Characteristic Mean Kurtosis Values in Simple Diffusion Kurtosis Imaging of Dentigerous Cysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI and Sequence
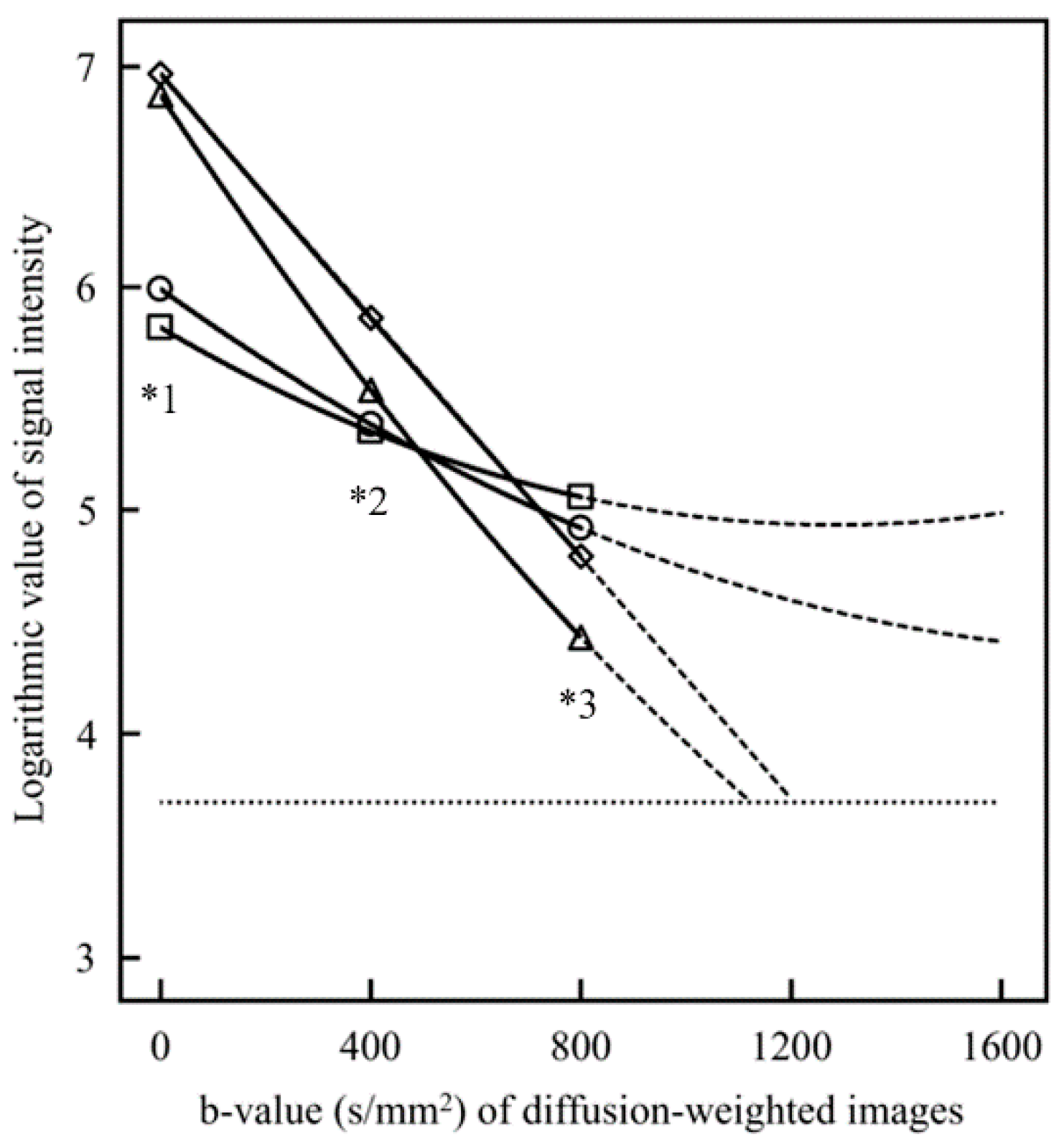
2.3. Creation of the SD Image and ADC Map
2.4. Region of Interest (ROI) Settings
2.5. Statistical Analysis
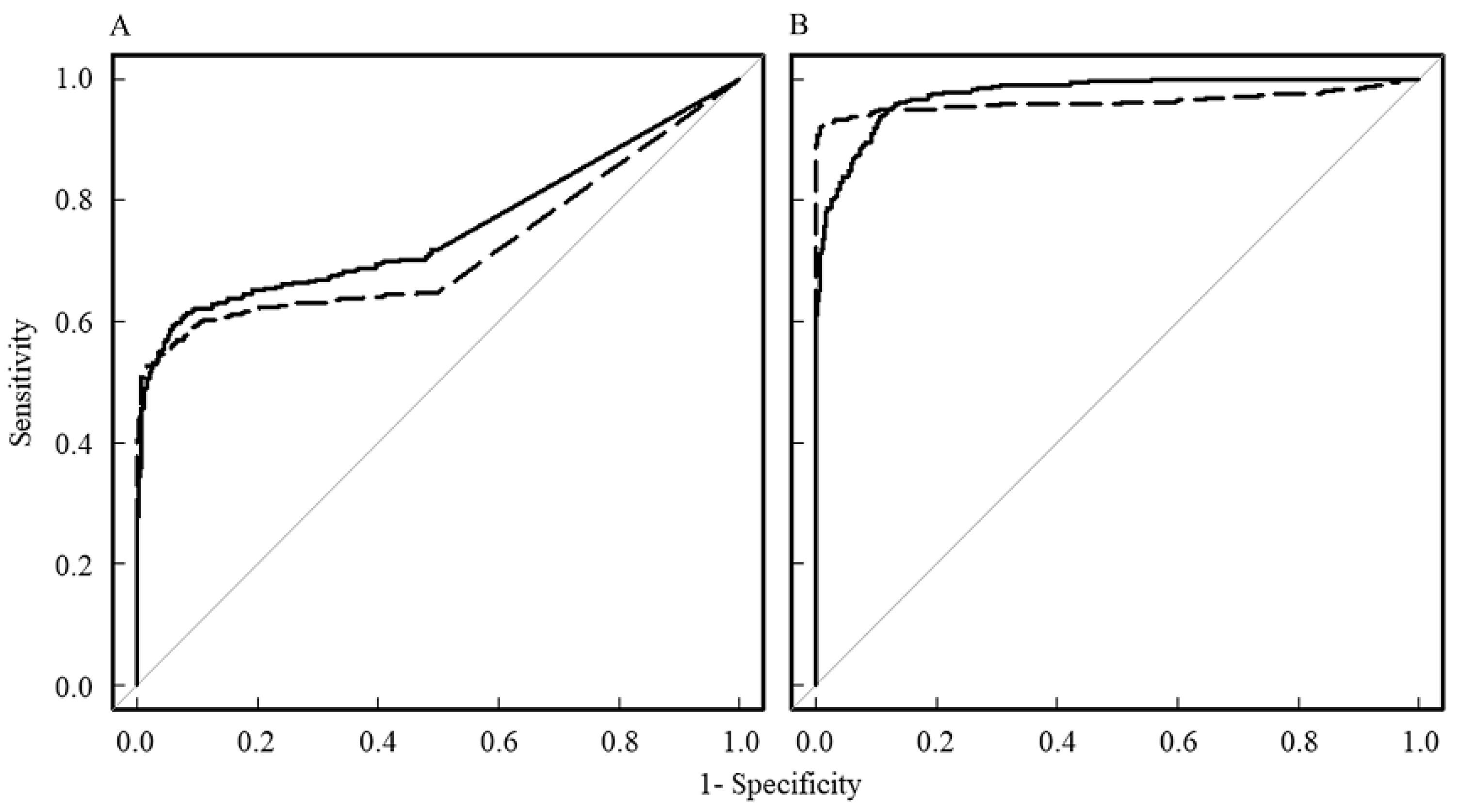
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Wamasing, N.; Watanabe, H.; Sakamoto, J.; Tomisato, H.; Kurabayashi, T. Differentiation of cystic lesions in the jaw by conventional magnetic resonance imaging and diffusion-weighted imaging. Dentomaxillofac. Radiol. 2022, 51, 20210212. [Google Scholar] [CrossRef]
- Srinivasan, K.; Seith Bhalla, A.; Sharma, R.; Kumar, A.; Roychoudhury, A.; Bhutia, O. Diffusion-weighted imaging in the evaluation of odontogenic cysts and tumours. Br. J. Radiol. 2012, 85, e864–e870. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Sue, M.; Sasaki, Y.; Ogura, I. Diffusion-weighted magnetic resonance imaging in oral and maxillofacial lesions: Preliminary study on diagnostic ability of apparent diffusion coefficient maps. Oral Radiol. 2018, 34, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Ogura, I.; Nakahara, K.; Sasaki, Y.; Sue, M.; Oda, T. Diffusion-weighted magnetic resonance imaging in odontogenic keratocysts: Preliminary study on usefulness of apparent diffusion coefficient maps for characterization of normal structures and lesions. Chin. J. Dent. Res. 2019, 22, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, J.; Kuribayashi, A.; Kotaki, S.; Fujikura, M.; Nakamura, S.; Kurabayashi, T. Application of diffusion kurtosis imaging to odontogenic lesions: Analysis of the cystic component. J. Magn. Reson. Imaging 2016, 44, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Valindria, V.; Slator, P.J.; Pye, H.; Grey, A.; Freeman, A.; Moore, C.; Whitaker, H.; Punwani, S.; Singh, S.; et al. Differentiating false positive lesions from clinically significant cancer and normal prostate tissue using VERDICT MRI and other diffusion models. Diagnostics 2022, 12, 1631. [Google Scholar] [CrossRef] [PubMed]
- Żurowska, A.; Pęksa, R.; Grzywińska, M.; Panas, D.; Sowa, M.; Skrobisz, K.; Matuszewski, M.; Szurowska, E. Comparison of diffusion kurtosis imaging and standard mono-exponential apparent diffusion coefficient in diagnosis of significant prostate cancer-a correlation with gleason score assessed on whole-mount histopathology specimens. Diagnostics 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Yang, D.J.; Wang, J.L.; Zhang, C.; Yang, H.F. Non-Gaussian diffusion imaging for malignant and benign pulmonary nodule differentiation: A preliminary study. Acta Radiol. 2017, 58, 19–26. [Google Scholar] [CrossRef]
- Fu, J.; Ye, J.; Zhu, W.; Wu, J.; Chen, W.; Zhu, Q. Magnetic resonance diffusion kurtosis imaging in differential diagnosis of benign and malignant renal tumors. Cancer Imaging 2021, 21, 6. [Google Scholar] [CrossRef]
