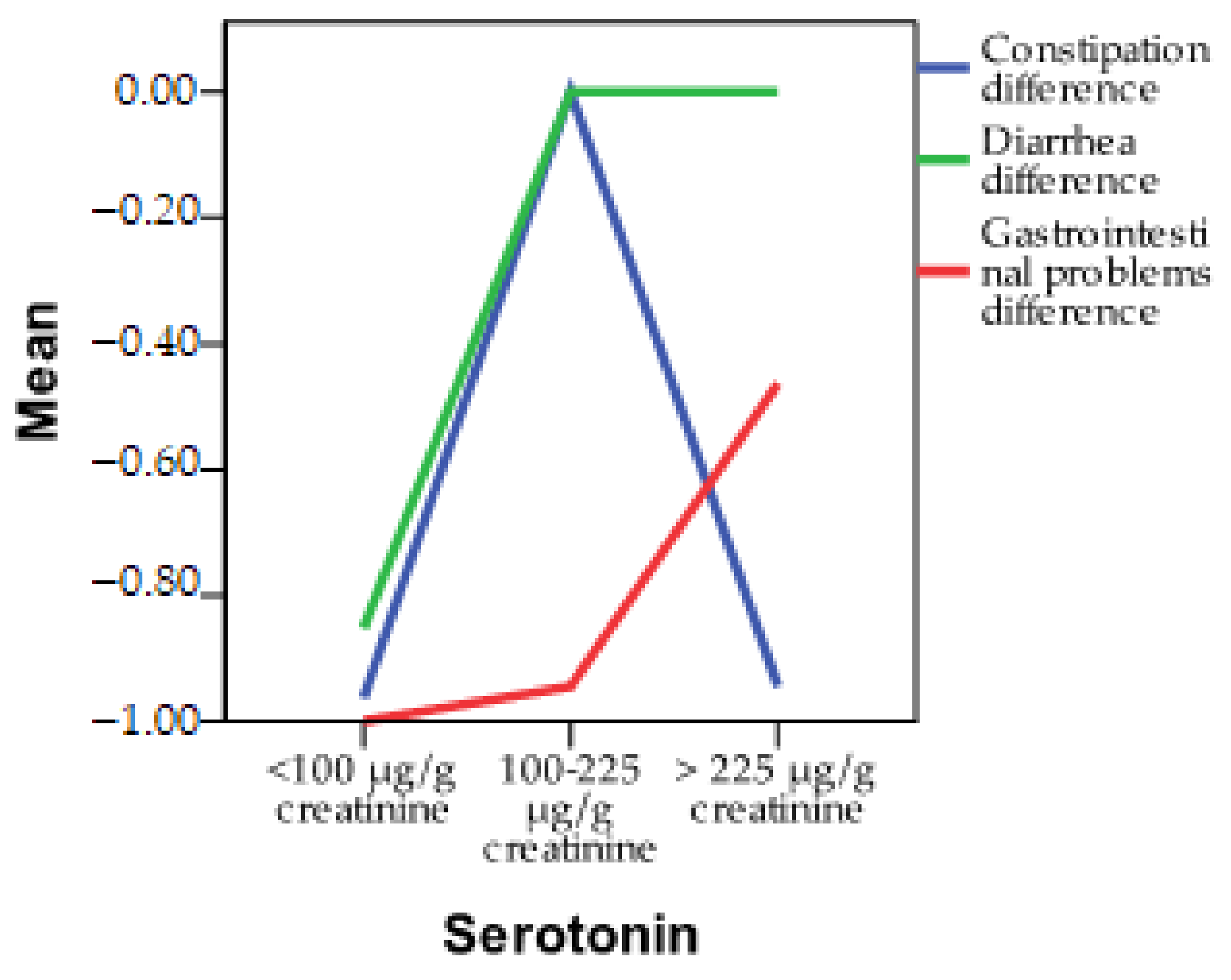
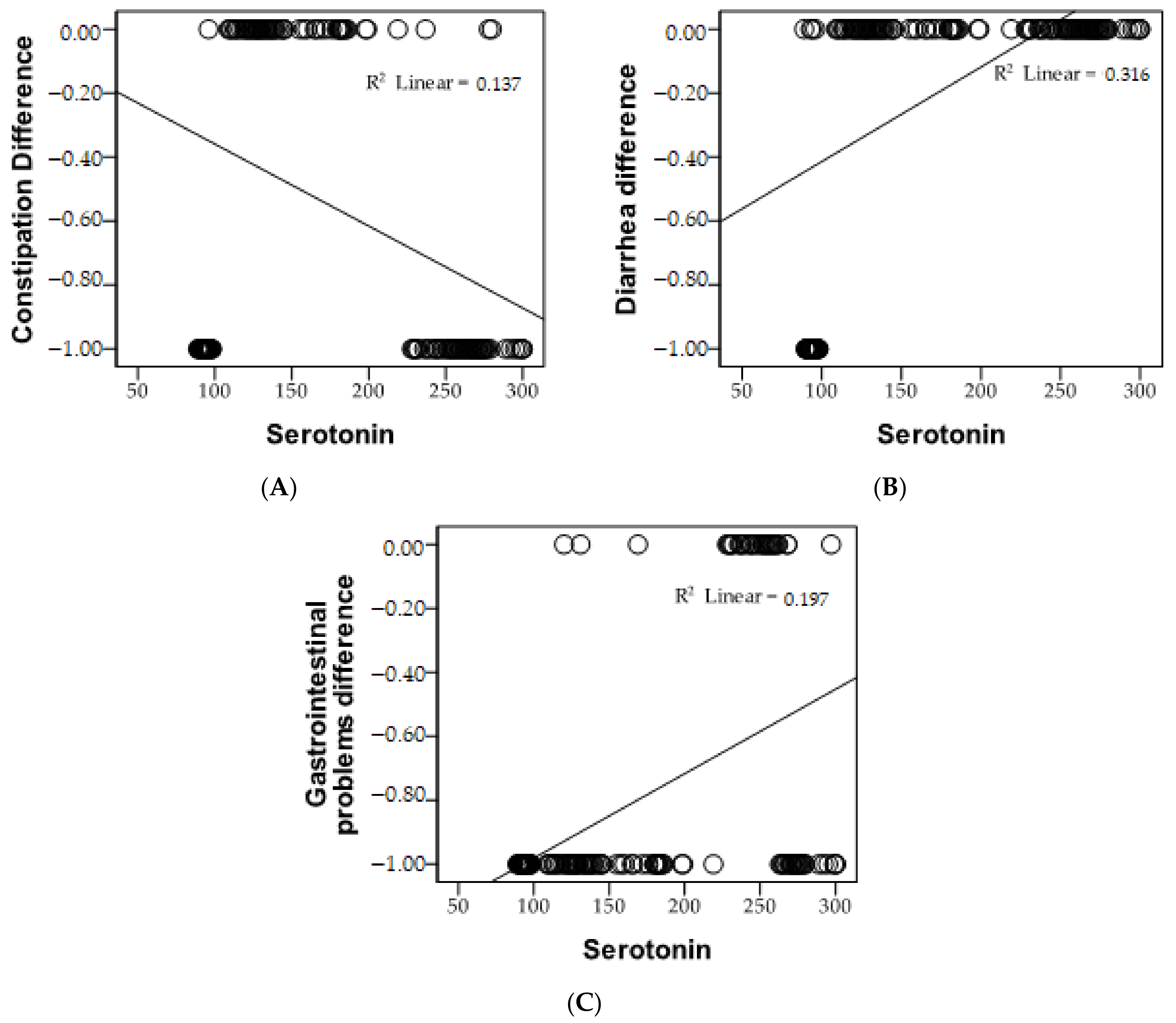
3.2. Incidence of Gastrointestinal Symptoms According to Serotonin Levels
3.3. Incidence of Neuropsychiatric Symptoms Based on Serotonin Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakshi, A.; Tadi, P. Biochemistry, Serotonin; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Barandouzi, Z.A.; Lee, J.; del Carmen Rosas, M.; Chen, J.; Henderson, W.A.; Starkweather, A.R.; Cong, X.S. Associations of neurotransmitters and the gut microbiome with emotional distress in mixed type of irritable bowel syndrome. Sci. Rep. 2022, 12, 1648. [Google Scholar] [CrossRef]
- Koza, J.; Liebert, A.; Hołyńska-Iwan, I.; Piskorska, E.; Kaczorowski, P. Reduced sodium absorption in the colon under serotonin is a potential factor aggravating secretory diarrhea. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2023, 32, 481–488. [Google Scholar] [CrossRef]
- Keszthelyi, D. Serotonin in Gastrointestinal Disorders: Delineating Peripheral vs Central Effects. Gastroenterology 2023, 164, 1027–1028. [Google Scholar] [CrossRef]
- Vaccaro, R.; Casini, A.; Severi, C.; Lamazza, A.; Pronio, A.; Palma, R. Serotonin and Melatonin in Human Lower Gastrointestinal Tract. Diagnostics 2023, 13, 204. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Camilleri, M.; Sellin, J.H.; Barrett, K.E. Pathophysiology, Evaluation, and Management of Chronic Watery Diarrhea. Gastroenterology 2017, 152, 515–532.e2. [Google Scholar] [CrossRef]
- Vahora, I.S.; Tsouklidis, N.; Kumar, R.; Soni, R.; Khan, S. How Serotonin Level Fluctuation Affects the Effectiveness of Treatment in Irritable Bowel Syndrome. Cureus 2020, 12, e9871. [Google Scholar] [CrossRef]
- Cannon Homaei, S.; Barone, H.; Kleppe, R.; Betari, N.; Reif, A.; Haavik, J. ADHD symptoms in neurometabolic diseases: Underlying mechanisms and clinical implications. Neurosci. Biobehav. Rev. 2022, 132, 838–856. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Järbrink-Sehgal, E.; Andreasson, A. The gut microbiota and mental health in adults. Curr. Opin. Neurobiol. 2020, 62, 102–114. [Google Scholar] [CrossRef]
