Exploring Huntington’s Disease Diagnosis via Artificial Intelligence Models: A Comprehensive Review
Abstract
1. Introduction
- This paper represents the pioneering effort to comprehensively compare the effectiveness of AI powered approaches for HD diagnosis, providing a critical synthesis of their respective strengths and potential clinical applications.
- This study presents a thorough examination of the various AI powered techniques that have been applied to the diagnosis of Huntington’s disease. It includes an overview of different ML and DL methodologies and how they have been used in this context.
- This review addresses current challenges and identifies future research opportunities in the use of ML and DL for Huntington’s disease diagnosis. It aims to provide information and inspiration for aspiring researchers and enthusiasts interested in pursuing this topic.
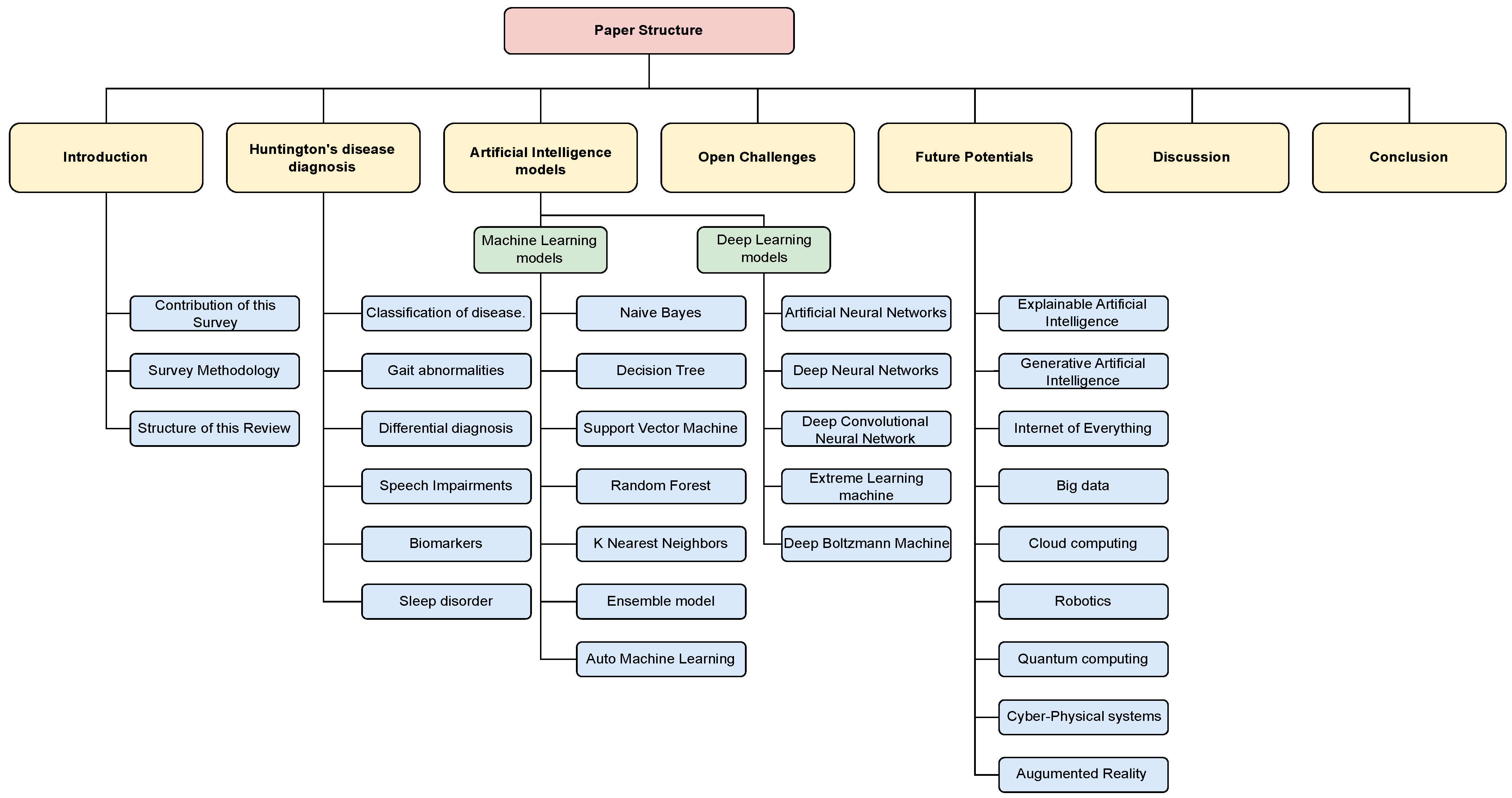
Arrangement of This Review
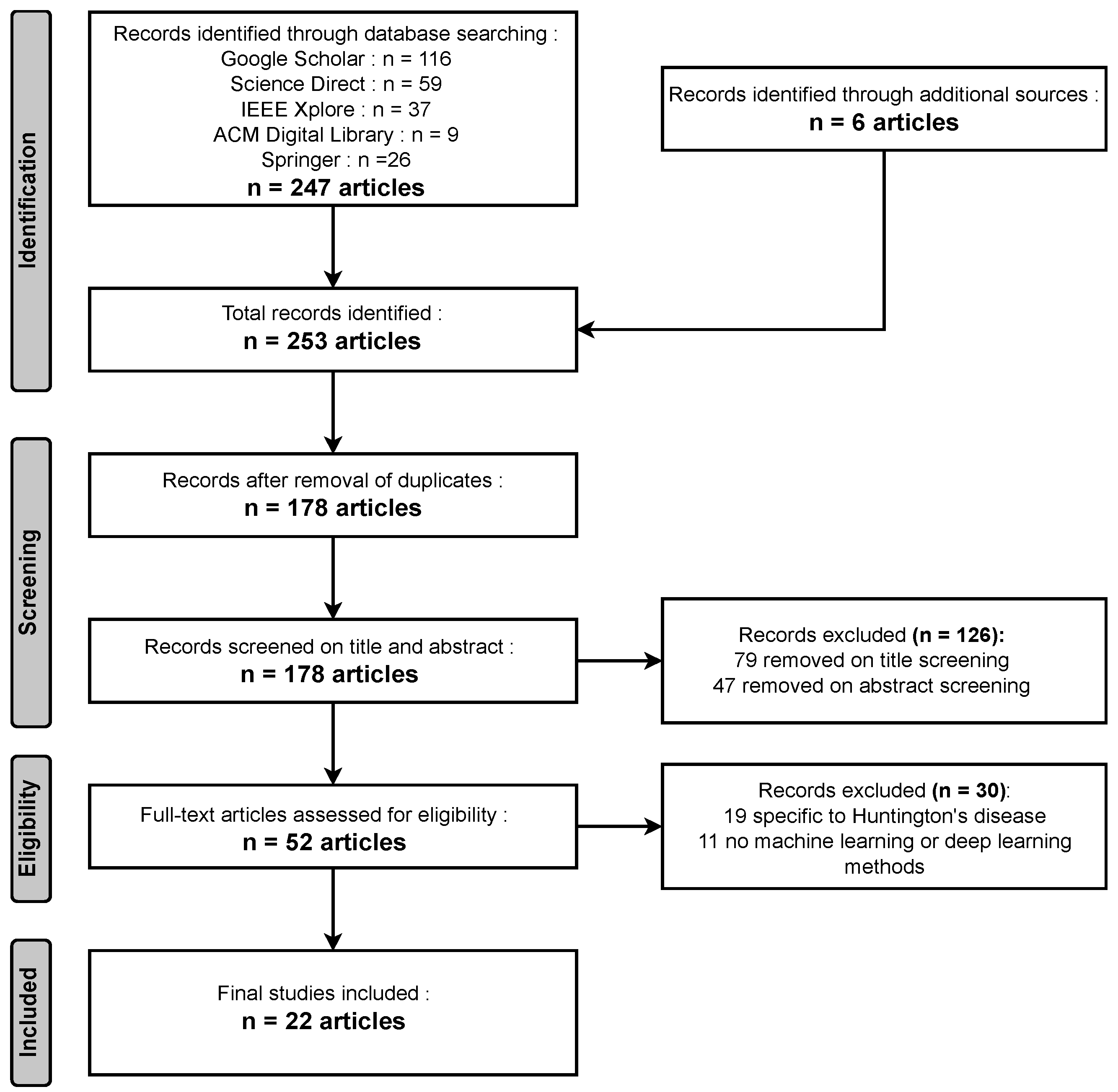
2. Survey Methodology
2.1. Search Strategy, Databases, and Screening Criteria
2.2. Quality Assessment
2.3. Results
- Identification: During the identification phase, a comprehensive search across various databases yielded 247 relevant articles, while an additional six articles were obtained from other sources, resulting in a total of 253 articles identified for this comprehensive review.
- Screening: In the screening phase, a meticulous process that included the removal of duplicates led to a selection of 178 unique articles. Subsequently, following title screening, 79 articles were excluded, and an additional 47 were eliminated after the abstract screening. In total, 126 articles were removed during the screening process, leaving a focused set of articles for further review and analysis.
