Diagnosis and Treatment of Leprosy in Taiwan during the COVID-19 Pandemic: A Retrospective Study in a Tertiaty Center
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Molecular and Serological Diagnoses
4.2. Drug Resistance
4.3. Treatment for Leprosy Reactions
4.4. Impact of the COVID-19 Pandemic
4.5. Leprosy Prevention
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization; Organisation Mondiale de la Santé. Global leprosy (Hansen disease) update, 2021: Moving towards interruption of transmission—Situation de la lèpre (maladie de Hansen) dans le monde, 2021: Vers l’interruption de la transmission. Wkly. Epidemiol. Rec. 2022, 97, 429–450. [Google Scholar]
- Huang, W.-L.; Jou, R. Epidemiology and Molecular Diagnosis of Leprosy, Taiwan, 2002–2016. Epidemiol. Bull. 2017, 33, 133. [Google Scholar] [CrossRef]
- Ridley, D.S.; Jopling, W.H. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 1966, 34, 255–273. [Google Scholar] [PubMed]
- Chaves, L.L.; Patriota, Y.; Soares-Sobrinho, J.L.; Vieira, A.C.C.; Lima, S.A.C.; Reis, S. Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication? Pharmaceutics 2020, 12, 1202. [Google Scholar] [CrossRef]
- Elaine Silva Nascimento Andrade, E.S.N.; Brandão, J.G.; da Silva, J.S.; Kurizky, P.S.; Rosa, P.S.; de Araújo, W.N.; Gomes, C.M. A systematic review and meta-analysis of studies on the diagnostic accuracy and screening of tests to detect antimicrobial resistance in leprosy. Diagn. Microbiol. Infect. Dis. 2021, 100, 115325. [Google Scholar]
- Gutiérrez-Villarreal, I.M.; Ocampo-Candiani, J.; Villarreal-Martinez, A.; Gomez-Flores, M.; Fernández, L.T.; Rodríguez-Tamez, G.; Pérez-Garza, D.M.; González-Martínez, G.; Yamallel-Ortega, L.A.; Chavez-Alvarez, S. Leprosy reactions after SARS-COV2 (COVID-19) infection. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e952–e954. [Google Scholar] [CrossRef] [PubMed]
- Lavania, M.; Katoch, K.; Sharma, R.; Sharma, P.; Das, R.; Gupta, A.K.; Chauhan, D.S.; Katoch, V.M. Molecular typing of Mycobacterium leprae strains from northern India using short tandem repeats. Indian J. Med. Res. 2011, 133, 618–626. [Google Scholar]
- Ridley, D.S.; Jopling, W.H. A classification of leprosy for research purposes. Lepr. Rev. 1962, 33, 119–128. [Google Scholar] [CrossRef]
- Guevara, B.E.K.; Saleem, S.; Chen, W.T.; Hsiao, P.F.; Wu, Y.H. Lucio phenomenon mimicking antiphospholipid syndrome: The occurrence of antiphospholipid antibodies in a leprosy patient. J. Cutan. Pathol. 2019, 46, 347–352. [Google Scholar] [CrossRef]
- Hsu, J.H.; Wu, Y.H.; Hsiao, P.F. Histoid leprosy complicated with Charcot neuroarthropathy: A case report. Dermatologica Sinica. 2021, 39, 137–138. [Google Scholar] [CrossRef]
- Bhandari, A.; Shilpa; Gupta, S.; Dogra, S.; Narang, T. Reactions in leprosy patients triggered by COVID-19 vaccination—A cross-sectional study from a tertiary care centre in India. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e971–e972. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Yang, C.S.; Yen, C.Y. Type 1 lepra reaction induced by a COVID-19 vaccine. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e266–e268. [Google Scholar] [CrossRef]
- Mahajan, V.K. Slit-skin smear in leprosy: Lest we forget it! Indian J. Lepr. 2013, 85, 177–183. [Google Scholar] [PubMed]
- Maymone, M.B.C.; Laughter, M.; Venkatesh, S.; Dacso, M.M.; Rao, P.N.; Stryjewska, B.M.; Hugh, J.; Dellavalle, R.P.; Dunnick, C.A. Leprosy: Clinical aspects and diagnostic techniques. J. Am. Acad. Dermatol. 2020, 83, 1–14. [Google Scholar] [CrossRef]
