Line-Field Confocal Optical Coherence Tomography Evaluation of Eyelid Skin Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Sample Population
2.3. LC-OCT Device, Imaging Acquisition, and Evaluation
2.4. Study Outcome and Statistical Analysis
3. Results
3.1. Clinical Features
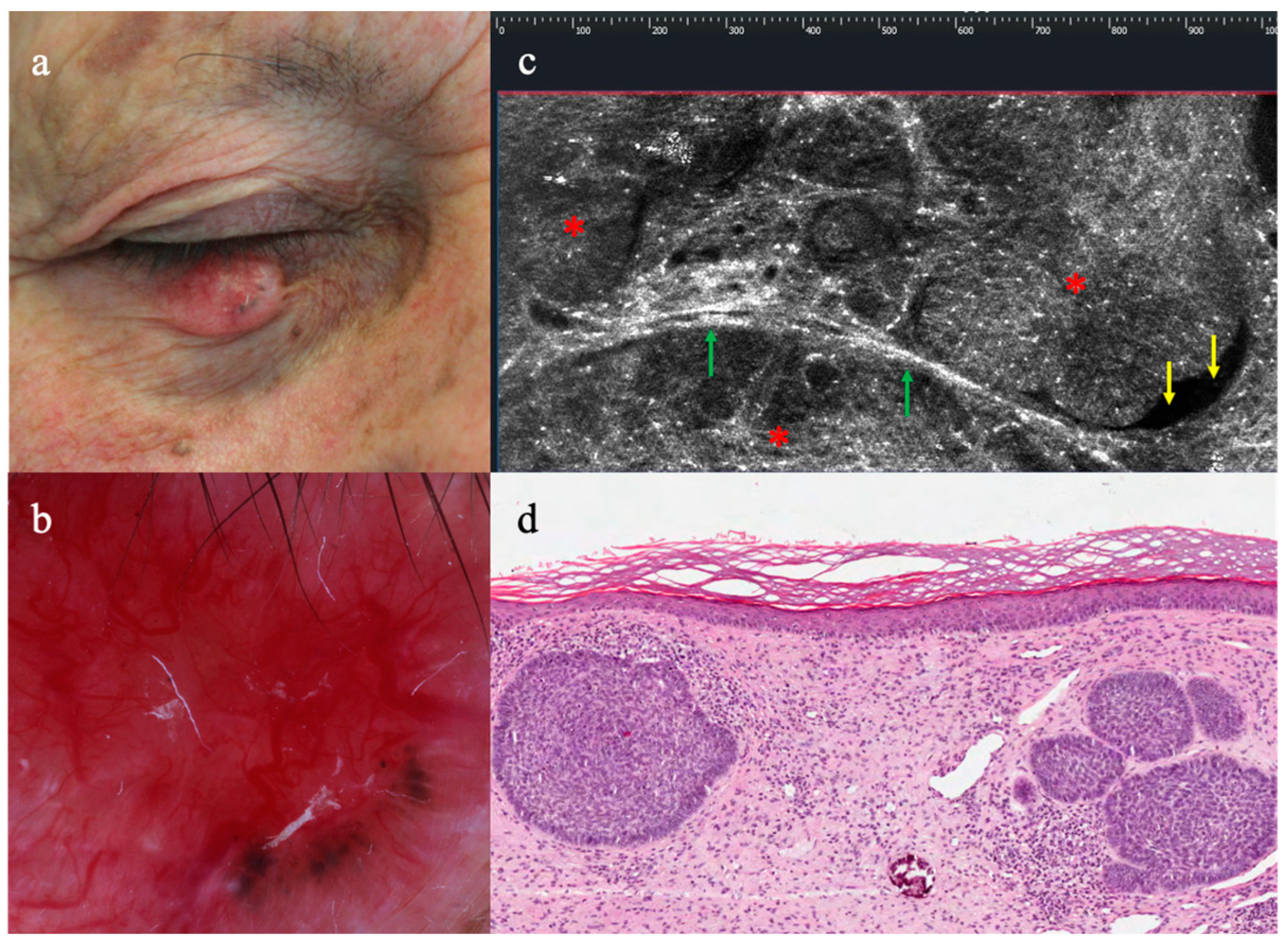
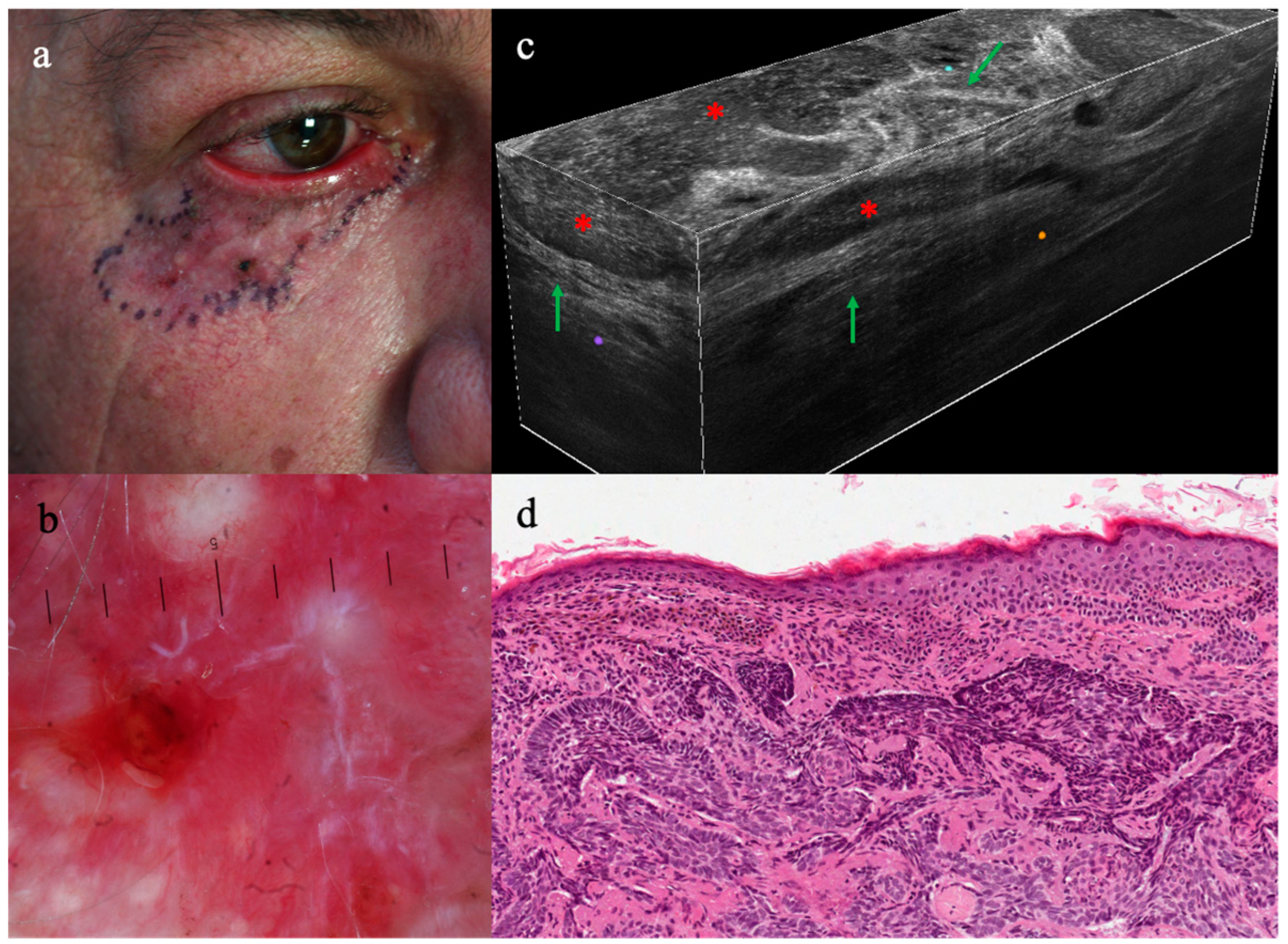
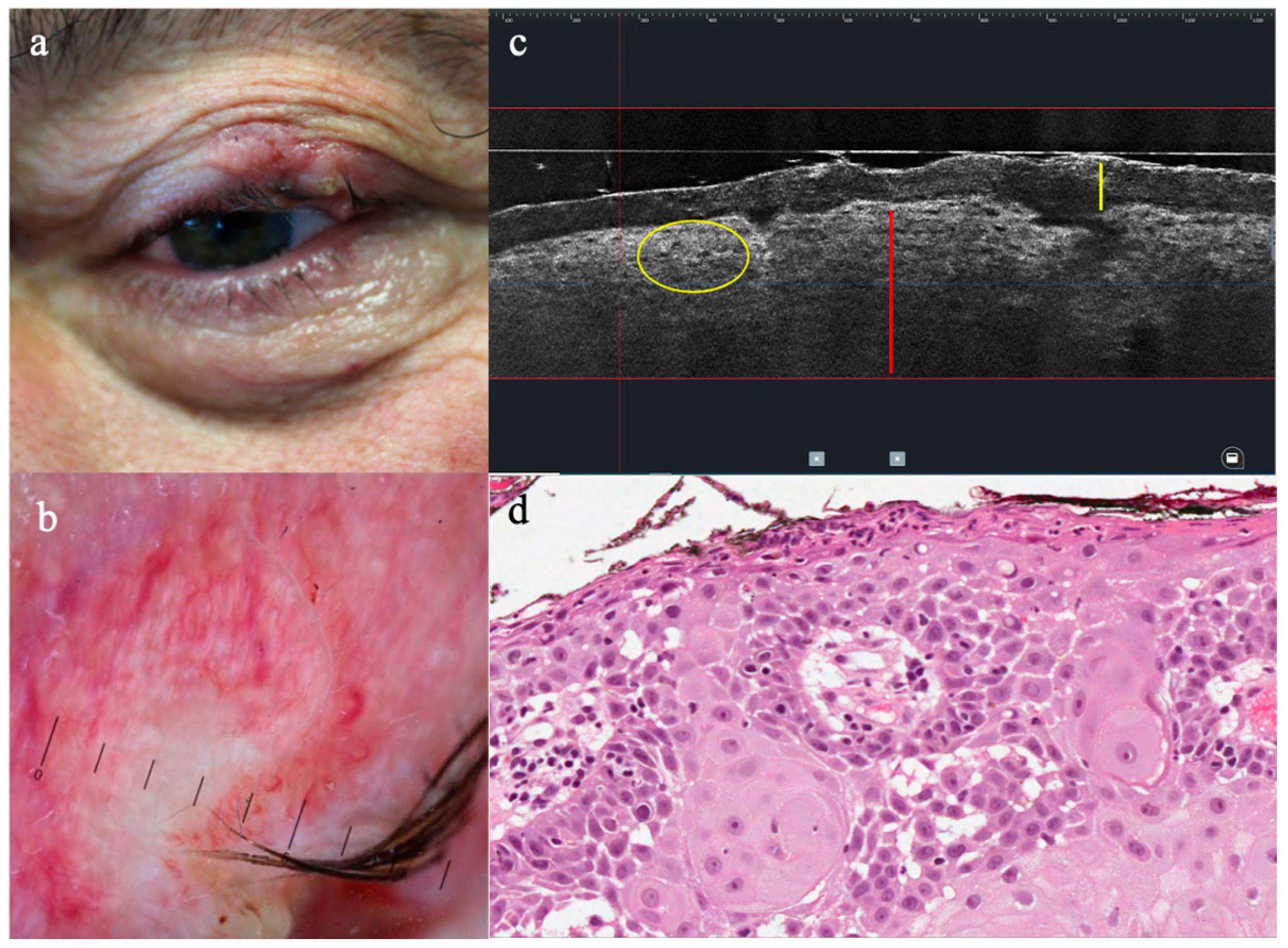
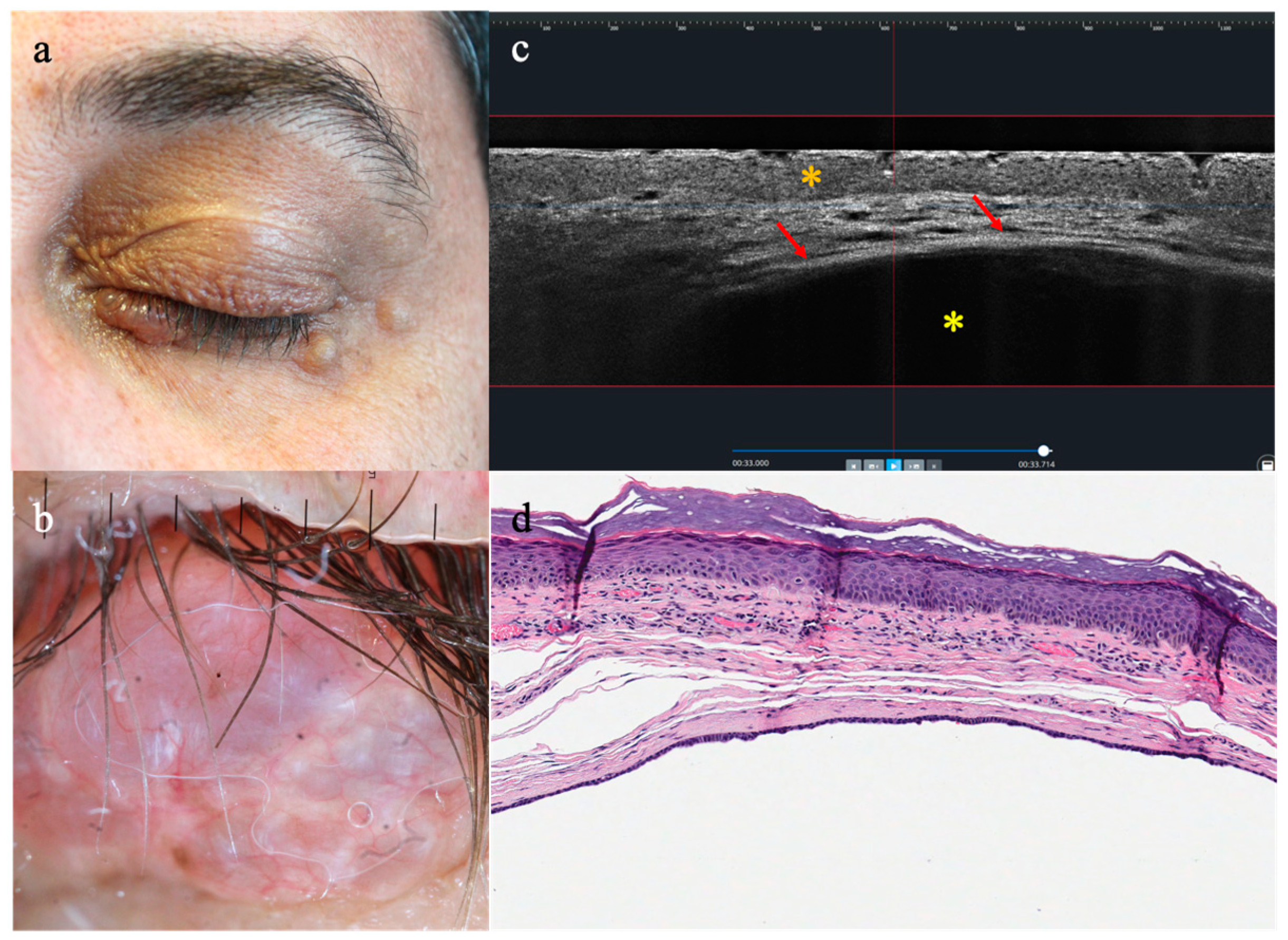
3.2. LC-OCT Features of Malignant Lesions of the Eyelid
3.3. LC-OCT Features of Benign Lesions of the Eyelid
3.4. Diagnostic Concordance LC-OCT/Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carter, S.R. Eyelid disorders: Diagnosis and management. Am. Fam. Physician 1998, 57, 2695–2702. [Google Scholar]
- Kersten, R.C.; Ewing-Chow, D.; Kulwin, D.R.; Gallon, M. Accuracy of clinical diagnosis of cutaneous eyelid lesions. Ophthalmology 1997, 104, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Adamski, W.Z.; Maciejewski, J.; Adamska, K.; Marszałek, A.; Rospond-Kubiak, I. The prevalence of various eyelid skin lesions in a single-centre observation study. Postep. Dermatol. Alergol. 2021, 38, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Deprez, M.; Uffer, S. Clinicopathological features of eyelid skin tumors. A retrospective study of 5504 cases and review of literature. Am. J. Dermatopathol. 2009, 31, 256–262. