Extracorporeal Membrane Oxygenation (ECMO)-Associated Coagulopathy in Adults
Abstract
:1. Introduction
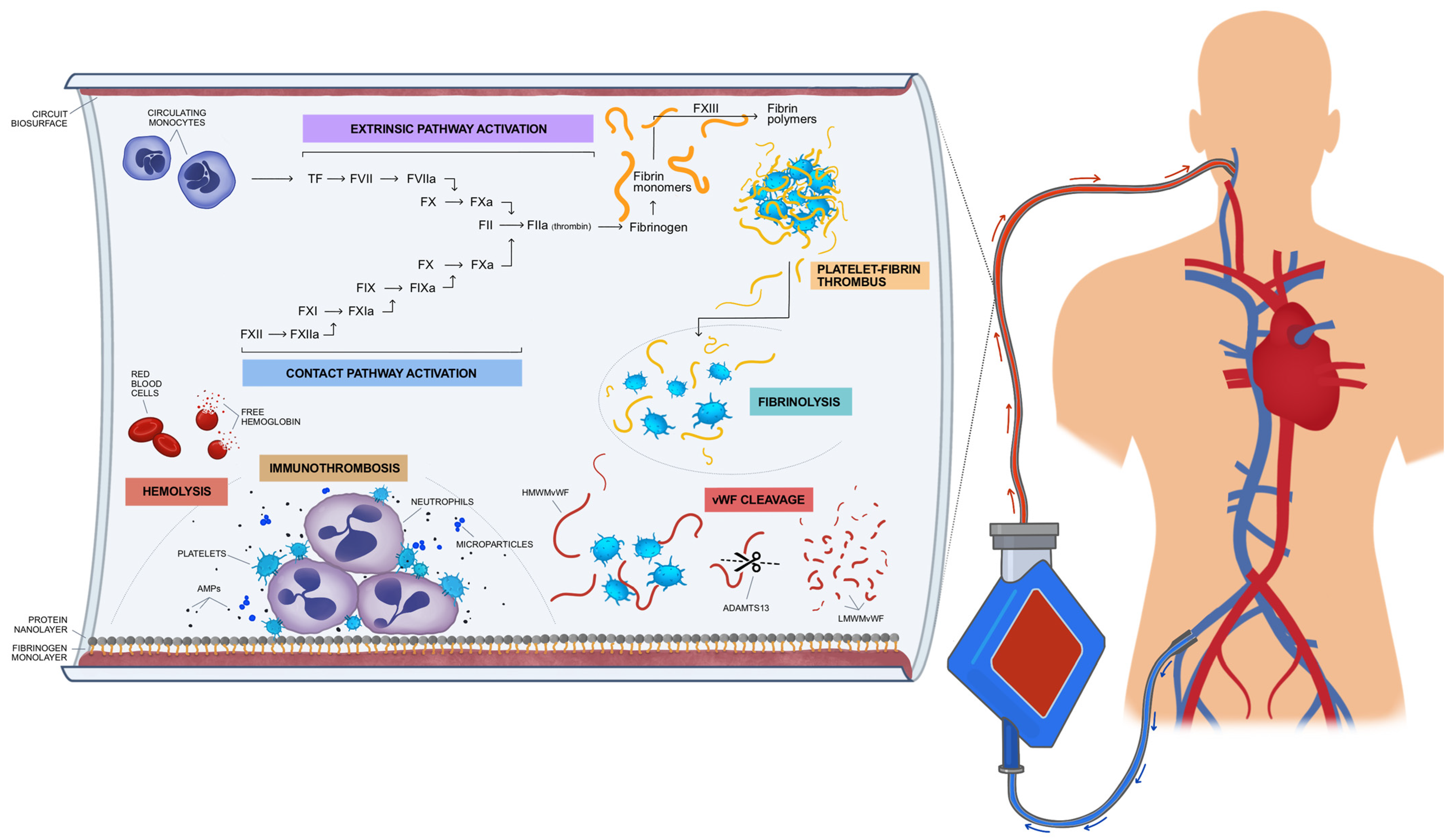
2. Interactions between Coagulation Cascade and Biosurfaces Leading to EAC
3. Monitoring Coagulation Profile of ECMO Patients
4. Activated Clotting Time (ACT)
5. Activated Partial Thromboplastin Time (aPTT) and Anti-Factor Xa Level
6. Viscoelastic Haemostatic Assays
7. D-Dimers, Platelets, and Antithrombin
8. Differences between Veno-Venous (V-V ECMO) and Veno-Arterial (V-A ECMO)-Associated Coagulopathy
9. Recommendations
10. Future Perspectives
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzeffi, M.; Greenwood, J.; Tanaka, K.; Menaker, J.; Rector, R.; Herr, D.; Kon, Z.; Lee, J.; Griffith, B.; Rajagopal, K.; et al. Bleeding, Transfusion, and Mortality on Extracorporeal Life Support: ECLS Working Group on Thrombosis and Hemostasis. Ann. Thorac. Surg. 2016, 101, 682–689. [Google Scholar] [CrossRef]
- Cheng, R.; Hachamovitch, R.; Kittleson, M.; Patel, J.; Arabia, F.; Moriguchi, J.; Esmailian, F.; Azarbal, B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1866 adult patients. Ann. Thorac. Surg. 2014, 97, 610–616. [Google Scholar] [CrossRef]
- Treml, B.; Breitkopf, R.; Bukumirić, Z.; Bachler, M.; Boesch, J.; Rajsic, S. ECMO Predictors of Mortality: A 10-Year Referral Centre Experience. J. Clin. Med. 2022, 11, 1224. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Phan, X.T.; Nguyen, T.H.; Huynh, D.Q.; Tran, L.T.; Pham, H.M.; Nguyen, T.N.; Kieu, H.T.; Ngoc Pham, T.T. Major Bleeding in Adults Undergoing Peripheral Extracorporeal Membrane Oxygenation (ECMO): Prognosis and Predictors. Crit. Care Res. Pract. 2022, 2022, 5348835. [Google Scholar] [CrossRef]