- Williams, D.L.; Gillis, T.P.; Booth, R.J.; Looker, D.; Watson, J.D. The use of a specific DNA probe and polymerase chain reaction for the detection of Mycobacterium leprae. J. Infect. Dis. 1990, 162, 193–200. [Google Scholar] [CrossRef]
- Martinez, A.N.; Talhari, C.; Moraes, M.O.; Talhari, S. PCR-based techniques for leprosy diagnosis: From the laboratory to the clinic. PLoS Negl. Trop. Dis. 2014, 8, e2655. [Google Scholar] [CrossRef] [PubMed]
- Gama, R.S.; Leite, L.A.; Colombo, L.T.; Fraga, L.A.O. Prospects for new leprosy diagnostic tools, a narrative review considering ELISA and PCR assays. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200197. [Google Scholar] [CrossRef] [PubMed]
- Fiallo, P.; Williams, D.L.; Chan, G.P.; Gillis, T.P. Effects of fixation on polymerase chain reaction detection of Mycobacterium leprae. J. Clin. Microbiol. 1992, 30, 3095–3098. [Google Scholar] [CrossRef]
- Yan, W.; Xing, Y.; Yuan, L.C.; De Yang, R.; Tan, F.Y.; Zhang, Y.; Li, H.Y. Application of RLEP real-time PCR for detection of M. leprae DNA in paraffin-embedded skin biopsy specimens for diagnosis of paucibacillary leprosy. Am. J. Trop. Med. Hyg. 2014, 90, 524–529. [Google Scholar] [CrossRef]
- Torres, R.T.; Fachi, M.M.; Böger, B.; Marson, B.M.; Ferreira, V.L.; Pontarolo, R.; Guimarães, T.M. Sensitivity and specificity of multibacillary and paucibacillary leprosy laboratory tests: A systematic review and meta-analysis. Diagn. Microbiol. Infect. Dis. 2021, 100, 115337. [Google Scholar] [CrossRef] [PubMed]
- Saar, M.; Beissner, M.; Gültekin, F.; Maman, I.; Herbinger, K.H.; Bretzel, G. RLEP LAMP for the laboratory confirmation of leprosy: Towards a point-of-care test. BMC Infect. Dis. 2021, 21, 1186. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Sharma, V.; Ramesh, V.; Singh, R.; Salotra, P. Development of a novel loop-mediated isothermal amplification assay for rapid detection of Mycobacterium leprae in clinical samples. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.R.; de Paula, N.A.; Simões, M.M.R.; Manso, G.M.D.C.; Albertino, G.S.; Felisbino, G.C.; Antunes, V.M.G.; Perecin, F.A.M.C.; Westin, A.T.; Lugão, H.B.; et al. Bacilloscopy and polymerase chain reaction of slit-skin smears and anti-phenolic glycolipid-I serology for Hansen’s disease diagnosis. Front. Med. 2022, 9, 972244. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, G.; Yang, J.; Jin, G.; Shao, Y.; Li, Y.; Wei, P.; Zhang, L. Drug Resistance (Dapsone, Rifampicin, Ofloxacin) and Resistance-Related Gene Mutation Features in Leprosy Patients: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12443. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M. Drug resistance in leprosy. Jpn. J. Infect. Dis. 2010, 63, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.C.D.S.; Bührer-Sékula, S.; Penna, M.L.F.; Penna, G.O.; Talhari, S. Leprosy: Current situation, clinical and laboratory aspects, treatment history and perspective of the uniform multidrug therapy for all patients. An. Bras. Dermatol. 2017, 92, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Aubry, A.; Sammarco Rosa, P.; Chauffour, A.; Fletcher, M.L.; Cambau, E.; Avanzi, C. Drug resistance in leprosy: An update following 70years of chemotherapy. Infect. Dis. Now. 2022, 52, 243–251. [Google Scholar] [CrossRef]
- Randhawa, A.; Kapila, R.; Schwartz, R.A. Leprosy: What is new. Int. J. Dermatol. 2022, 61, 733–738. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Diagnosis, Treatment and Prevention of Leprosy; Cooreman, E.A., Ed.; World Health Organization, Regional Office for South-East Asia: New Delhi, India, 2018.