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Liang, W.Y.; Tsai, C.C.; Kao, S.C.; Yu, W.K.; Kau, H.C.; Liu, C.J. Comparison of the Clinical Characteristics and Outcome of Benign and Malignant Eyelid Tumors: An Analysis of 4521 Eyelid Tumors in a Tertiary Medical Center. BioMed Res. Int. 2015, 2015, 453091. [Google Scholar] [CrossRef] [PubMed]
- Brodowski, R.; Pakla, P.; Dymek, M.; Migut, M.; Ambicki, M.; Stopyra, W.; Ozga, D.; Lewandowski, B. Clinical-pathological characteristics of patients treated for cancers of the eyelid skin and periocular areas. Adv. Clin. Exp. Med. 2019, 28, 325–330. [Google Scholar] [CrossRef]
- Pelosini, L.; Smith, H.B.; Schofield, J.B.; Meeckings, A.; Dithal, A.; Khandwala, M. A novel imaging approach to periocular basal cell carcinoma: In vivo optical coherence tomography and histological correlates. Eye 2015, 29, 1092–1098. [Google Scholar] [CrossRef][Green Version]
- Leibovitch, I.; McNab, A.; Sullivan, T.; Davis, G.; Selva, D. Orbital invasion by periocular basal cell carcinoma. Ophthalmology 2005, 112, 717–723. [Google Scholar] [CrossRef]
- Weesie, F.; Naus, N.C.; Vasilic, D.; Hollestein, L.M.; van den Bos, R.R.; Wakkee, M. Recurrence of periocular basal cell carcinoma and squamous cell carcinoma after Mohs micrographic surgery: A retrospective cohort study. Br. J. Dermatol. 2019, 180, 1176–1182. [Google Scholar] [CrossRef]
- Shah, M.A.; Lynch, E.; Cauchi, P.; Chadha, V. One Year of the Ocular Oncology Multidisciplinary Team Meeting—Has it Made a Difference? Clin. Oncol. 2019, 31, 400. [Google Scholar] [CrossRef]