- Quentin, M.; Pentang, G.; Schimmöller, L.; Kott, O.; Müller-Lutz, A.; Blondin, D.; Arsov, C.; Hiester, A.; Rabenalt, R.; Wittsack, H.J. Feasibility of diffusional kurtosis tensor imaging in prostate MRI for the assessment of prostate cancer: Preliminary results. Magn. Reson. Imaging 2014, 32, 880–885. [Google Scholar] [CrossRef]
- Hamada, K.; Kuroda, M.; Yoshimura, Y.; Khasawneh, A.; Barham, M.; Tekiki, N.; Sugianto, I.; Bamgbose, B.O.; Konishi, K.; Sugimoto, K.; et al. Evaluation of the imaging process for a novel subtraction method using apparent diffusion coefficient values. Acta Med. Okayama 2021, 75, 139–145. [Google Scholar] [CrossRef]
- Kuroda, M.; Konishi, K.; Sugimoto, K.; Yoshimura, Y.; Hamada, K.; Khasawnehc, A.; Barham, M.; Tekiki, N.; Sugianto, I.; Bamgbose, B.O.; et al. Evaluation of fast diffusion kurtosis imaging using new software designed for widespread clinical use. Acta Med. Okayama 2022, 76, 297–305. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kuroda, M.; Nakamitsu, Y.; Al-Hammad, W.E.; Yoshida, S.; Fukumura, Y.; Nakamura, Y.; Kuroda, K.; Kamizaki, R.; Imajoh, S.; et al. Usefulness of simple diffusion kurtosis imaging for head and neck tumors: An early clinical study. Acta Med. Okayama 2023, 77, 273–280. [Google Scholar] [CrossRef]
- Jiang, J.X.; Tang, Z.H.; Zhong, Y.F.; Qiang, J.W. Diffusion kurtosis imaging for differentiating between the benign and malignant sinonasal lesions. J. Magn. Reson. Imaging 2017, 45, 1446–1454. [Google Scholar] [CrossRef]