- Nakata, N.; Kai, M.; Makino, M. Mutation analysis of the Mycobacterium leprae folP1 gene and dapsone resistance. Antimicrob. Agents Chemother. 2011, 55, 762–766. [Google Scholar] [CrossRef]
- Cambau, E.; Saunderson, P.; Matsuoka, M.; Cole, S.T.; Kai, M.; Suffys, P.; Rosa, P.S.; Williams, D.; Gupta, U.D.; Lavania, M.; et al. Antimicrobial resistance in leprosy: Results of the first prospective open survey conducted by a WHO surveillance network for the period 2009-15. Clin. Microbiol. Infect. 2018, 24, 1305–1310. [Google Scholar] [CrossRef]
- Polycarpou, A.; Walker, S.L.; Lockwood, D.N. A Systematic Review of Immunological Studies of Erythema Nodosum Leprosum. Front. Immunol. 2017, 8, 233. [Google Scholar] [CrossRef]
- Narang, T.; Ashraf, R.; Kaushik, A.; Dogra, S. Apremilast in multibacillary leprosy patients with chronic and recurrent erythema nodosum leprosum: A prospective single-centre pilot study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e917–e919. [Google Scholar] [CrossRef]
- Marlowe, S.N.; Leekassa, R.; Bizuneh, E.; Knuutilla, J.; Ale, P.; Bhattarai, B.; Sigdel, H.; Anderson, A.; Nicholls, P.G.; Johnston, A.; et al. Response to ciclosporin treatment in Ethiopian and Nepali patients with severe leprosy Type 1 reactions. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 1004–1012. [Google Scholar] [CrossRef]
- Narang, T.; Sawatkar, G.U.; Kumaran, M.S.; Dogra, S. Minocycline for Recurrent and/or Chronic Erythema Nodosum Leprosum. JAMA Dermatol. 2015, 151, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, P.; Balan, A.K.; Velmurugan, H.; Venkatesan, S.; Yella, S.S.T. Leprosy Reactions: Clinical Pharmacologist Perspective with Repurposed Medications. Infect. Disord. Drug Targets. 2023, 23, e070922208607. [Google Scholar] [CrossRef] [PubMed]
- Narang, T.; Kaushik, A.; Dogra, S. Apremilast in chronic recalcitrant erythema nodosum leprosum: A report of two cases. Br. J. Dermatol. 2020, 182, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, N.; Tripathy, D.M.; Kumar, S.; Awasthi, P.; Gopal, M.M. A spectrum of leprosy reactions triggered by COVID-19 vaccination: A series of four cases. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e858–e860. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Begum, F.; Panda, M.; Jena, A.K. Trigger of Type 2 Lepra reaction with acute foot drop following COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e334–e335. [Google Scholar] [CrossRef]
- Rerknimitr, P.; Puaratanaarunkon, T.; Wongtada, C.; Wittayabusarakam, N.; Krithin, S.; Paitoonpong, L.; Kumtornrut, C.; Kerr, S.J.; Asawanonda, P.; Jantarabenjakul, W.; et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: A prospective cohort study in healthcare workers. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e158–e161. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Suneetha, S.; Narang, T.; Bhardwaj, A.; Gupta, S.K.; Kamoji, S.G.; Ashwini, P.K.; Pradhan, S.; Rather, S.P.; Patnaik, S.; et al. Management of Leprosy in the Context of COVID-19 Pandemic: Recommendations by SIG Leprosy (IADVL Academy). Indian Dermatol. Online J. 2020, 11, 345–348. [Google Scholar] [CrossRef]