- Jaworska, K.; Sławińska, M.; Wyszomirski, A.; Lakomy, J.; Sobjanek, M. Dermoscopic features of eyelid margin tumors: A single-center retrospective study. J. Dermatol. 2022, 49, 851–861. [Google Scholar] [CrossRef]
- Kozubowska, K.; Sławińska, M.; Sobjanek, M. The role of dermoscopy in diagnostics of dermatological conditions of the eyelid, eyelashes, and conjunctiva—A literature review. Int. J. Dermatol. 2021, 60, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, F.H.L.; Osaki, M.H.; Biazim, D.F.; Leonardo, Y.M.O.; Osaki, T.H. Slit lamp polarized dermoscopy: A cost-effective tool to assess eyelid lesions. Int. Ophthalmol. 2023, 43, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Singer, A.; Labeille, B.; Grivet, D.; Rubegni, P.; Douchet, C.; Cambazard, F.; Thuret, G.; Gain, P.; Perrot, J.L. Handheld In Vivo Reflectance Confocal Microscopy for the Diagnosis of Eyelid Margin and Conjunctival Tumors. JAMA Ophthalmol. 2017, 135, 845–851. [Google Scholar] [CrossRef]
- Malvehy, J.; Pellacani, G. Dermoscopy, Confocal Microscopy and other Non-invasive Tools for the Diagnosis of Non-Melanoma Skin Cancers and Other Skin Conditions. Acta Derm. Venereol. 2017, 97 (Suppl. S218), 22–30. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, S.; Arthurs, B.; Sanft, D.M.; Mastromonaco, C.; Burnier, M.N., Jr. Optical Coherence Tomography of Peri-Ocular Skin Cancers: An Optical Biopsy. Ocul. Oncol. Pathol. 2021, 7, 149–158. [Google Scholar] [CrossRef]