- Eligibility: During the eligibility phase, a rigorous assessment revealed that 19 articles were unrelated to Huntington’s disease, and in 11 articles, no Machine Learning (ML) or Deep Learning (DL) models were utilized. Consequently, a total of 30 papers were excluded from the review, ensuring that the remaining articles are directly relevant to the exploration of Huntington’s disease diagnosis via AI models.
- Included: In the inclusion phase, after careful evaluation, a final selection of 22 articles met the criteria for inclusion in the comprehensive review.
3. Background of Huntington’s Disease Diagnosis
3.1. Classification of Huntington’s Disease
3.2. Gait Abnormalities
3.3. Differential Diagnosis
- Parkinson’s disease: This neurodegenerative disorder is characterised by tremors, rigidity, and difficulty with movement. While HD also involves movement disorders, the signs of Parkinson’s disease tend to be more symmetrical and respond well to medication, while HD symptoms are often asymmetrical and do not respond well to medication [36]. Recent advancements in gene prioritization strategies, as observed in the context of Parkinson’s disease (PD) and preeclampsia, underscore the significance of consensus strategies in unraveling the pathogenesis of neurodegenerative disorders. Studies such as [37,38] exemplify the potency of consensus strategies in prioritizing genes linked to disease etiology, akin to the endeavors witnessed in Huntington’s disease (HD) research. The study [37] proposed a consensus strategy for PD gene prioritization, merging multiple prioritization approaches, akin to ensemble models, to enhance the identification of genes relevant to PD pathogenesis. Similarly, the research [38] utilized a consensus strategy for preeclampsia, employing various prioritization strategies and bioinformatics analyses to identify crucial genes associated with the condition. These strategies, by amalgamating diverse data sources and methodologies, facilitate the identification of biologically significant genes, offering potential targets for understanding the pathogenesis of degenerative diseases such as HD and developing targeted therapeutics. Importantly, the success of such consensus strategies in identifying genes directly associated with the disease and those involved in relevant biological processes echoes the potential applicability of similar methodologies in HD research, augmenting efforts to comprehend the intricate mechanisms underlying the disease’s progression and potentially uncovering novel targets for intervention and therapy.
- Schizophrenia: This mental disorder is characterized by delusions, hallucinations, and other psychotic symptoms. While HD may also involve changes in behavior and cognition, schizophrenia typically involves more severe and persistent symptoms and does not involve movement disorders [41].
- Wilson’s disease: This rare genetic disorder results from a shortage of the enzyme copper-transporting ATPase, this results in a buildup of copper in the body. Symptoms of Wilson’s disease may include movement disorders, cognitive decline, and behavioral changes, but typically also include liver dysfunction and other symptoms that are not seen in HD [42].
- Multiple sclerosis: This autoimmune disorder is caused by harm inflicted upon the protective layer of myelin that encases nerve fibers within the central nervous system [43]. Symptoms of multiple sclerosis may include movement disorders, cognitive decline, and behavioral changes, but typically also include sensory symptoms, such as numbness and tingling, that are not seen in HD.
3.4. Speech Impairments
- Dysarthria: This refers to a difficulty in producing clear and intelligible speech and is often caused by muscle weakness or difficulty with coordination and control [45]. Patients with HD may have difficulty forming words, and may have a slurred or slushy quality to their speech.
- Aphasia: This refers to a difficulty with language and may include problems with understanding, producing, and comprehending speech. Patients with HD may have difficulty with word-finding, naming objects, or understanding complex sentences [46].
- Apraxia: This refers to a difficulty with the planning and execution of voluntary movements, including speech movements [47]. Patients with HD may have difficulty with the coordination and sequencing of speech sounds, and may have difficulty producing certain sounds or words.
3.5. Biomarkers
3.5.1. Brain Imaging
3.5.2. EEG signal
3.6. Sleep Disorders Present in Huntington’s Disease
3.6.1. Rapid Eye Movement Sleep Behaviour Disorder
- Loss of Atonia: In HD, there is a notable absence of muscular activity typically observed during the rapid eye movement (REM) sleep phase. This means that the characteristic muscle paralysis that occurs during REM, preventing individuals from acting out their dreams, is compromised. This leads to vivid and often vigorous movements or behaviors during this phase of sleep.
- Complex Motor Behaviors: Individuals with HD may exhibit a range of complex motor behaviors during REM sleep. These can include purposeful movements, such as reaching, grasping, or even more dramatic actions such as punching or kicking. These behaviors can be disruptive and may lead harm to the individual or their sleeping companion.
- Frequency and Intensity: The frequency and intensity of REM sleep behavior disorder (RBD) in HD can vary among individuals. Some may experience sporadic episodes, while others may have more frequent and intense behaviors. Factors such as the stage of HD progression and individual differences in sleep patterns may contribute to this variation.
- Dream Enactment: RBD in HD often involves vivid and often violent dream enactment behaviors. These behaviors are typically related to the content of the dream, suggesting a failure in the normal inhibitory mechanisms that prevent motor activity during REM sleep.
- Potential Prodromal Sign: Emerging research suggests that RBD might occur before the appearance of motor symptoms in Huntington’s disease. This has led to speculation that RBD could serve as a potential prodromal sign or early marker of the disease. Monitoring REM sleep behaviors in individuals at risk for HD could provide valuable insights into disease progression.
3.6.2. Restless Legs Syndrome and Periodic Limb Movement
- Unpleasant Sensations: Individuals may experience uncomfortable sensations in their legs, described as tingling, crawling, or aching. These sensations are often accompanied by an uncontrollable desire to shift or reposition the legs or other limbs, especially when at rest.
- Worsening in the Evening/Night: Symptoms tend to worsen in the evening and at night, disrupting the ability to fall asleep and maintain restful sleep.
- Motor Restlessness: This includes both voluntary and involuntary movements of the lower limbs, which can extend to other parts of the body. These movements can be rhythmic and repetitive in nature.
- Periodic Movements: In addition to the continuous urge to move, individuals may experience periodic, involuntary limb movements during sleep. These movements often happen at consistent time intervals, typically occurring approximately every 20 to 40 s.
- Impact on Sleep Quality: Both RLS and PLMD can severely disrupt sleep, leading to insomnia, daytime fatigue, and impaired cognitive functioning.
- Bed Partner Awareness: In the case of PLMD, the affected individual is often unaware of the limb movements during sleep. It is usually a bed partner or a sleep study that observes these movements.
- Daytime Consequences: Both conditions can lead to daytime sleepiness and decreased the standard of life resulting from disrupted sleep architecture.
4. Exploring HD Disease Diagnosis via AI-Powered Models
4.1. Preamble—Diagnosis of Huntington’s Disease via AI-Powered Models
4.2. Machine Learning Techniques
4.2.1. Naive Bayes
4.2.2. Decision Tree
4.2.3. Support Vector Machine
4.2.4. Random Forest
4.2.5. K-Nearest Neighbours
4.2.6. Ensemble Models
4.2.7. Automatic Machine Learning
4.2.8. Summary of Machine Learning Models
4.3. Deep Learning Techniques
4.3.1. Artificial Neural Network
4.3.2. Deep Neural Network
4.3.3. Deep Convolutional Neural Network
4.3.4. Extreme Learning Machine
4.3.5. Deep Boltzmann Machine
4.3.6. Summary of Deep Learning Models
5. Open Challenges
5.1. Setbacks in Computational Machine Learning Approaches
- Data Availability and Quality: One of the primary challenges in developing ML models for HD diagnosis is the availability of high-quality data. Gathering comprehensive datasets that include genetic, clinical, and imaging data from a diverse range of HD patients is essential. Ensuring the accuracy and completeness of these data is crucial for model training and validation.
- Data Imbalance: HD is a rare genetic disorder and datasets for rare diseases that often suffer from class imbalance, with a limited number of positive cases (HD patients) compared to negative cases (healthy individuals). Datasets with imbalances can result in model bias, where the algorithm demonstrates strong performance in the majority class but struggles when dealing with the minority class. Addressing this imbalance is critical for accurate diagnosis.
- Feature Selection: HD is a complex disorder with multifaceted clinical manifestations. Selecting the most informative features from various data sources (genetic, clinical, imaging) and determining their relevance to the disease diagnosis is a challenge. ML models need to incorporate relevant features while reducing dimensionality and noise.
- Interpretable Models: Numerous machine learning algorithms, including deep learning models, are often referred to as “opaque” models, posing difficulties in deciphering the rationale behind their decisions. In the medical field, interpretability is crucial for understanding why a particular diagnosis or prediction was made. Developing interpretable models that provide insights into the disease process is essential.
- Ethical Concerns and Privacy: Handling patient data in healthcare applications raises ethical and privacy concerns. Ensuring the security and privacy of sensitive health information while allowing for meaningful analysis is a delicate balance that must be addressed when developing ML models for HD diagnosis. Techniques such as federated learning, as highlighted in the work [95], have emerged as crucial tools in preserving privacy while enabling the collaborative analysis of medical data.
- Generalization and Validation: ML models must generalize well to new, unseen data. Proper cross-validation techniques and external validation on diverse datasets are essential to ensure that the models’ performance remains consistent across different populations and settings.