- Fujima, N.; Sakashita, T.; Homma, A.; Shimizu, Y.; Yoshida, A.; Harada, T.; Tha, K.K.; Kudo, K.; Shirato, H. Advanced diffusion models in head and neck squamous cell carcinoma patients: Goodness of fit, relationships among diffusion parameters and comparison with dynamic contrast-enhanced perfusion. Magn. Reson. Imaging 2017, 36, 16–23. [Google Scholar] [CrossRef]
- Ogura, I.; Sasaki, Y.; Kameta, A.; Sue, M.; Oda, T. Diffusion-weighted imaging in the oral and maxillofacial region: Usefulness of apparent diffusion coefficient maps and maximum intensity projection for characterization of normal structures and lesions. Pol. J. Radiol. 2017, 82, 571–577. [Google Scholar] [CrossRef]
- Daley, T.D.; Wysocki, G.P.; Pringle, G.A. Relative incidence of odontogenic-tumors and oral and jaw cysts in a Canadian population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1994, 77, 276–280. [Google Scholar] [CrossRef]
- Ustuner, E.; Fitoz, S.; Atasoy, C.; Erden, I.; Akyar, S. Bilateral maxillary dentigerous cysts: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 632–635. [Google Scholar] [CrossRef]
- Perez, A.; Lenoir, V.; Lombardi, T. Dentigerous cysts with diverse radiological presentation highlighting diagnostic challenges. Diagnostics 2022, 12, 2006. [Google Scholar] [CrossRef]
- Eida, S.; Hotokezaka, Y.; Katayama, I.; Ichikawa, Y.; Tashiro, S.; Sumi, T.; Sumi, M.; Nakamura, T. Apparent diffusion coefficient-based differentiation of cystic lesions of the mandible. Oral Radiol. 2012, 28, 109–114. [Google Scholar] [CrossRef]