- Jernej, B.; Banović, M.; Cicin-Sain, L.; Hranilović, D.; Balija, M.; Oresković, D.; Folnegović-Smalc, V. Physiological characteristics of platelet/circulatory serotonin: Study on a large human population. Psychiatry Res. 2000, 94, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Chernecky, C.C.; Berger, B.J. Laboratory Tests and Diagnostic Procedures; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Chugani, D.C.; Muzik, O.; Behen, M.; Rothermel, R.; Janisse, J.J.; Lee, J.; Chugani, H.T. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann. Neurol. 1999, 45, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Mathee, K.; Cickovski, T.; Deoraj, A.; Stollstorff, M.; Narasimhan, G. The gut microbiome and neuropsychiatric disorders: Implications for attention deficit hyperactivity disorder (ADHD). J. Med. Microbiol. 2020, 69, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Cumming, R.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Handelsman, D.J.; Seibel, M.; Waite, L.M.; Hirani, V. Associations of sarcopenic obesity with the metabolic syndrome and insulin resistance over five years in older men: The Concord Health and Ageing in Men Project. Exp. Gerontol. 2018, 108, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Cingelová, Z. Important functions of serotonin in the gastrointestinal tract. Int. J. Health New Technol. Soc. Work. 2023, 18, 73–83. [Google Scholar]
- Muldoon, M.F.; Mackey, R.H.; Korytkowski, M.T.; Flory, J.D.; Pollock, B.G.; Manuck, S.B. The metabolic syndrome is associated with reduced central serotonergic responsivity in healthy community volunteers. J. Clin. Endocrinol. Metab. 2006, 91, 718–721. [Google Scholar] [CrossRef]
- Steiger, H. Eating disorders and the serotonin connection: State, trait and developmental effects. J. Psychiatry Neurosci. 2004, 29, 20–29. [Google Scholar]
- Schnappauf, O.; Chae, J.J.; Kastner, D.L.; Aksentijevich, I. The Pyrin Inflammasome in Health and Disease. Front. Immunol. 2019, 10, 1745. [Google Scholar] [CrossRef]
- Ghitea, T.C. Correlation of Periodontal Bacteria with Chronic Inflammation Present in Patients with Metabolic Syndrome. Biomedicines 2021, 9, 1709. [Google Scholar] [CrossRef]
- Trifan, D.F.; Tirla, A.G.; Mos, C.; Danciu, A.; Bodog, F.; Manole, F.; Ghitea, T.C. Involvement of Vitamin D3 in the Aging Process According to Sex. Cosmetics 2023, 10, 114. [Google Scholar] [CrossRef]