- Early Detection and Biomarker Discovery: Identifying reliable biomarkers for early HD detection is a significant challenge. ML models can assist in this process, but the discovery of biomarkers that can accurately predict disease onset or progression is an ongoing research area.
- Clinical Integration: Transitioning ML models from research settings to clinical practice requires collaboration with healthcare professionals and regulatory bodies. Models need to be validated in clinical trials and integrated into existing healthcare systems, which can be a lengthy and complex process.
- Longitudinal Data: HD is a progressive disease that evolves over time. Obtaining and analyzing longitudinal data is essential for tracking disease progression and treatment response accurately. ML models must be capable of handling longitudinal data effectively.
- Validation with Small Cohorts: Due to the rarity of HD, it can be challenging to validate ML models with large cohorts of patients. Small sample sizes can lead to overfitting and may not capture the full spectrum of disease variability.
5.2. Setbacks in Computational Deep Learning Approaches
- Limited HD-Specific Data: While data availability is a general challenge, obtaining a sufficiently large and diverse dataset specifically for HD is particularly challenging due to the rarity of the disease. HD-specific data, including genetic profiles, clinical records, and imaging data, may be limited in comparison to other more common medical conditions.
- Complex Disease Progression: HD is a neurodegenerative disease with a highly complex and nonlinear progression pattern. Capturing this complexity in DL models, especially for early-stage diagnosis and monitoring, is challenging. DL models must account for the multi-modal nature of HD progression, including motor, cognitive, and psychiatric symptoms.
- Feature Extraction: DL models often rely on automatic feature extraction from raw data. However, extracting meaningful features from complex data sources such as brain imaging (e.g., MRI, fMRI) and genetic data can be challenging. Developing effective feature extraction methods tailored to HD-specific data is essential.
- Heterogeneity of HD: HD exhibits significant variability in symptom onset, progression, and severity among individuals. DL models need to account for this heterogeneity and provide personalized predictions and treatment recommendations, which can be challenging in clinical practice.
- Integration of Multiple Data Sources: Effective HD diagnosis and prognosis may require integrating information from various sources, such as genetic, imaging, and clinical data. Developing DL models that can seamlessly integrate heterogeneous data and extract meaningful insights is a complex task.
- Model Explainability: While interpretability is a general challenge in ML and DL, it is particularly critical in healthcare applications. DL models for HD diagnosis need to provide clear explanations for their predictions, helping clinicians to understand the rationale behind a diagnosis or prognosis.
- Scalability: Training large DL models for healthcare applications often requires substantial computational resources. Ensuring that the models are scalable and can be deployed in resource-constrained clinical settings is a challenge.
- Clinical Adoption: Even with accurate DL models, achieving widespread clinical adoption can be challenging. Overcoming barriers related to regulatory approvals, integration with electronic health records (EHRs), and gaining the trust of healthcare professionals is crucial for the successful deployment of DL-based diagnostic tools.
- Patient Data Privacy: Protecting patient data privacy is a paramount concern. Ensuring that DL models comply with data protection regulations and securely handle sensitive patient information poses a challenge in healthcare applications.
5.3. Issues in Data Integration of Huntington’s Disease Diagnosis
5.4. Obstacles in the Realm of Precision Medicine and the Quest for Tailored Therapies
5.5. Data Isolation Challenges
5.6. Data Management Challenges
5.7. Data Sparseness Challenges
6. Future Potentials
6.1. Explainable Artificial Intelligence
6.2. Generative Artificial Intelligence
- Data Generation and Augmentation: Generative AI can be used to generate synthetic medical images or data that closely resemble real patient data [104]. This could prove beneficial in training machine learning models in situations where actual patient data is scarce or in cases where privacy issues are a concern. Augmenting the dataset with generated data can improve the performance of diagnostic models.
- Image Enhancement: Generative models can be used to enhance the quality of medical images, making it easier for healthcare professionals to identify subtle signs of HD in brain scans or other medical imaging data [105]. Enhanced images can provide better insights and aid in more accurate diagnoses.
- Early Detection: Generative AI can assist in the early detection of HD by analyzing patterns in medical data over time. Longitudinal data from patients can be used to create generative models that predict the progression of the disease, allowing for early intervention and personalized treatment plans.
- Predictive Biomarkers: Generative models can identify predictive biomarkers associated with Huntington’s disease. Through the examination of extensive patient data encompassing genetic profiles, medical records, and clinical evaluations, these models can unveil nuanced relationships that might remain concealed when employing conventional statistical methods.
- Disease Progression Modeling: Generative AI can create models that simulate the progression of HD in virtual patients. This can be useful for understanding how the disease develops over time and for testing the efficacy of potential treatments in silico before clinical trials.
- Support for Clinicians: Generative AI can assist clinicians in making more informed decisions by providing additional insights into patient data. For example, it can generate visualizations that highlight regions of interest in medical images or generate reports, summarizing key findings from patient records.
- Personalized Treatment Plans: Leveraging data from a substantial group of individuals afflicted with HD, generative models have the potential to facilitate the development of personalized therapeutic strategies. These plans can take into account an individual’s genetic profile, disease progression, and response to previous treatments to optimize therapeutic strategies.
- Drug Discovery: Generative AI can be applied to discover potential drug candidates for Huntington’s disease by generating molecular structures that may interact with specific disease-related targets. This can accelerate the drug development process.
6.3. Internet of Everything
- Remote Monitoring: IoE allows for continuous remote monitoring of patient’s vital signs and movements. Wearable devices, such as fitness trackers and smartwatches, can collect data on heart rate, sleep patterns, and physical activity [107]. In the case of HD, changes in motor function and sleep disturbances can be early indicators of disease progression. By continuously monitoring these metrics, healthcare providers can detect changes sooner and intervene proactively.
- Genomic Data Sharing: The presence of a particular genetic mutation is responsible for HD, necessitating genetic testing as a common diagnostic procedure [108]. IoE can facilitate the sharing of genomic data securely between patients, clinicians, and researchers. Utilizing these data allows for the identification of individuals who may be susceptible to Huntington’s disease and enables the monitoring of the progression of the condition. Privacy and security measures must be robust to protect sensitive genetic information.
- Telemedicine and Consultations: IoE enables telemedicine and remote consultations, making it easier for patients with HD to access specialized care. The utilization of video conferencing and remote monitoring can diminish the necessity for regular face-to-face appointments, especially benefiting individuals residing in distant or underserved regions.
- Data Analytics and Predictive Modeling: IoE allows for the collection of vast amounts of patient data. Advanced data analytics and machine learning algorithms can process this information to identify patterns and trends associated with HD. Predictive modeling can help anticipate disease progression and develop personalized treatment plans.
- Medication Management: For individuals with HD, managing medications is crucial. IoE can support medication adherence through smart pill dispensers and reminders [109]. These devices can notify patients when it is time to take their medication and send alerts to caregivers or healthcare providers if doses are missed.
- Support Communities: IoE can connect HD patients with support communities and resources. Online forums, social networks, and communication tools can help patients and caregivers connect, share experiences, and access valuable information and emotional support.
- Clinical Trials and Research: IoE can facilitate the recruitment and monitoring of participants in clinical trials for potential HD treatments. Real-time data collection can provide researchers with valuable insights into treatment efficacy and safety.
6.4. Big Data
6.5. Cloud, Edge, and Fog Computing
6.5.1. Cloud Computing
- Data Storage and Centralization: Cloud computing offers vast storage capabilities, making it ideal for storing large datasets such as genetic information, medical records, and imaging data related to Huntington’s disease patients [113]. Researchers and healthcare providers can securely store and access these data from anywhere with an internet connection.
- Data Analysis: Cloud platforms offer the computational muscle essential for intricate data analysis, including genetic sequencing, medical imaging analysis, and machine learning algorithms for early HD diagnosis. Researchers can run resource-intensive computations on the cloud, accelerating the development of diagnostic tools and treatment options.
- Collaboration: Cloud-based platforms facilitate collaboration among researchers and clinicians from different locations, allowing them to share data, insights, and best practices in diagnosing and managing HD. This collaborative approach can lead to faster advancements in HD research and treatment.
6.5.2. Edge Computing
- Real-time Data Processing: Edge computing brings computation closer to the data source, making it ideal for real-time analysis of patient data, including wearable device data, continuous monitoring, and sensor data [114]. In the context of HD, edge devices can process and analyze patient data on the spot, providing immediate feedback to both patients and healthcare providers.
- Data Privacy and Security: The utilization of edge computing can fortify data privacy and security by maintaining sensitive patient data in proximity to its origin. This approach mitigates the potential for data breaches, preserving the confidentiality of patient information, a pivotal concern in healthcare environments.
- Reduced Latency: For applications such as telemedicine or remote monitoring of HD patients, edge computing reduces data transmission latency, enabling quicker response times and ensuring that critical information reaches healthcare professionals promptly.
6.5.3. Fog Computing
- Distributed Processing: Fog computing extends the capabilities of edge computing by enabling distributed data processing and analysis [115]. In HD diagnosis, fog computing can distribute processing tasks across multiple edge devices, optimizing computational resources for complex tasks.