- Smith, G.; Smith, A.J.; Browne, R.M. Glycosaminoglycans in fluid aspirates from odontogenic cysts. J. Oral Pathol. 1984, 13, 614–621. [Google Scholar] [CrossRef]
- Aragaki, T.; Michi, Y.; Katsube, K.; Uzawa, N.; Okada, N.; Akashi, T.; Amagasa, T.; Yamaguchi, A.; Sakamoto, K. Comprehensive keratin profiling reveals different histopathogenesis of keratocystic odontogenic tumor and orthokeratinized odontogenic cyst. Hum. Pathol. 2010, 41, 1718–1725. [Google Scholar] [CrossRef]
- Borghesi, A.; Nardi, C.; Giannitto, C.; Tironi, A.; Maroldi, R.; Di Bartolomeo, F.; Preda, L. Odontogenic keratocyst: Imaging features of a benign lesion with an aggressive behaviour. Insights Imaging 2018, 9, 883–897. [Google Scholar] [CrossRef]
- Sumi, M.; Ichikawa, Y.; Katayama, I.; Tashiro, S.; Nakamura, T. Diffusion-weighted MR imaging of ameloblastomas and keratocystic odontogenic tumors: Differentiation by apparent diffusion coefficients of cystic lesions. Am. J. Neuroradiol. 2008, 29, 1897–1901. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Sumi, M.; Eida, S.; Takagi, Y.; Tashiro, S.; Hotokezaka, Y.; Katayama, I.; Nakamura, T. Apparent diffusion coefficient characterization of fluid areas in cystic and abscess lesions of the neck. Oral Radiol. 2012, 28, 62–69. [Google Scholar] [CrossRef]
- Koong, B. Atlas of Oral and Maxillofacial Radiology, 1st ed.; Wiley Blackwell: Hoboken, NJ, USA, 2017; p. 354. [Google Scholar]