- Rezzani, R.; Franco, C. Liver, Oxidative Stress and Metabolic Syndromes. Nutrients 2021, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Corb Aron, R.A.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M. Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of akkermansia muciniphila as a key gut bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Matiș, L.; Alexandru, B.A.; Fodor, R.; Daina, L.G.; Ghitea, T.C.; Vlad, S. Effect of Probiotic Therapy on Neuropsychiatric Manifestations in Children with Multiple Neurotransmitter Disorders: A Study. Biomedicines 2023, 11, 2643. [Google Scholar] [CrossRef]
- Ghitea, T.C.; Vlad, S.; Birle, D.; Tit, D.M.; Lazar, L.; Nistor-Cseppento, C.; Behl, T.; Bungau, S. The influence of diet therapeutic intervention on the sarcopenic index of patients with metabolic syndrome. Acta Endocrinol. 2020, 16, 470–478. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef]
- Dhopatkar, N.; Keeler, J.L.; Mutwalli, H.; Whelan, K.; Treasure, J.; Himmerich, H. Gastrointestinal symptoms, gut microbiome, probiotics and prebiotics in anorexia nervosa: A review of mechanistic rationale and clinical evidence. Psychoneuroendocrinology 2023, 147, 105959. [Google Scholar] [CrossRef]
- Șchiopu, C.G.; Ștefănescu, C.; Boloș, A.; Diaconescu, S.; Gilca-Blanariu, G.-E.; Ștefănescu, G. Functional Gastrointestinal Disorders with Psychiatric Symptoms: Involvement of the Microbiome–Gut–Brain Axis in the Pathophysiology and Case Management. Microorganisms 2022, 10, 2199. [Google Scholar] [CrossRef]
- Simpson, C.A.; Mu, A.; Haslam, N.; Schwartz, O.S.; Simmons, J.G. Feeling down? A systematic review of the gut microbiota in anxiety/depression and irritable bowel syndrome. J. Affect. Disord. 2020, 266, 429–446. [Google Scholar] [CrossRef]
- Wang, P.X.; Deng, X.R.; Zhang, C.H.; Yuan, H.J. Gut microbiota and metabolic syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef]
- Jang, S.H.; Woo, Y.S.; Lee, S.Y.; Bahk, W.M. The Brain-Gut-Microbiome Axis in Psychiatry. Int. J. Mol. Sci. 2020, 21, 7122. [Google Scholar] [CrossRef]
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef]