- Resilience: Fog computing provides redundancy and resilience in data processing, ensuring that critical diagnostic processes continue to operate even in the event of a device failure [116]. This reliability is essential for continuous monitoring and early detection of HD symptoms.
- Scalability: Fog computing possesses inherent scalability, readily expanding its capacity to cater to a rising population of HD patients and an expanding array of devices. With the proliferation of patients and data sources, fog computing exhibits the adaptability required to adeptly and effectively handle the augmented workload.
6.6. Robots and Machine Co-Creativity
6.7. Quantum Computing
- Efficient Genetic Analysis: Quantum computers have the capability to process vast amounts of genetic data much faster than classical computers. This becomes particularly pertinent when dealing with HD, where the focus lies on scrutinizing the patient’s DNA to pinpoint the distinct genetic mutation responsible for this condition. Quantum computers can accelerate the process of genetic sequencing and analysis, potentially leading to quicker and more accurate diagnoses.
- Simulating Protein Structures: Understanding the molecular basis of diseases such as Huntington’s relies on simulating complex protein structures. Quantum computers can simulate these structures with far greater precision and speed than classical computers. This potential can assist scientists in uncovering the underlying processes of the condition, potentially pinpointing areas for therapeutic intervention.
- Drug Discovery: Quantum computing can expedite drug discovery by simulating the interactions between potential drug compounds and the target proteins involved in HD. This has the potential to substantially decrease the resources and time required for the development of novel therapies or the discovery of already-available drugs suitable for repurposing in treatment.
- Personalized Medicine: Quantum computing can enhance the personalization of treatment plans. By analyzing a patient’s genetic data alongside other clinical parameters, quantum computers can help healthcare providers tailor treatment strategies specifically to each patient’s unique genetic makeup, potentially leading to more effective treatments and better outcomes.
- Data Security: As quantum computing advances, so does the need for improved data security. Given the sensitivity of genetic and medical data, quantum-resistant encryption methods will become crucial to protect patients’ privacy and the integrity of healthcare systems [120].
- Machine Learning and Pattern Recognition: Quantum computing can be harnessed to enhance machine learning algorithms [121].Enhancing disease diagnosis precision can be achieved by scrutinizing a wider spectrum of patient information, encompassing medical imaging, patient records, and genetic data. This comprehensive approach aims to detect Huntington’s disease-related patterns and markers effectively.
6.8. Cyber-Physical Systems
- Remote Monitoring: CPS can enable the remote monitoring of individuals at risk of or already diagnosed with Huntington’s disease. Wearable devices equipped with sensors can continuously collect data on motor function, gait, and other relevant parameters. These data can be transmitted in real-time to healthcare professionals, allowing for early detection of symptoms and timely intervention.
- Data Analytics and Machine Learning: The collected data can be processed using advanced analytics and machine learning algorithms to identify subtle changes in motor skills and behavior associated with HD. These algorithms can analyze patterns over time and provide insights into disease progression, potentially leading to earlier diagnosis and personalized treatment plans.
- Telemedicine and Telehealth: CPS can facilitate telemedicine consultations for individuals with limited access to specialized healthcare facilities [123]. Remote consultations can help healthcare providers assess patients’ symptoms, track their progress, and make treatment adjustments as needed.
- Medication Adherence: CPS can remind patients to take their medications and track adherence. This is especially important for individuals with HD, as medication management can be complex, and missing doses can impact symptom management.
- Fall Detection and Safety Monitoring: Individuals with HD are at an increased risk of falls due to motor impairments. CPS can incorporate fall detection systems and alert caregivers or emergency services when a fall occurs. Additionally, environmental sensors can be used to monitor home safety and detect hazards.
- Genetic Testing and Predictive Modeling: CPS can integrate genetic testing data to identify individuals at risk of developing HD based on their genetic profile. Predictive modeling can estimate the likelihood and age of onset, allowing for early interventions and lifestyle modifications.
- Patient Support and Education: CPS can provide patients and their families with educational resources, support groups, and communication tools to enhance their understanding of HD and improve their quality of life.
- Research and Data Sharing: The integration of Cyber-Physical Systems (CPS) has the potential to enhance data exchange between researchers and healthcare institutions, fostering improved insights into HD and expediting advancements in treatments and therapies.
6.9. Augmented Reality (AR), Mixed Reality (MR) and Virtual Reality (VR)
6.9.1. Augmented Reality (AR)
- Diagnostic Support: AR can assist healthcare professionals during the diagnostic process by overlaying relevant medical data, such as genetic information or diagnostic criteria, onto a patient’s medical record or real-time examination. This can help doctors make more accurate and timely diagnoses [124].
- Guided Procedures: During medical procedures such as deep brain stimulation (DBS) surgery, AR can provide surgeons with real-time guidance and data visualization. This can improve the precision and safety of surgical interventions for individuals with HD.
- Therapeutic Support: AR apps and wearables can provide individuals with HD and their caregivers with real-time information and reminders related to medication schedules, therapy exercises, and symptom management strategies.
6.9.2. Mixed Reality (MR)
- Simulated Environments for Assessment: MR can be used to create simulated environments that mimic real-life situations [125]. This can aid in the assessment of a patient’s functional capabilities and how HD impacts their daily life. Clinicians can use MR to understand a patient’s challenges better and tailor treatment plans accordingly.
- Neuroimaging Visualization: MR can enhance the visualization of neuroimaging data, such as MRI or CT scans. By overlaying these images onto a patient’s physical body, doctors can get a clearer understanding of the brain structures affected by HD. This aids in precise diagnosis and treatment planning.
6.9.3. Virtual Reality (VR)
- Patient Education and Empowerment: VR can be used to create immersive educational experiences for patients and their families, helping them understand the complexities of HD, its symptoms, and its progression [126]. This can promote better self-management and informed decision-making.
- Rehabilitation: VR-based rehabilitation programs can help individuals with HD improve their motor skills, coordination, and cognitive functions. Personalized virtual reality activities and games can be customized to cater to individual patient requirements, enhancing therapy engagement and efficacy.
- Telemedicine and Remote Consultations: VR can facilitate remote consultations with specialists, enabling individuals with HD to access expert care without the need for extensive travel. This is particularly valuable for patients in remote or underserved areas.
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhachawat, S.; Shriram, E.; Srinivasan, K.; Hu, Y.C. Leveraging Computational Intelligence Techniques for Diagnosing Degenerative Nerve Diseases: A Comprehensive Review, Open Challenges, and Future Research Directions. Diagnostics 2023, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef] [PubMed]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Tabrizi, S.J. Clinical features of Huntington’s disease. In Polyglutamine Disorders; Springer: Cham, Switzerland, 2018; pp. 1–28. [Google Scholar]