- Suppa, M.; Palmisano, G.; Tognetti, L.; Lenoir, C.; Cappilli, S.; Fontaine, M.; Orte Cano, C.; Diet, G.; Perez-Anker, J.; Schuh, S.; et al. Line-field confocal optical coherence tomography in melanocytic and non-melanocytic skin tumors. Ital. J. Dermatol. Venerol. 2023, 158, 180–189. [Google Scholar] [CrossRef]
- Cappilli, S.; Guerriero, C.; Iacoangeli, A.; Verzì, A.E.; Cinotti, E.; Suppa, M.; Peris, K.; Di Stefani, A. Utility of line-field confocal optical coherence tomography in the pediatric population. Ital. J. Dermatol. Venerol. 2023, 158, 197–204. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E. Line-field optical coherence tomography: In vivo diagnosis of basal cell carcinoma subtypes compared with histopathology. Clin. Exp. Dermatol. 2021, 46, 1471–1481. [Google Scholar] [CrossRef]
- Hobelsberger, S.; Steininger, J.; Bauer, A.; Beissert, S.; Gellrich, F.F. Line-field confocal optical coherence tomography for the diagnosis of onychomycosis in comparison with healthy nails: A case series. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1234–e1236. [Google Scholar] [CrossRef]
- Verzì, A.E.; Lacarrubba, F.; Dall’Oglio, F.; Rini, S.; Tosti, A.; Micali, G. Subclinical, early hair regrowth in alopecia areata patients under treatment with baricitinib detected by line-field confocal optical coherence tomography evaluation. J. Eur. Acad. Dermatol. Venereol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.; Ruini, C.; Perwein, M.K.E.; Daxenberger, F.; Gust, C.; Sattler, E.C.; Welzel, J. Line-Field Confocal Optical Coherence Tomography: A New Tool for the Differentiation between Nevi and Melanomas? Cancers 2022, 14, 1140. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E.C. Line-field confocal optical coherence tomography for the in vivo real-time diagnosis of different stages of keratinocyte skin cancer: A preliminary study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2388–2397. [Google Scholar] [CrossRef]
- Gust, C.; Schuh, S.; Welzel, J.; Daxenberger, F.; Hartmann, D.; French, L.E.; Ruini, C.; Sattler, E.C. Line-Field Confocal Optical Coherence Tomography Increases the Diagnostic Accuracy and Confidence for Basal Cell Carcinoma in Equivocal Lesions: A Prospective Study. Cancers 2022, 14, 1082. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Schuh, S.; Gust, C.; Hartmann, D.; French, L.E.; Sattler, E.C.; Welzel, J. In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study. Cancers 2021, 13, 2856. [Google Scholar] [CrossRef] [PubMed]
- Lacarrubba, F.; Verzì, A.E.; Puglisi, D.F.; Broggi, G.; Caltabiano, R.; Micali, G. Line-field confocal optical coherence tomography of xanthogranuloma: Correlation with vertical and horizontal histopathology. J. Cutan. Pathol. 2021, 48, 1208–1211. [Google Scholar] [CrossRef]
- Verzì, A.E.; Broggi, G.; Caltabiano, R.; Micali, G.; Lacarrubba, F. Line-field confocal optical coherence tomography of lentigo maligna with horizontal and vertical histopathologic correlations. J. Cutan. Pathol. 2023, 50, 118–122. [Google Scholar] [CrossRef]