- Pai, V.V.; Wakade, A. Leprosy and COVID-19 Co-infection–Experience in a referral centre in Mumbai, India. In Proceedings of the 31st Biennial Conference of Indian Association of Leprologists, Hyderabad, India, 16–18 April 2021; pp. 48–49. [Google Scholar]
- Cerqueira, S.R.P.S.; Deps, P.D.; Cunha, D.V.; Bezerra, N.V.F.; Barroso, D.H.; Pinheiro, A.B.S.; Pillegi, G.S.; Repsold, T.A.R.; Kurizky, P.S.; Collin, S.M.; et al. The influence of leprosy-related clinical and epidemiological variables in the occurrence and severity of COVID-19: A prospective real-world cohort study. PLoS Negl. Trop. Dis. 2021, 15, e0009635. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Gupta, S.K.; Narang, T.; Suneetha, S.; Pradhan, S.; Agarwal, P.; Suvirya, S.; Gupta, A.; Chhabra, N.; Rao, A.G.; et al. Updates on Management of Leprosy in the Context of COVID-19 Pandemic: Recommendations by IADVL SIG Leprosy. Indian Dermatol. Online J. 2021, 12, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Packyanathan, J.S.; Packyanathan, I.C.; Balini, A.I. Anti-Leprosy Vaccine (Hansen’s Disease Vaccine). In Advances in Pharmaceutical Biotechnology: Recent Progress and Future Applications; Patra, J.K., Shukla, A.C., Das, G., Eds.; Springer: Singapore, 2020; pp. 365–381. [Google Scholar]
- Duthie, M.S.; Balagon, M.F. Combination chemoprophylaxis and immunoprophylaxis in reducing the incidence of leprosy. Risk Manag. Healthc. Policy 2016, 9, 43–53. [Google Scholar] [CrossRef]
- Mungroo, M.R.; Khan, N.A.; Siddiqui, R. Mycobacterium leprae: Pathogenesis, diagnosis, and treatment options. Microb. Pathog. 2020, 149, 104475. [Google Scholar] [CrossRef] [PubMed]
- Duthie, M.S.; Pena, M.T.; Ebenezer, G.J.; Gillis, T.P.; Sharma, R.; Cunningham, K.; Polydefkis, M.; Maeda, Y.; Makino, M.; Truman, R.W.; et al. LepVax, a defined subunit vaccine that provides effective pre-exposure and post-exposure prophylaxis of M. leprae infection. Npj Vaccines 2018, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Smith, W.C. Chemoprophylaxis is effective in the prevention of leprosy in endemic countries: A systematic review and meta-analysis. MILEP2 Study Group. Mucosal Immunology of Leprosy. J. Infect. 2000, 41, 137–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moet, F.J.; Pahan, D.; Oskam, L.; Richardus, J.H.; COLEP Study Group. Effectiveness of single dose rifampicin in preventing leprosy in close contacts of patients with newly diagnosed leprosy: Cluster randomised controlled trial. BMJ 2008, 336, 761–764. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Yan, L.; Yu, M.; Yang, J.; Li, J.; Li, J.; Ning, Y.; Jiang, H.; Shi, Y.; et al. Single-Dose Rifapentine in Household Contacts of Patients with Leprosy. N. Engl. J. Med. 2023, 388, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Scollard, D.M. A New Step in Postexposure Prophylaxis for Leprosy. N. Engl. J. Med. 2023, 388, 1904–1905. [Google Scholar] [CrossRef]
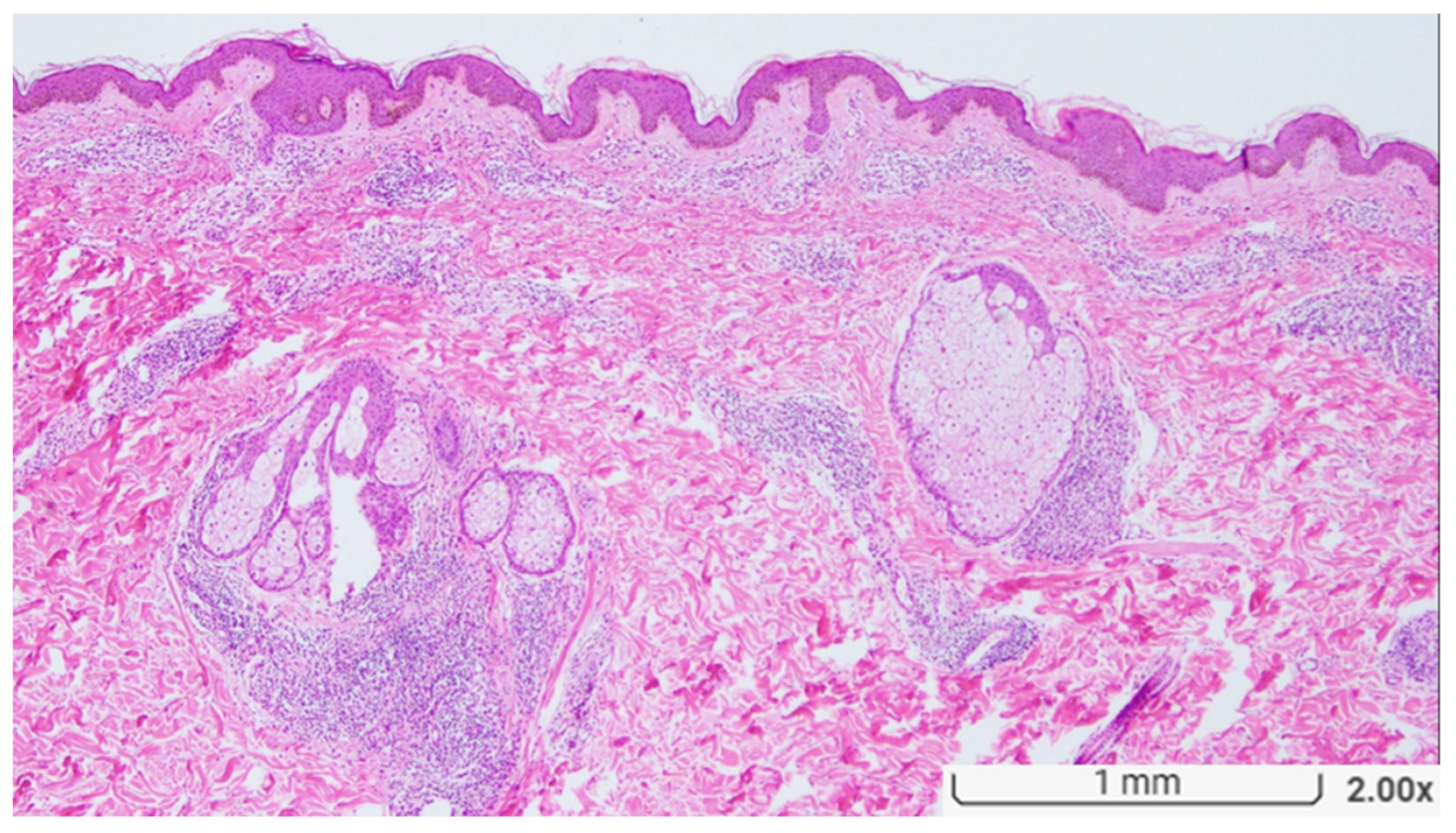

| Demographics and Clinical Presentation | n | % |
|---|---|---|
| Total patients | 28 | |
| Age (in years) | ||
| Mean | 39 ± 13 (23–70) | |
| Sex | ||
| Male | 10 | 35 |
| Female | 18 | 65 |
| Nationality | ||
| Indonesian | 15 | 54 |
| Filipino | 6 | 21 |
| Taiwanese | 5 | 18 |
| Myanma | 1 | 4 |
| Unknown | 1 | 1 |
| Lesion number | ||
| Multiple | 24 | 86 |
| Single | 4 | 14 |
| Clinical classification | ||
| Indeterminate | 2 | 7 |
| Lepromatous | 5 | 18 |
| Borderline lepromatous | 11 | 39 |
| Borderline borderline | 3 | 11 |
| Borderline tuberculoid | 5 | 18 |
| Tuberculoid | 1 | 4 |
| Histoid | 1 | 4 |
| Features (n = 34) | n | % |
|---|---|---|
| Granuloma | ||
| + | 26 | 76 |
| − | 8 | 24 |
| Vasculitis | ||
| + | 3 | 9 |
| − | 31 | 91 |
| Neuritis | ||
| + | 22 | 65 |
| − | 12 | 35 |
| Necrosis | ||
| + | 2 | 6 |
| − | 32 | 94 |
| Inflammatory infiltrates | ||
| Lymphocyte | 15 | 44 |
| Foamy histiocyte | 5 | 15 |
| Neutrophil | 2 | 6 |
| Plasma cell | 7 | 20 |
| Acid-fast stain | ||
| + | 25 | 74 |
| − | 9 | 26 |
| S100 | ||
| + | 12 | 35 |
| Not performed | 22 | 65 |
| Giant cell | ||
| + | 2 | 6 |
| − | 32 | 94 |
| Mycobacterium leprae PCR | ||
| Not performed | 25 | 74 |
| + | 4 | 12 |
| − | 5 | 15 |
| Drug-resistant gene | ||
| folP1 (dapsone) | 1 | 3 |
| rpoB (rifampin) | 0 | 0 |
| gyrA (ofloxacin) | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-L.; Hsiao, P.-F. Diagnosis and Treatment of Leprosy in Taiwan during the COVID-19 Pandemic: A Retrospective Study in a Tertiaty Center. Diagnostics 2023, 13, 3655. https://doi.org/10.3390/diagnostics13243655
Hsieh C-L, Hsiao P-F. Diagnosis and Treatment of Leprosy in Taiwan during the COVID-19 Pandemic: A Retrospective Study in a Tertiaty Center. Diagnostics. 2023; 13(24):3655. https://doi.org/10.3390/diagnostics13243655
Chicago/Turabian StyleHsieh, Chin-Ling, and Pa-Fan Hsiao. 2023. "Diagnosis and Treatment of Leprosy in Taiwan during the COVID-19 Pandemic: A Retrospective Study in a Tertiaty Center" Diagnostics 13, no. 24: 3655. https://doi.org/10.3390/diagnostics13243655
APA StyleHsieh, C.-L., & Hsiao, P.-F. (2023). Diagnosis and Treatment of Leprosy in Taiwan during the COVID-19 Pandemic: A Retrospective Study in a Tertiaty Center. Diagnostics, 13(24), 3655. https://doi.org/10.3390/diagnostics13243655