- Mahendran, N.; PM, D.R.V. Deep belief network-based approach for detecting Alzheimer’s disease using the multi-omics data. Comput. Struct. Biotechnol. J. 2023, 21, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, N.; PM, D.R.V. A deep learning framework with an embedded-based feature selection approach for the early detection of the Alzheimer’s disease. Comput. Biol. Med. 2022, 141, 105056. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, N.; Vincent, P.D.R.; Srinivasan, K.; Chang, C.Y. Improving the classification of alzheimer’s disease using hybrid gene selection pipeline and deep learning. Front. Genet. 2021, 12, 784814. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Bohre, K.; Singh, Y.; Himeur, Y.; Mansoor, W.; Atalla, S.; Srinivasan, K. A Comprehensive review on AI-enabled models for Parkinson’s disease diagnosis. Electronics 2023, 12, 783. [Google Scholar] [CrossRef]
- Medina, A.; Mahjoub, Y.; Shaver, L.; Pringsheim, T. Prevalence and Incidence of Huntington’s Disease: An Updated Systematic Review and Meta-Analysis. Mov. Disord. 2022, 37, 2327–2335. [Google Scholar] [CrossRef]
- Rusz, J.; Tykalova, T. Reader response: Motor speech patterns in Huntington disease. Neurology 2020, 95, 607–608. [Google Scholar] [CrossRef]
- Kouba, T.; Frank, W.; Tykalova, T.; Mühlbäck, A.; Klempíř, J.; Lindenberg, K.S.; Landwehrmeyer, G.B.; Rusz, J. Speech biomarkers in Huntington’s disease: A cross-sectional study in pre-symptomatic, prodromal and early manifest stages. Eur. J. Neurol. 2023, 30, 1262–1271. [Google Scholar] [CrossRef]
- Schultz, J.L.; Neema, M.; Nopoulos, P.C. Unravelling the role of huntingtin: From neurodevelopment to neurodegeneration. Brain 2023, 146, 4408–4410. [Google Scholar] [CrossRef] [PubMed]
- Wilton, D.K.; Mastro, K.; Heller, M.D.; Gergits, F.W.; Willing, C.R.; Fahey, J.B.; Frouin, A.; Daggett, A.; Gu, X.; Kim, Y.A.; et al. Microglia and complement mediate early corticostriatal synapse loss and cognitive dysfunction in Huntington’s disease. Nat. Med. 2023, 29, 2866–2884. [Google Scholar] [CrossRef] [PubMed]
- Innate immune mechanisms mediate loss of corticostriatal synapses in Huntington’s disease. Nat. Med. 2023, 29, 2718–2719. [CrossRef] [PubMed]
- Kiani, L. Insights into the toxic effects of mutant huntingtin. Nat. Rev. Neurol. 2023, 19, 576. [Google Scholar] [CrossRef] [PubMed]
- Karpov, P.; Godin, G.; Tetko, I.V. Transformer-CNN: Swiss knife for QSAR modeling and interpretation. J. Cheminform. 2020, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.J.; Braga, R.C.; Alves, V.M.; Lima, M.N.; Cassiano, G.C.; Muratov, E.N.; Costa, F.T.; Andrade, C.H. Deep Learning-driven research for drug discovery: Tackling Malaria. PLoS Comput. Biol. 2020, 16, e1007025. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Singh, K.; Kumar, S.; Kim, Y.S.; Lee, Y.M.; Kim, J.J. Therapeutic advances for Huntington’s disease. Brain Sci. 2020, 10, 43. [Google Scholar] [CrossRef]
- Stoker, T.B.; Mason, S.L.; Greenland, J.C.; Holden, S.T.; Santini, H.; Barker, R.A. Huntington’s disease: Diagnosis and management. Pract. Neurol. 2022, 22, 32–41. [Google Scholar] [CrossRef]
- Ross, C.A.; Hayden, M.R. Huntington’s disease. In Analysis of Triplet Repeat Disorders; Garland Science: New York, NY, USA, 2020; pp. 169–208. [Google Scholar]
- Mason, S.L.; Daws, R.E.; Soreq, E.; Johnson, E.B.; Scahill, R.I.; Tabrizi, S.J.; Barker, R.A.; Hampshire, A. Predicting clinical diagnosis in Huntington’s disease: An imaging polymarker. Ann. Neurol. 2018, 83, 532–543. [Google Scholar] [CrossRef]
- Niemela, V.; Landtblom, A.M.; Nyholm, D.; Kneider, M.; Constantinescu, R.; Paucar, M.; Svenningsson, P.; Abujrais, S.; Burman, J.; Shevchenko, G.; et al. Proenkephalin decreases in cerebrospinal fluid with symptom progression of Huntington’s disease. Mov. Disord. 2021, 36, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Baskota, S.U.; Lopez, O.L.; Greenamyre, J.T.; Kofler, J. Spectrum of tau pathologies in Huntington’s disease. Lab. Investig. 2019, 99, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Foroud, T.; Gray, J.; Ivashina, J.; Conneally, P.M. Differences in duration of Huntington’s disease based on age at onset. J. Neurol. Neurosurg. Psychiatry 1999, 66, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Smith-Geater, C.; Hernandez, S.J.; Lim, R.G.; Adam, M.; Wu, J.; Stocksdale, J.T.; Wassie, B.T.; Gold, M.P.; Wang, K.Q.; Miramontes, R.; et al. Aberrant development corrected in adult-onset Huntington’s disease iPSC-derived neuronal cultures via WNT signaling modulation. Stem Cell Rep. 2020, 14, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Bakels, H.S.; Roos, R.A.; van Roon-Mom, W.M.; de Bot, S.T. Juvenile-onset Huntington disease pathophysiology and neurodevelopment: A review. Mov. Disord. 2022, 37, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Cronin, T.; Rosser, A.; Massey, T. Clinical presentation and features of juvenile-onset Huntington’s disease: A systematic review. J. Huntingt. Dis. 2019, 8, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Koller, W.C.; Trimble, J. The gait abnormality of Huntington’s disease. Neurology 1985, 35, 1450. [Google Scholar] [CrossRef]
- Talman, L.S.; Hiller, A.L. Approach to posture and gait in Huntington’s disease. Front. Bioeng. Biotechnol. 2021, 9, 668699. [Google Scholar] [CrossRef]
- Gaßner, H.; Jensen, D.; Marxreiter, F.; Kletsch, A.; Bohlen, S.; Schubert, R.; Muratori, L.M.; Eskofier, B.; Klucken, J.; Winkler, J.; et al. Gait variability as digital biomarker of disease severity in Huntington’s disease. J. Neurol. 2020, 267, 1594–1601. [Google Scholar] [CrossRef]
- Saljuqi, M.; Ghaderyan, P. A novel method based on matching pursuit decomposition of gait signals for Parkinson’s disease, Amyotrophic lateral sclerosis and Huntington’s disease detection. Neurosci. Lett. 2021, 761, 136107. [Google Scholar] [CrossRef]
- Bologna, M.; Paparella, G.; Fasano, A.; Hallett, M.; Berardelli, A. Evolving concepts on bradykinesia. Brain 2020, 143, 727–750. [Google Scholar] [CrossRef] [PubMed]
- Reilmann, R. Parkinsonism in Huntington’s disease. Int. Rev. Neurobiol. 2019, 149, 299–306. [Google Scholar] [PubMed]
- Martino, D.; Stamelou, M.; Bhatia, K.P. The differential diagnosis of Huntington’s disease-like syndromes:‘red flags’ for the clinician. J. Neurol. Neurosurg. Psychiatry 2013, 84, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Monteagudo, M.; Borges, F.; Paz-y Miño, C.; Cordeiro, M.N.D.; Rebelo, I.; Perez-Castillo, Y.; Helguera, A.M.; Sánchez-Rodríguez, A.; Tejera, E. Efficient and biologically relevant consensus strategy for Parkinson’s disease gene prioritization. BMC Med. Genom. 2016, 9, 12. [Google Scholar] [CrossRef]
- Tejera, E.; Cruz-Monteagudo, M.; Burgos, G.; Sánchez, M.E.; Sánchez-Rodríguez, A.; Pérez-Castillo, Y.; Borges, F.; Cordeiro, M.N.D.S.; Paz-y Miño, C.; Rebelo, I. Consensus strategy in genes prioritization and combined bioinformatics analysis for preeclampsia pathogenesis. BMC Med. Genom. 2017, 10, 50. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and management of dementia. JMAM 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Chen, J.H.; Lin, K.P.; Chen, Y.C. Risk factors for dementia. J. Formos. Med. Assoc. 2009, 108, 754–764. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Marques, T.R.; Howes, O.D. Schizophrenia—An overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef]
- Poujois, A.; Woimant, F. Wilson’s disease: A 2017 update. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 512–520. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.K.; Mefferd, A.S.; Lin, Y.C.; Sellers, J.; McDonell, K.E.; de Riesthal, M.; Claassen, D.O. Motor speech patterns in Huntington disease. Neurology 2019, 93, e2042–e2052. [Google Scholar] [CrossRef] [PubMed]
- Enderby, P. Disorders of communication: Dysarthria. Handb. Clin. Neurol. 2013, 110, 273–281. [Google Scholar] [PubMed]
- Fridriksson, J.; den Ouden, D.B.; Hillis, A.E.; Hickok, G.; Rorden, C.; Basilakos, A.; Yourganov, G.; Bonilha, L. Anatomy of aphasia revisited. Brain 2018, 141, 848–862. [Google Scholar] [CrossRef] [PubMed]
- De Renzi, E.; Faglioni, P. Apraxia. In Handbook of Clinical and Experimental Neuropsychology; Psychology Press: London, UK, 2020; pp. 421–440. [Google Scholar]
- Weir, D.W.; Sturrock, A.; Leavitt, B.R. Development of biomarkers for Huntington’s disease. Lancet Neurol. 2011, 10, 573–590. [Google Scholar] [CrossRef]
- Zeun, P.; Scahill, R.I.; Tabrizi, S.J.; Wild, E.J. Fluid and imaging biomarkers for Huntington’s disease. Mol. Cell. Neurosci. 2019, 97, 67–80. [Google Scholar] [CrossRef]