- Aktas, D.; Palmisano, G.; Cinotti, E.; Tognetti, L.; Perrot, J.L.; Perez-Anker, J.; Rubegni, P.; Puig, S.; Malvehy, J.; Peris, K.; et al. The role of line-field confocal optical coherence tomography in the differential diagnosis of infiltrative basal cell carcinoma with scar-like lesions: A case series. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1396–e1398. [Google Scholar] [CrossRef]
- Ogien, J.; Tavernier, C.; Fischman, S.; Dubois, A. Line-field confocal optical coherence tomography (LC-OCT): Principles and practical use. Ital. J. Dermatol. Venerol. 2023, 158, 171–179. [Google Scholar] [CrossRef]
- Verzì, A.E.; Micali, G.; Lacarrubba, F. Line-Field Confocal Optical Coherence Tomography May Enhance Monitoring of Superficial Basal Cell Carcinoma Treated with Imiquimod 5% Cream: A Pilot Study. Cancers 2021, 13, 4913. [Google Scholar] [CrossRef]
| N (%) | |
|---|---|
| Sex | |
| Male | 23 (45%) |
| Female | 28 (55%) |
| Mean age | 66.4 (34–88) |
| Phototype | |
| I/II | 32 (63%) |
| III | 19 (37%) |
| Anatomical location | |
| Lower eyelid | 20 (39%) |
| Medial canthus | 14 (27%) |
| Upper eyelid | 9 (18%) |
| Lateral canthus | 8 (16%) |
| Clinical I | |
| Flat | 10 (20%) |
| Slightly elevated | 13 (25%) |
| Nodular | 28 (55%) |
| Histopathology | |
| Malignant tumours | 30/51 (59%) |
| BCC | 24 (47%) |
| SCC | 4 (8%) |
| Melanoma | 2 (4%) |
| Benign tumours | 21/51 (41%) |
| SK | 9 (17%) |
| Scar | 5 (10%) |
| Hidrocystoma | 3 (6%) |