- Fusco, R.; Sansone, M.; Granata, V.; Grimm, R.; Pace, U.; Delrio, P.; Tatangelo, F.; Botti, G.; Avallone, A.; Pecori, B.; et al. Diffusion and perfusion MR parameters to assess preoperative short-course radiotherapy response in locally advanced rectal cancer: A comparative explorative study among Standardized Index of Shape by DCE-MRI, intravoxel incoherent motion- and diffusion kurtosis imaging-derived parameters. Abdom. Radiol. 2019, 44, 3683–3700. [Google Scholar] [CrossRef]
- Pasicz, K.; Podgórska, J.; Jasieniak, J.; Fabiszewska, E.; Skrzyński, W.; Anysz-Grodzicka, A.; Cieszanowski, A.; Kukołowicz, P.; Grabska, I. Optimal b-values for diffusion kurtosis imaging of the liver and pancreas in MR examinations. Phys. Medica 2019, 66, 119–123. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Padhani, A.R.; Chenevert, T.L.; Koh, D.; Keyzer, F.D.; Taouli, B.; Bihan, D.L. Body diffusion kurtosis imaging: Basic principles, applications, and considerations for clinical practice. J. Magn. Reson. Imaging 2015, 42, 1190–1202. [Google Scholar] [CrossRef]
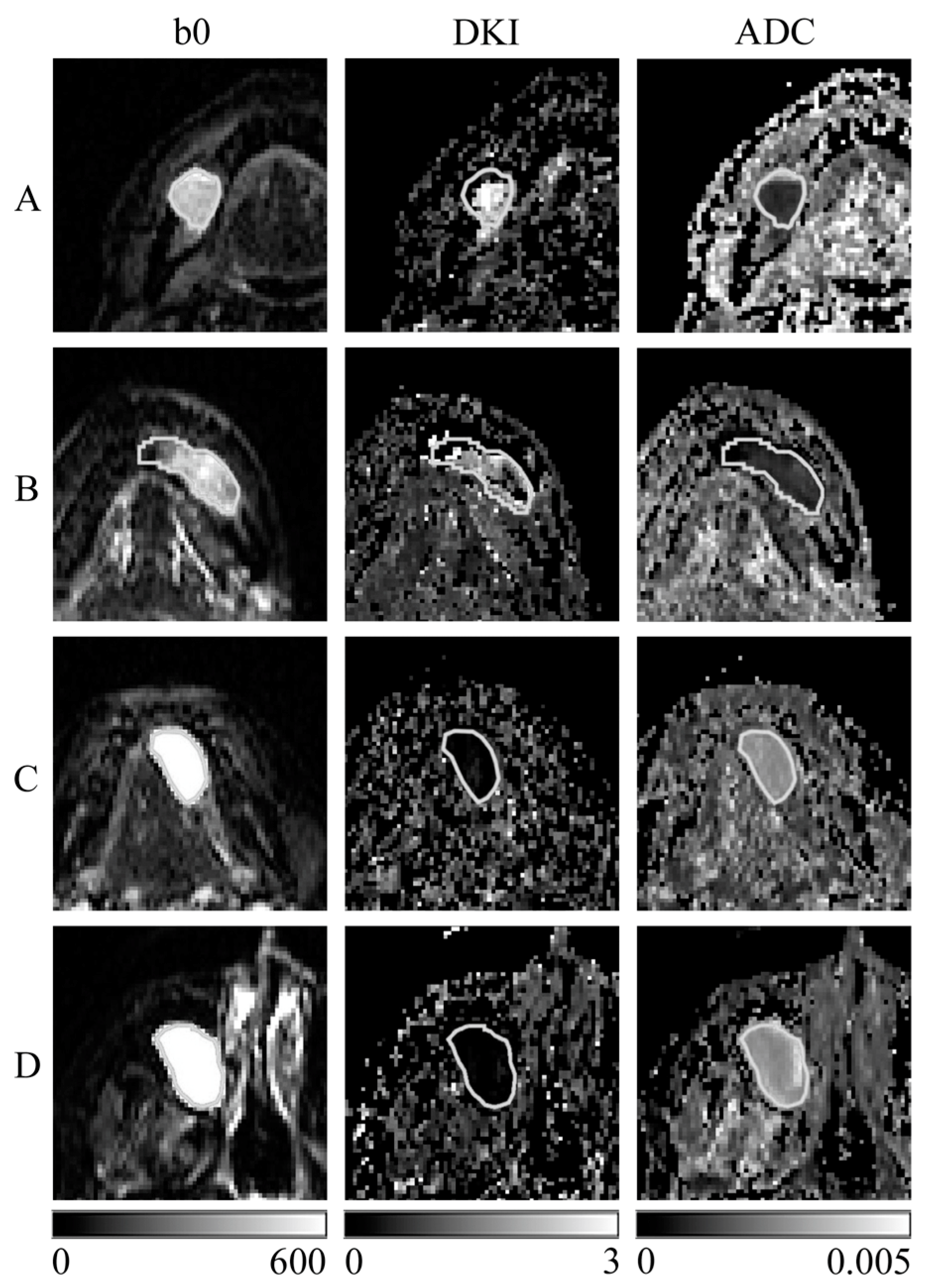
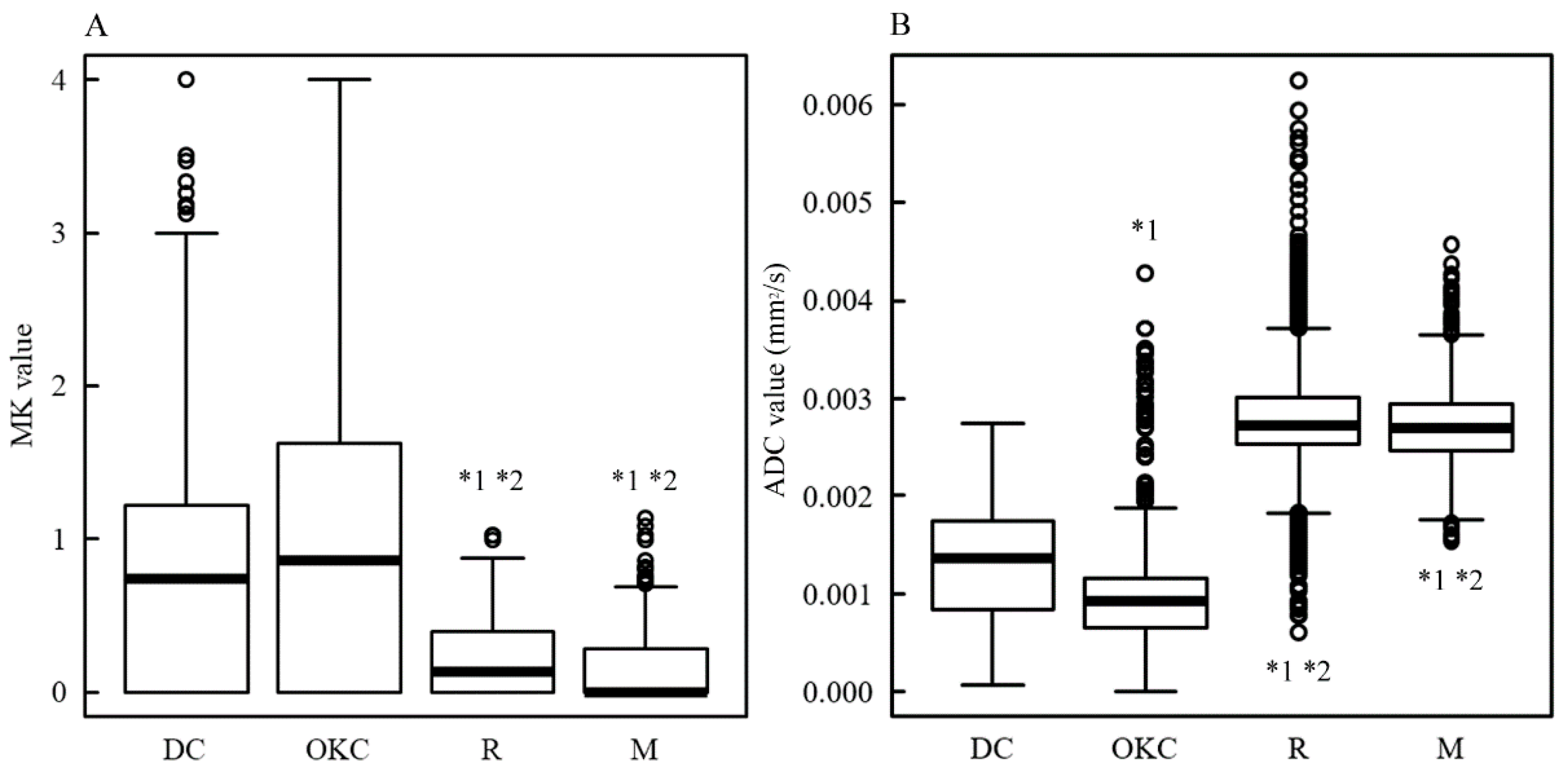

| Case | Histological Classification | Site | Pixel Number * | MK [Median (Q1, Q3)] | ADC [Median (Q1, Q3)] [×10−6 mm2/s] |
|---|---|---|---|---|---|
| 1 | Dentigerous cyst | Mandible | 95 | 1.92 (0.40, 2.53) | 781 (667, 965) |
| 2 | 22 | 0.09 (0, 0.96) | 953 (699, 1199) | ||