- Przybyl, L.; Wozna-Wysocka, M.; Kozlowska, E.; Fiszer, A. What, when and how to measure—peripheral biomarkers in therapy of Huntington’s disease. Int. J. Mol. Sci. 2021, 22, 1561. [Google Scholar] [CrossRef]
- Georgiou-Karistianis, N.; Hannan, A.J.; Egan, G.F. Magnetic resonance imaging as an approach towards identifying neuropathological biomarkers for Huntington’s disease. Brain Res. Rev. 2008, 58, 209–225. [Google Scholar]
- Kinnunen, K.M.; Schwarz, A.J.; Turner, E.C.; Pustina, D.; Gantman, E.C.; Gordon, M.F.; Joules, R.; Mullin, A.P.; Scahill, R.I.; Georgiou-Karistianis, N.; et al. Volumetric MRI-based biomarkers in Huntington’s disease: An evidentiary review. Front. Neurol. 2021, 12, 712555. [Google Scholar] [CrossRef]
- Killoran, A.; Biglan, K. Biomarkers for Huntington’s disease: A brief overview. J. Rare Dis. Res. Treat. 2016, 1, 46–50. [Google Scholar] [CrossRef][Green Version]
- Silajdžić, E.; Björkqvist, M. A critical evaluation of wet biomarkers for Huntington’s disease: Current status and ways forward. J. Huntingt. Dis. 2018, 7, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.B.; Gregory, S. Huntington’s disease: Brain imaging in Huntington’s disease. Prog. Mol. Biol. Transl. Sci. 2019, 165, 321–369. [Google Scholar] [PubMed]
- Pini, L.; Jacquemot, C.; Cagnin, A.; Meneghello, F.; Semenza, C.; Mantini, D.; Vallesi, A. Aberrant brain network connectivity in presymptomatic and manifest Huntington’s disease: A systematic review. Hum. Brain Mapp. 2020, 41, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Horta, S.; Sampedro, F.; Horta-Barba, A.; Perez-Perez, J.; Pagonabarraga, J.; Gomez-Anson, B.; Kulisevsky, J. Structural brain correlates of irritability and aggression in early manifest Huntington’s disease. Brain Imaging Behav. 2021, 15, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Fraga, C.; Scahill, R.; Rees, G.; Tabrizi, S.J.; Gregory, S. Diffusion imaging in Huntington’s disease: Comprehensive review. J. Neurol. Neurosurg. Psychiatry 2021, 92, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Odish, O.F.; Johnsen, K.; van Someren, P.; Roos, R.A.; van Dijk, J.G. EEG may serve as a biomarker in Huntington’s disease using machine learning automatic classification. Sci. Rep. 2018, 8, 16090. [Google Scholar] [CrossRef] [PubMed]
- Ponomareva, N.; Klyushnikov, S.; Abramycheva, N.; Malina, D.; Scheglova, N.; Fokin, V.; Ivanova-Smolenskaia, I.; Illarioshkin, S. Alpha–theta border EEG abnormalities in preclinical Huntington’s disease. J. Neurol. Sci. 2014, 344, 114–120. [Google Scholar] [CrossRef]
- Vas, S.; Nicol, A.U.; Kalmar, L.; Miles, J.; Morton, A.J. Abnormal patterns of sleep and EEG power distribution during non-rapid eye movement sleep in the sheep model of Huntington’s disease. Neurobiol. Dis. 2021, 155, 105367. [Google Scholar] [CrossRef]
- Delussi, M.; Nazzaro, V.; Ricci, K.; de Tommaso, M. EEG functional connectivity and cognitive variables in premanifest and manifest Huntington’s disease: EEG Low-Resolution Brain Electromagnetic Tomography (LORETA) study. Front. Physiol. 2020, 11, 612325. [Google Scholar] [CrossRef]
- Herzog-Krzywoszanska, R.; Krzywoszanski, L. Sleep disorders in Huntington’s disease. Front. Psychiatry 2019, 10, 221. [Google Scholar] [CrossRef]
- Arnulf, I.; Nielsen, J.; Lohmann, E.; Schieffer, J.; Wild, E.; Jennum, P.; Konofal, E.; Walker, M.; Oudiette, D.; Tabrizi, S.; et al. Rapid eye movement sleep disturbances in Huntington disease. Arch. Neurol. 2008, 65, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Niu, L.; Liu, X.; Liu, Y.; Li, S.; Yu, H.; Le, W. Rapid eye movement sleep behavior disorder and neurodegenerative diseases: An update. Aging Dis. 2020, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A.; Jiménez-Jiménez, F.J. Association between restless legs syndrome and other movement disorders. Neurology 2019, 92, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Savva, E.; Schnorf, H.; Burkhard, P. Restless legs syndrome: An early manifestation of Huntington’s disease? Acta Neurol. Scand. 2009, 119, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Keren, K.; Busse, M.; Fritz, N.E.; Muratori, L.M.; Gazit, E.; Hillel, I.; Scheinowitz, M.; Gurevich, T.; Inbar, N.; Omer, N.; et al. Quantification of daily-living gait quantity and quality using a wrist-worn accelerometer in Huntington’s disease. Front. Neurol. 2021, 12, 719442. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, S.; Chu, C.; Tan, G.; Draganski, B.; Johnson, H.; Paulsen, J.; Kienzle, W.; Tabrizi, S.J.; Ashburner, J.; Frackowiak, R.S.; et al. Automatic detection of preclinical neurodegeneration: Presymptomatic Huntington disease. Neurology 2009, 72, 426–431. [Google Scholar] [CrossRef]
- Mohan, A.; Sun, Z.; Ghosh, S.; Li, Y.; Sathe, S.; Hu, J.; Sampaio, C. A machine-learning derived Huntington’s disease progression model: Insights for clinical trial design. Mov. Disord. 2022, 37, 553–562. [Google Scholar] [CrossRef]
- Lois, C.; González, I.; Izquierdo-García, D.; Zürcher, N.R.; Wilkens, P.; Loggia, M.L.; Hooker, J.M.; Rosas, H.D. Neuroinflammation in Huntington’s disease: New insights with 11C-PBR28 PET/MRI. ACS Chem. Neurosci. 2018, 9, 2563–2571. [Google Scholar] [CrossRef]
- Ko, J.; Furby, H.; Ma, X.; Long, J.D.; Lu, X.Y.; Slowiejko, D.; Gandhy, R. Clustering and prediction of disease progression trajectories in Huntington’s disease: An analysis of Enroll-HD data using a machine learning approach. Front. Neurol. 2023, 13, 1034269. [Google Scholar] [CrossRef]
- Felix, J.P.; Vieira, F.H.T.; da Silva Vieira, G.; Franco, R.A.P.; da Costa, R.M.; Salvini, R.L. An Automatic Method for Identifying Huntington’s Disease using Gait Dynamics. In Proceedings of the 2019 IEEE 31st International Conference on Tools with Artificial Intelligence (ICTAI), Portland, OR, USA, 4–6 November 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1659–1663. [Google Scholar]
- Cheng, J.; Liu, H.P.; Lin, W.Y.; Tsai, F.J. Identification of contributing genes of Huntington’s disease by machine learning. BMC Med. Genom. 2020, 13, 176. [Google Scholar] [CrossRef]
- Mannini, A.; Trojaniello, D.; Cereatti, A.; Sabatini, A.M. A machine learning framework for gait classification using inertial sensors: Application to elderly, post-stroke and huntington’s disease patients. Sensors 2016, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yang, M.; Wang, H.; McClean, S. Machine learning and statistical approaches to support the discrimination of neuro-degenerative diseases based on gait analysis. In Intelligent Patient Management; Springer: Berlin/Heidelberg, Germany, 2009; pp. 57–70. [Google Scholar]
- Rizk-Jackson, A.; Stoffers, D.; Sheldon, S.; Kuperman, J.; Dale, A.; Goldstein, J.; Corey-Bloom, J.; Poldrack, R.A.; Aron, A.R. Evaluating imaging biomarkers for neurodegeneration in pre-symptomatic Huntington’s disease using machine learning techniques. Neuroimage 2011, 56, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Miranda, Â.; Lavrador, R.; Júlio, F.; Januário, C.; Castelo-Branco, M.; Caetano, G. Classification of Huntington’s disease stage with support vector machines: A study on oculomotor performance. Behav. Res. Methods 2016, 48, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, R.; Júlio, F.; Januário, C.; Castelo-Branco, M.; Caetano, G. Classification of Huntington’s Disease Stage with Features Derived from Structural and Diffusion-Weighted Imaging. J. Pers. Med. 2022, 12, 704. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Chen, H.-Y.; Chen, C.Y.-C. A network pharmacology-based approach to investigate the novel TCM formula against huntington’s disease and validated by support vector machine model. Evid. Based Complement. Altern. Med. 2018, 2018, 6020197. [Google Scholar] [CrossRef]
- Ghazaleh, N.; Houghton, R.; Palermo, G.; Schobel, S.A.; Wijeratne, P.A.; Long, J.D. Ranking the predictive power of clinical and biological features associated with disease progression in Huntington’s disease. Front. Neurol. 2021, 12, 678484. [Google Scholar] [CrossRef]
- Patel, K.; Sheridan, C.; Shanley, D. Using Machine Learning to identify microRNA biomarkers for predisposition to Juvenile Onset Huntington’s Disease. J. Bioinform. Syst. Biol. 2023, 6, 18–30. [Google Scholar]
- Guimarães, M.T.; Medeiros, A.G.; Almeida, J.S.; y Martin, M.F.; Damaševičius, R.; Maskeliūnas, R.; Mattos, C.L.C.; Rebouças Filho, P.P. An optimized approach to Huntington’s disease detecting via audio signals processing with dimensionality reduction. In Proceedings of the 2020 International Joint Conference on Neural Networks (IJCNN), Glasgow, UK, 19–24 July 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–8. [Google Scholar]