| AK | 2 (4%) |
| Blue nevus | 1 (2%) |
| Cherry hemangioma | 1 (2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stefani, A.; Cappilli, S.; Cuffaro, G.; Fionda, B.; Pagliara, M.M.; Paradisi, A.; Ricci, C.; Rossi, E.; Sammarco, M.G.; Schinzari, G.; et al. Line-Field Confocal Optical Coherence Tomography Evaluation of Eyelid Skin Lesions. Diagnostics 2023, 13, 3590. https://doi.org/10.3390/diagnostics13233590
Di Stefani A, Cappilli S, Cuffaro G, Fionda B, Pagliara MM, Paradisi A, Ricci C, Rossi E, Sammarco MG, Schinzari G, et al. Line-Field Confocal Optical Coherence Tomography Evaluation of Eyelid Skin Lesions. Diagnostics. 2023; 13(23):3590. https://doi.org/10.3390/diagnostics13233590
Chicago/Turabian StyleDi Stefani, Alessandro, Simone Cappilli, Giovanni Cuffaro, Bruno Fionda, Monica Maria Pagliara, Andrea Paradisi, Costantino Ricci, Ernesto Rossi, Maria Grazia Sammarco, Giovanni Schinzari, and et al. 2023. "Line-Field Confocal Optical Coherence Tomography Evaluation of Eyelid Skin Lesions" Diagnostics 13, no. 23: 3590. https://doi.org/10.3390/diagnostics13233590
APA StyleDi Stefani, A., Cappilli, S., Cuffaro, G., Fionda, B., Pagliara, M. M., Paradisi, A., Ricci, C., Rossi, E., Sammarco, M. G., Schinzari, G., Tagliaferri, L., Blasi, M. A., Cinotti, E., Moro, A., Savino, G., Suppa, M., & Peris, K. (2023). Line-Field Confocal Optical Coherence Tomography Evaluation of Eyelid Skin Lesions. Diagnostics, 13(23), 3590. https://doi.org/10.3390/diagnostics13233590