| 3 | 81 | 0.49 (0, 0.83) | 1633 (1373, 1886) | ||
| 4 | 138 | 0.77 (0, 1.02) | 1535 (1064, 1887) | ||
| 5 | Odontogenic keratocyst | Mandible | 136 | 0.42 (0, 0.94) | 891 (176, 1953) |
| 6 | 58 | 1.20 (0, 1.64) | 813 (724, 885) | ||
| 7 | 69 | 0 (0, 0.79) | 1049 (954, 1149) | ||
| 8 | 200 | 1.35 (0, 1.89) | 870 (656, 1051) | ||
| 9 | 94 | 1.02 (0.61, 1.36) | 1123 (843, 1356) | ||
| 10 | 50 | 0.05 (0, 2.68) | 619 (460, 1053) | ||
| 11 | Ranula | Oral floor | 32 | 0 (0, 0.50) | 1828 (1738, 2085) |
| 12 | 146 | 0.40 (0.12, 0.54) | 2009 (1611, 2412) | ||
| 13 | 54 | 0.11 (0, 0.39) | 4264 (3785, 4583) | ||
| 14 | 144 | 0.04 (0, 0.20) | 2717 (2594, 2833) | ||
| 15 | 210 | 0.05 (0, 0.29) | 2710 (2614, 2880) | ||
| 16 | Submandible | 253 | 0.13 (0, 0.47) | 2886 (2662, 3300) | |
| 17 | Mucous cyst | Maxillary sinus | 214 | 0 (0, 0.05) | 2846 (2702, 3122) |
| 18 | 45 | 0 (0, 0.28) | 2830 (2621, 3052) | ||
| 19 | 88 | 0 (0, 0.33) | 2707 (2538, 2954) | ||
| 20 | 113 | 0.05 (0, 0.29) | 2713 (2555, 2986) | ||
| 21 | 38 | 0.13 (0, 0.39) | 2031 (1911, 2145) | ||
| 22 | 20 | 0.45 (0, 0.64) | 2046 (1914, 2258) | ||
| 23 | 91 | 0.35 (0, 0.47) | 2472 (2322, 2635) | ||
| 24 | 28 | 0.05 (0, 0.35) | 2225 (2029, 2333) | ||
| 25 | Radicular cyst | Maxilla | 101 | 0 (0, 0.22) | 2437 (2318, 2575) |
| 26 | Postoperative maxillary cyst | Maxillary sinus | 40 | 0.87 (0.33, 1.23) | 1247 (1095, 1365) |