- Feng, P.; Zhao, S.; Zhang, Y.; Li, E. A review of probiotics in the treatment of autism spectrum disorders: Perspectives from the gut–brain axis. Front. Microbiol. 2023, 14, 1123462. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Veereman-Wauters, G.; De Greef, E.; Peeters, S.; Casteels, A.; Mahler, T.; Devreker, T.; Hauser, B. Probiotics and prebiotics in prevention and treatment of diseases in infants and children. J. Pediatr. 2011, 87, 292–300. [Google Scholar] [CrossRef]
- Gros, M.; Gros, B.; Mesonero, J.E.; Latorre, E. Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. J. Clin. Med. 2021, 10, 3429. [Google Scholar] [CrossRef]
- Dale, H.F.; Rasmussen, S.H.; Asiller, Ö.Ö.; Lied, G.A. Probiotics in Irritable Bowel Syndrome: An Up-to-Date Systematic Review. Nutrients 2019, 11, 2048. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Cai, R.; Li, Y.; Gu, B. A narrative review of relationship between gut microbiota and neuropsychiatric disorders: Mechanisms and clinical application of probiotics and prebiotics. Ann. Palliat. Med. 2021, 10, 2304–2313. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P. Healthy Traditional Mediterranean Diet: An Expression of Culture, History, and Lifestyle. Nutr. Rev. 2020, 55, 383–389. [Google Scholar] [CrossRef]
- Briguglio, M.; Vitale, J.A.; Galentino, R.; Banfi, G.; Zanaboni Dina, C.; Bona, A.; Panzica, G.; Porta, M.; Dell’Osso, B.; Glick, I.D. Healthy Eating, Physical Activity, and Sleep Hygiene (HEPAS) as the Winning Triad for Sustaining Physical and Mental Health in Patients at Risk for or with Neuropsychiatric Disorders: Considerations for Clinical Practice. Neuropsychiatr. Dis. Treat. 2020, 16, 55–70. [Google Scholar] [CrossRef]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Kageyama, K.; Iwasaki, Y.; Daimon, M. Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience. Int. J. Mol. Sci. 2021, 22, 12242. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Barros-Santos, T.; Silva, K.S.O.; Libarino-Santos, M.; Elisangela Gouveia, C.-P.; Reis, H.S.; Tamura, E.K.; de Oliveira-Lima, A.J.; Berro, L.F.; Uetanabaro, A.P.T.; Marinho, E.A.V. Effects of chronic treatment with new strains of Lactobacillus plantarum on cognitive, anxiety- and depressive-like behaviors in male mice. PLoS ONE 2020, 15, e0234037. [Google Scholar] [CrossRef]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef]