- Acosta-Escalante, F.D.; Beltrán-Naturi, E.; Boll, M.C.; Hernández-Nolasco, J.A.; García, P.P. Meta-classifiers in Huntington’s disease patients classification, using iPhone’s movement sensors placed at the ankles. IEEE Access 2018, 6, 30942–30957. [Google Scholar] [CrossRef]
- Kohli, M.; Pustina, D.; Warner, J.H.; Alexander, D.C.; Scahill, R.I.; Tabrizi, S.J.; Sampaio, C.; Wijeratne, P.A. Predicting Huntington’s disease state with ensemble learning & sMRI: More than just the striatum. medRxiv 2023. [Google Scholar] [CrossRef]
- Riad, R.; Lunven, M.; Titeux, H.; Cao, X.N.; Hamet Bagnou, J.; Lemoine, L.; Montillot, J.; Sliwinski, A.; Youssov, K.; Cleret de Langavant, L.; et al. Predicting clinical scores in Huntington’s disease: A lightweight speech test. J. Neurol. 2022, 269, 5008–5021. [Google Scholar] [CrossRef]
- Abbas, P.; Yashar, S. A grey box neural network model of basal ganglia for gait signal of patients with Huntington disease. Basic Clin. Neurosci. 2016, 7, 107. [Google Scholar]
- Lauraitis, A.; Maskeliūnas, R.; Damaševičius, R. ANN and fuzzy logic based model to evaluate huntington disease symptoms. J. Healthc. Eng. 2018, 2018, 4581272. [Google Scholar] [CrossRef] [PubMed]
- Lauraitis, A.; Maskeliūnas, R. Investigation of predicting functional capacity level for Huntington disease patients. In Proceedings of the Information and Software Technologies: 23rd International Conference, ICIST 2017, Druskininkai, Lithuania, 12–14 October 2017; Proceedings. Springer: Cham, Switzerland, 2017; pp. 142–149. [Google Scholar]
- Alfonso Perez, G.; Caballero Villarraso, J. Neural network aided detection of Huntington disease. J. Clin. Med. 2022, 11, 2110. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Jin, W.; Le, D.; Carlozzi, N.; Dayalu, P.; Roberts, A.; Provost, E.M. Classification of huntington disease using acoustic and lexical features. Interspeech 2018, 2018, 1898–1902. [Google Scholar] [PubMed]
- Zhang, S.; Poon, S.K.; Vuong, K.; Sneddon, A.; Loy, C.T. A deep learning-based approach for gait analysis in Huntington disease. In MEDINFO 2019: Health and Wellbeing e-Networks for All; IOS Press: Amsterdam, The Netherlands, 2019; pp. 477–481. [Google Scholar]
- Akusok, A.; Eirola, E.; Björk, K.M.; Miche, Y.; Johnson, H.; Lendasse, A. Brute-force Missing Data Extreme Learning Machine for Predicting Huntington’s Disease. In Proceedings of the 10th International Conference on PErvasive Technologies Related to Assistive Environments, Island of Rhodes, Greece, 21–23 June 2017; pp. 189–192. [Google Scholar]
- Jiang, X.; Zhang, H.; Duan, F.; Quan, X. Identify Huntington’s disease associated genes based on restricted Boltzmann machine with RNA-seq data. BMC Bioinform. 2017, 18, 447. [Google Scholar] [CrossRef] [PubMed]
- Karargyris, A.; Umeton, R.; Sheller, M.J.; Aristizabal, A.; George, J.; Wuest, A.; Pati, S.; Kassem, H.; Zenk, M.; Baid, U.; et al. Federated benchmarking of medical artificial intelligence with MedPerf. Nat. Mach. Intell. 2023, 5, 799–810. [Google Scholar] [CrossRef]
- Sun, Z.; Ghosh, S.; Li, Y.; Cheng, Y.; Mohan, A.; Sampaio, C.; Hu, J. A probabilistic disease progression modeling approach and its application to integrated Huntington’s disease observational data. JAMIA Open 2019, 2, 123–130. [Google Scholar] [CrossRef]
- Kosorok, M.R.; Laber, E.B. Precision medicine. Annu. Rev. Stat. Its Appl. 2019, 6, 263–286. [Google Scholar] [CrossRef]
- Luo, H.; Li, M.; Yang, M.; Wu, F.X.; Li, Y.; Wang, J. Biomedical data and computational models for drug repositioning: A comprehensive review. Briefings Bioinform. 2021, 22, 1604–1619. [Google Scholar] [CrossRef]
- Gunning, D.; Stefik, M.; Choi, J.; Miller, T.; Stumpf, S.; Yang, G.Z. XAI—Explainable artificial intelligence. Sci. Robot. 2019, 4, eaay7120. [Google Scholar] [CrossRef]
- Arrieta, A.B.; Díaz-Rodríguez, N.; Del Ser, J.; Bennetot, A.; Tabik, S.; Barbado, A.; García, S.; Gil-López, S.; Molina, D.; Benjamins, R.; et al. Explainable Artificial Intelligence (XAI): Concepts, taxonomies, opportunities and challenges toward responsible AI. Inf. Fusion 2020, 58, 82–115. [Google Scholar] [CrossRef]
- Jaganathan, K.; Tayara, H.; Chong, K.T. An explainable supervised machine learning model for predicting respiratory toxicity of chemicals using optimal molecular descriptors. Pharmaceutics 2022, 14, 832. [Google Scholar] [CrossRef] [PubMed]
- Kather, J.N.; Ghaffari Laleh, N.; Foersch, S.; Truhn, D. Medical domain knowledge in domain-agnostic generative AI. NPJ Digit. Med. 2022, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.; DeVito, N.J.; Zhang, J. Generative AI for medical research. BMJ 2023, 382, 1551. [Google Scholar] [CrossRef] [PubMed]
- Chlap, P.; Min, H.; Vandenberg, N.; Dowling, J.; Holloway, L.; Haworth, A. A review of medical image data augmentation techniques for deep learning applications. J. Med. Imaging Radiat. Oncol. 2021, 65, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, Z.; Sun, W.; Lou, M.; Lian, J.; Zhao, W.; Deng, X.; Ma, Y. A comprehensive overview of image enhancement techniques. Arch. Comput. Methods Eng. 2021, 29, 583–607. [Google Scholar] [CrossRef]
- Snyder, T.; Byrd, G. The internet of everything. Computer 2017, 50, 8–9. [Google Scholar] [CrossRef]
- Iqbal, F.M.; Lam, K.; Joshi, M.; Khan, S.; Ashrafian, H.; Darzi, A. Clinical outcomes of digital sensor alerting systems in remote monitoring: A systematic review and meta-analysis. NPJ Digit. Med. 2021, 4, 7. [Google Scholar] [CrossRef]
- Wilson, S.L.; Way, G.P.; Bittremieux, W.; Armache, J.P.; Haendel, M.A.; Hoffman, M.M. Sharing biological data: Why, when, and how. FEBS Lett. 2021, 595, 847. [Google Scholar] [CrossRef]
- Ghoniemy, S.; Maghawry, N.E. A Proposed Internet of Everything Framework for Medical Applications. J. Adv. Inf. Technol. 2019, 10. [Google Scholar] [CrossRef]
- Ristevski, B.; Chen, M. Big data analytics in medicine and healthcare. J. Integr. Bioinform. 2018, 15, 20170030. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Shakyawar, S.K.; Sharma, M.; Kaushik, S. Big data in healthcare: Management, analysis and future prospects. J. Big Data 2019, 6, 54. [Google Scholar] [CrossRef]
- Kunjan, K. A Big Data Augmented Analytics Platform to Operationalize Efficiencies at Community Clinics; Indiana University-Purdue University Indianapolis: Indianapolis, IN, USA, 2017. [Google Scholar]
- Rolim, C.O.; Koch, F.L.; Westphall, C.B.; Werner, J.; Fracalossi, A.; Salvador, G.S. A cloud computing solution for patient’s data collection in health care institutions. In Proceedings of the 2010 Second International Conference on eHealth, Telemedicine, and Social Medicine, Saint Maarten, The Netherlands, 10–16 February 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 95–99. [Google Scholar]
- Sun, L.; Jiang, X.; Ren, H.; Guo, Y. Edge-cloud computing and artificial intelligence in internet of medical things: Architecture, technology and application. IEEE Access 2020, 8, 101079–101092. [Google Scholar] [CrossRef]
- Sheller, M.J.; Edwards, B.; Reina, G.A.; Martin, J.; Pati, S.; Kotrotsou, A.; Milchenko, M.; Xu, W.; Marcus, D.; Colen, R.R.; et al. Federated learning in medicine: Facilitating multi-institutional collaborations without sharing patient data. Sci. Rep. 2020, 10, 12598. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, F.A.; Braten, A.E.; Tamkittikhun, N.; Palma, D. Fog computing in healthcare—A review and discussion. IEEE Access 2017, 5, 9206–9222. [Google Scholar] [CrossRef]
- Gyles, C. Robots in medicine. Can. Vet. J. 2019, 60, 819. [Google Scholar] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Quantum Computing: Progress and Prospects; National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 2019. [Google Scholar]
- Kumar, S.A.; Kumar, A.; Dutt, V.; Agrawal, R. Multi model implementation on general medicine prediction with quantum neural networks. In Proceedings of the 2021 Third International Conference on Intelligent Communication Technologies and Virtual Mobile Networks (ICICV), Tirunelveli, India, 4–6 February 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1391–1395. [Google Scholar]
- Saki, A.A.; Alam, M.; Phalak, K.; Suresh, A.; Topaloglu, R.O.; Ghosh, S. A survey and tutorial on security and resilience of quantum computing. In Proceedings of the 2021 IEEE European Test Symposium (ETS), Bruges, Belgium, 24–28 May 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–10. [Google Scholar]