| 27 | Nasoalveolar cyst | Nasolabial groove | 53 | 0.50 (0, 0.77) | 2280 (2077, 2603) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukumura, Y.; Kuroda, M.; Yoshida, S.; Nakamura, Y.; Nakamitsu, Y.; Al-Hammad, W.E.; Kuroda, K.; Kamizaki, R.; Shimizu, Y.; Tanabe, Y.; et al. Characteristic Mean Kurtosis Values in Simple Diffusion Kurtosis Imaging of Dentigerous Cysts. Diagnostics 2023, 13, 3619. https://doi.org/10.3390/diagnostics13243619
Fukumura Y, Kuroda M, Yoshida S, Nakamura Y, Nakamitsu Y, Al-Hammad WE, Kuroda K, Kamizaki R, Shimizu Y, Tanabe Y, et al. Characteristic Mean Kurtosis Values in Simple Diffusion Kurtosis Imaging of Dentigerous Cysts. Diagnostics. 2023; 13(24):3619. https://doi.org/10.3390/diagnostics13243619
Chicago/Turabian StyleFukumura, Yuka, Masahiro Kuroda, Suzuka Yoshida, Yoshihide Nakamura, Yuki Nakamitsu, Wlla E. Al-Hammad, Kazuhiro Kuroda, Ryo Kamizaki, Yudai Shimizu, Yoshinori Tanabe, and et al. 2023. "Characteristic Mean Kurtosis Values in Simple Diffusion Kurtosis Imaging of Dentigerous Cysts" Diagnostics 13, no. 24: 3619. https://doi.org/10.3390/diagnostics13243619
APA StyleFukumura, Y., Kuroda, M., Yoshida, S., Nakamura, Y., Nakamitsu, Y., Al-Hammad, W. E., Kuroda, K., Kamizaki, R., Shimizu, Y., Tanabe, Y., Sugimoto, K., Oita, M., Sugianto, I., Barham, M., Tekiki, N., Kamaruddin, N. N., Yanagi, Y., & Asaumi, J. (2023). Characteristic Mean Kurtosis Values in Simple Diffusion Kurtosis Imaging of Dentigerous Cysts. Diagnostics, 13(24), 3619. https://doi.org/10.3390/diagnostics13243619