- Matiș, L.; Alexandru, B.A.; Ghitea, T.C. Catecholamine Variations in Pediatric Gastrointestinal Disorders and Their Neuropsychiatric Expression. Biomedicines 2023, 11, 2600. [Google Scholar] [CrossRef]

| Parameters | Serotonin Levels | p | ||||||
|---|---|---|---|---|---|---|---|---|
| <100 µg/g Creatinine | 100–225 µg/g Creatinine | >225 µg/g Creatinine | ||||||
| n | % | n | % | n | % | |||
| Initial | ||||||||
| Constipation | No | 0 | 0.0 | 54 | 100.0 | 0 | 0.0 | a |
| Yes | 27 | 100.0 | 0 | 0.0 | 54 | 100.0 | ||
| Diarrhea | No | 0 | 0.0 | 54 | 100.0 | 54 | 100.0 | a |
| Yes | 27 | 100.0 | 0 | 0.0 | 0 | 0.0 | ||
| Gastrointestinal problems | No | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | a |
| Yes | 27 | 100.0 | 54 | 100.0 | 54 | 100.0 | ||
| Final | ||||||||
| Constipation | No | 26 | 96.3 | 54 | 100.0 | 51 | 94.4 | 0.231 |
| Yes | 1 | 3.7 | 0 | 0.0 | 3 | 5.6 | ||
| Diarrhea | No | 23 | 85.2 | 54 | 100.0 | 54 | 100.0 | 0.001 ** |
| Yes | 4 | 14.8 | 0 | 0.0 | 0 | 0.0 | ||
| Gastrointestinal problems | No | 27 | 100.0 | 51 | 94.4 | 25 | 46.3 | 0.001 ** |
| Yes | 0 | 0.0 | 3 | 5.6 | 29 | 53.7 | ||
| Pearson Correlation | Serotonin | |
|---|---|---|
| Constipation | r | −0.370 ** |
| p | 0.000 | |
| Diarrhea | r | 0.562 ** |
| p | 0.000 | |
| Gastrointestinal problems | r | 0.444 ** |
| p | 0.000 | |
| n | 135 | |
| Parameters | Serotonin | p | ||||||
|---|---|---|---|---|---|---|---|---|
| <100 µg/g Creatinine | 100–225 µg/g Creatinine | >225 µg/g Creatinine | ||||||
| n | % | n | % | n | % | |||
| Initial | ||||||||
| Headache | No | 0 | 0.0 | 27 | 50.0 | 27 | 50.0 | 0.001 ** |
| Yes | 27 | 100.0 | 27 | 50.0 | 27 | 50.0 | ||
| Fatigue | No | 0 | 0.0 | 27 | 50.0 | 54 | 100.0 | a |
| Yes | 27 | 100.0 | 27 | 50.0 | 0 | 0.0 | ||
| Mood swings | No | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.001 ** |
| Yes | 27 | 100.0 | 54 | 100.0 | 54 | 100.0 | ||
| Hyperactivity | No | 27 | 100.0 | 0 | 0.0 | 27 | 50.0 | 0.001 ** |
| Yes | 0 | 0.0 | 54 | 100.0 | 27 | 50.0 | ||
| Aggression | No | 0 | 0.0 | 0 | 0.0 | 27 | 50.0 | 0.001 |
| Yes | 27 | 100.0 | 54 | 100.0 | 27 | 50.0 | ||
| Sleep disturbances | No | 0 | 0.0 | 0 | 0.0 | 27 | 50.0 | 0.001 ** |
| Yes | 27 | 100.0 | 54 | 100.0 | 27 | 50.0 | ||
| Lack of concentration | No | 0 | 0.0 | 27 | 50.0 | 0 | 0.0 | 0.001 ** |