- DeBenedictis, E.P. A future with quantum machine learning. Computer 2018, 51, 68–71. [Google Scholar] [CrossRef]
- Dey, N.; Ashour, A.S.; Shi, F.; Fong, S.J.; Tavares, J.M.R. Medical cyber-physical systems: A survey. J. Med. Syst. 2018, 42, 74. [Google Scholar] [CrossRef]
- Lee, I.; Sokolsky, O. Medical cyber physical systems. In Proceedings of the 47th Design Automation Conference, Anaheim, CA, USA, 13–18 June 2010; pp. 743–748. [Google Scholar]
- Eckert, M.; Volmerg, J.S.; Friedrich, C.M. Augmented reality in medicine: Systematic and bibliographic review. JMIR Mhealth Uhealth 2019, 7, e10967. [Google Scholar] [CrossRef]
- Hu, H.z.; Feng, X.b.; Shao, Z.w.; Xie, M.; Xu, S.; Wu, X.h.; Ye, Z.w. Application and prospect of mixed reality technology in medical field. Curr. Med. Sci. 2019, 39, 1–6. [Google Scholar] [CrossRef]
- Li, L.; Yu, F.; Shi, D.; Shi, J.; Tian, Z.; Yang, J.; Wang, X.; Jiang, Q. Application of virtual reality technology in clinical medicine. Am. J. Transl. Res. 2017, 9, 3867. [Google Scholar] [PubMed]



| Reference | Machine Learning Approaches Used | Main Contributions | Dataset | Performance Evaluation Metrics | Limitation |
|---|---|---|---|---|---|
| [73] | Decision Tree | Proposes an automated method for evaluating gait dynamics as a means of diagnosing HD. | Gait in Neuro- degenerative disease dataset of 36 people | Accuracy = 100% | Limited models explored |
| [74] | Decision Tree | Identification of potential genes contributing to HD | GSE33000 dataset of 314 subjects | Accuracy = 90.79% | Small data samples |
| [75] | Support Vector Machine | Introduces an approach centered around training classifiers such as Hidden Markov Models and SVMs, tailored to specific classes, with a focus on gait classification. | Dataset of gait measurements of 58 subjects | Accuracy = 90.5% | Restricted to only two patho- logical populations |
| [76] | Support Vector Machine | Investigates the viability of employing machine learning and statistical methods to aid in distinguishing neurodegenerative conditions through gait analysis. | Gait in Neuro- degenerative disease dataset of 64 patients | Accuracy = 86.9% | Use of irrelavant features |
| [77] | Support Vector Machine | Focuses on developing imaging biomarkers for neurodegenerative disease, specifically HD. | Voxel based data of 64 individuals | Accuracy = 76% | Classification pre-HD subjects >22 YTO or >14 YTO |
| [78] | Support Vector Machine | Explores the use of ML methods, specifically the SVM algorithm, to classify individuals with HD based on oculomotor performance | Recorded eye movement data of 50 participants | Classifying: Accuracy = 73.4% Distinguishing: Accuracy = 81.8% | Relatively small number of individuals per group |
| [79] | Support Vector Machine | Investigates the use of SVMs in categorizing HD stages , utilizing metrics extracted from T1-weighted and diffusion-weighted imaging data. | MRI-derived datasets of 68 people | Classifying: Accuracy = 85–95% Distinguishing: Accuracy = 74% | Small training sample size |
| [80] | Support Vector Machine | Utilization of a pharmacologic strategy to explore a newly developed traditional Chinese medicine (TCM) formulation for HD therapy. | TCM database | CoMFA − R2 = 0.9488 CoMSIA − R2 = 0.9555 | Limited only to HD |
| [73] | Support Vector Machine | Recommends an automated method for diagnosing HD by examining gait dynamics | Gait in Neuro- degenerative disease dataset of 36 people | Accuracy = 100% | Limited models explored |
| [74] | Random Forest | Utilising machine learning methods to pinpoint potential genes that play a role in HD | GSE33000 dataset of 314 subjects | Accuracy = 90.45% | Small data samples |
| [81] | Random Forest | Aims to assess the ability of clinical and biological factors to forecast the advancement of HD. | Enroll-HD periodic dataset (PDS6) of 15,301 subjects | NIL | Focused on clinical variables only |
| [82] | Random Forest | Discover potential microRNA biomarkers associated with susceptibility to Juvenile Onset HD. | JOHD miRNA- mRNA expression dataset (GSE65776) of 168 samples | 100% AUC | Limited to Juvenile Onset HD |
| [83] | K-NN | Suggested an innovative method to identify HD by analyzing digitized voice recordings of patients reciting Lithuanian poems. | Own audio dataset of 24 patients | Accuracy = 97.3% | Smaller dataset |
| [84] | Logiboost, Random Forest | Enhance the accuracy of classifying HD patients using gait data while simultaneously minimizing the reliance on a reduced number of sensor devices for data acquisition. | HD gait dataset of 28 gait features | For raw data: Accuracy = 94.4% For gait features: Accuracy = 92.8% | Analyses only two experiment results |
| [85] | Ensemble Model | Creation and presentation of a ML model based on stacked ensemble techniques for predicting the individual stages of HD. | TRACK-HD dataset of 184 HD patients | Accuracy = 55.3% ± 6.1 | Research solely on baseline cross-sectional data only |
| [86] | Automatic Machine Learning | Development of a ML model that can predict clinical performance in HD using brief samples of speech recordings | 126 samples of audio recordings of HD gene carriers | Relative error from 12.7% to 20% | Less number of participants |
| References | Deep Learning Approaches Used | Main Contribution | Dataset | Performance Evaluation Metrics | Limitation |
|---|---|---|---|---|---|
| [87] | Artificial Neural Network | Creating a mathematical model with a grey box approach to replicate the characteristics of Huntington’s disease disorders. | Gait Signal dataset of 36 people | NIL | Limited to pharmaceutical treatments only |
| [88] | Artificial Neural Network | Creating a hybrid framework that merges an ANN with a Fuzzy Logic System (FLS). | Dataset of 3032 examples from 20 test subjects | R value: 0.98 MSE value: 0.08 | Small dataset |
| [89] | Artificial Neural Network | Creation of an ANN model aimed at forecasting the functional capacity status of individuals. | Dataset of 200 examples from 10 subjects | R value: 0.995 MSE value: 0.108 | Inadequate dataset |
| [90] | Artificial Neural Network | Creating a biomarker utilizing DNA CpG methylation data to identify HD. | DNA methylation data of 76 samples | CMP: 0.92 CP: 0.86 | Small size of the datapool |
| [91] | Deep Neural Network | Development of an objective and non-invasive acoustic biomarker that can detect HD | Data from HD study of 62 speakers | Accuracy = 87% | Insufficient features |
| [92] | Deep Convolutional Neural Network | Creating a DL-driven method to analyze gait patterns in individuals with HD. | Foot pressure data of 12 patients | Accuracy = 82% | Preprocessing module can be further optimized |
| [93] | Extreme Learning Machine | Built an innovative technique to educate ELM models using datasets containing absent data points. | Huntington’s disease dataset of 3729 samples from 1370 subjects | F1 score: 0.98 | Performance loss on smaller features |
| [94] | Deep Boltzmann Machine | Proposal of SRBM to analyze RNA-seq data associated with Huntington’s disease. | Gene expression dataset of 12 samples | AUC: 0.522 | Did not explore other methodologies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganesh, S.; Chithambaram, T.; Krishnan, N.R.; Vincent, D.R.; Kaliappan, J.; Srinivasan, K. Exploring Huntington’s Disease Diagnosis via Artificial Intelligence Models: A Comprehensive Review. Diagnostics 2023, 13, 3592. https://doi.org/10.3390/diagnostics13233592
Ganesh S, Chithambaram T, Krishnan NR, Vincent DR, Kaliappan J, Srinivasan K. Exploring Huntington’s Disease Diagnosis via Artificial Intelligence Models: A Comprehensive Review. Diagnostics. 2023; 13(23):3592. https://doi.org/10.3390/diagnostics13233592
Chicago/Turabian StyleGanesh, Sowmiyalakshmi, Thillai Chithambaram, Nadesh Ramu Krishnan, Durai Raj Vincent, Jayakumar Kaliappan, and Kathiravan Srinivasan. 2023. "Exploring Huntington’s Disease Diagnosis via Artificial Intelligence Models: A Comprehensive Review" Diagnostics 13, no. 23: 3592. https://doi.org/10.3390/diagnostics13233592
APA StyleGanesh, S., Chithambaram, T., Krishnan, N. R., Vincent, D. R., Kaliappan, J., & Srinivasan, K. (2023). Exploring Huntington’s Disease Diagnosis via Artificial Intelligence Models: A Comprehensive Review. Diagnostics, 13(23), 3592. https://doi.org/10.3390/diagnostics13233592