| Yes | 27 | 100.0 | 27 | 50.0 | 54 | 100.0 | ||
| Final | ||||||||
| Headache | No | 27 | 100.0 | 54 | 100.0 | 49 | 90.7 | 0.020 * |
| Yes | 0 | 0.0 | 0 | 0.0 | 5 | 9.3 | ||
| Fatigue | No | 25 | 92.6 | 54 | 100.0 | 54 | 100.0 | 0.017 * |
| Yes | 2 | 7.4 | 0 | 0.0 | 0 | 0.0 | ||
| Mood swings | No | 23 | 85.2 | 54 | 100.0 | 0 | 0.0 | 0.001 ** |
| Yes | 4 | 14.8 | 0 | 0.0 | 54 | 100.0 | ||
| Hyperactivity | No | 27 | 100.0 | 27 | 50.0 | 47 | 87.0 | 0.001 ** |
| Yes | 0 | 0.0 | 27 | 50.0 | 7 | 13.0 | ||
| Aggression | No | 27 | 100.0 | 27 | 50.0 | 48 | 88.9 | 0.001 ** |
| Yes | 0 | 0.0 | 27 | 50.0 | 6 | 11.1 | ||
| Sleep disturbances | No | 27 | 100.0 | 53 | 98.1 | 48 | 88.9 | 0.037 * |
| Yes | 0 | 0.0 | 1 | 1.9 | 6 | 11.1 | ||
| Lack of concentration | No | 27 | 100.0 | 54 | 100.0 | 27 | 50.0 | 0.001 ** |
| Yes | 0 | 0.0 | 0 | 0.0 | 27 | 50.0 | ||
| Pearson Correlation | Serotonin | |
|---|---|---|
| Headache | r | 0.417 ** |
| p | 0.000 | |
| Fatigue | r | 0.682 ** |
| p | 0.000 | |
| Mood swings | r | 0.831 ** |
| p | 0.000 | |
| Hyperactivity | r | −0.068 |
| p | 0.432 | |
| Aggression | r | 0.426 ** |
| p | 0.000 | |
| Sleep disturbances | r | 0.709 ** |
| p | 0.000 | |
| Lack of concentration | r | 0.162 |
| p | 0.060 | |
| n | 135 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matiș, L.; Daina, L.G.; Maris, L.; Ghitea, T.C.; Trifan, D.F.; Moga, I.; Fodor, R. Variety of Serotonin Levels in Pediatric Gastrointestinal Disorders. Diagnostics 2023, 13, 3675. https://doi.org/10.3390/diagnostics13243675
Matiș L, Daina LG, Maris L, Ghitea TC, Trifan DF, Moga I, Fodor R. Variety of Serotonin Levels in Pediatric Gastrointestinal Disorders. Diagnostics. 2023; 13(24):3675. https://doi.org/10.3390/diagnostics13243675
Chicago/Turabian StyleMatiș, Loredana, Lucia Georgeta Daina, Lavinia Maris, Timea Claudia Ghitea, Daniela Florina Trifan, Ioana Moga, and Radu Fodor. 2023. "Variety of Serotonin Levels in Pediatric Gastrointestinal Disorders" Diagnostics 13, no. 24: 3675. https://doi.org/10.3390/diagnostics13243675
APA StyleMatiș, L., Daina, L. G., Maris, L., Ghitea, T. C., Trifan, D. F., Moga, I., & Fodor, R. (2023). Variety of Serotonin Levels in Pediatric Gastrointestinal Disorders. Diagnostics, 13(24), 3675. https://doi.org/10.3390/diagnostics13243675