- Doyle, A.J.; Hunt, B.J. Current Understanding of How Extracorporeal Membrane Oxygenators Activate Haemostasis and Other Blood Components. Front. Med. 2018, 5, 352. [Google Scholar] [CrossRef]
- Nunez, J.I.; Gosling, A.F.; O’Gara, B.; Kennedy, K.F.; Rycus, P.; Abrams, D.; Brodie, D.; Shaefi, S.; Garan, A.R.; Grandin, E.W. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: An ELSO registry analysis. Intensive Care Med. 2022, 48, 213–224. [Google Scholar] [CrossRef]
- Paden, M.L.; Conrad, S.A.; Rycus, P.T.; Thiagarajan, R.R. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J. 2013, 59, 202–210. [Google Scholar] [CrossRef]
- Kanji, R.; Vandenbriele, C.; Arachchillage, D.R.J.; Price, S.; Gorog, D.A. Optimal Tests to Minimise Bleeding and Ischaemic Complications in Patients on Extracorporeal Membrane Oxygenation. Thromb. Haemost. 2022, 122, 480–491. [Google Scholar] [CrossRef]
- Willers, A.; Swol, J.; van Kuijk, S.M.J.; Buscher, H.; McQuilten, Z.; Ten Cate, H.; Rycus, P.T.; McKellar, S.; Lorusso, R.; Tonna, J.E. HEROES V-V-HEmorRhagic cOmplications in Veno-Venous Extracorporeal life Support-Development and internal validation of multivariable prediction model in adult patients. Artif. Organs 2022, 46, 932–952. [Google Scholar] [CrossRef]
- Zakhary, B.; Vercaemst, L.; Mason, P.; Lorusso, R.; Brodie, D. How I manage drainage insufficiency on extracorporeal membrane oxygenation. Crit. Care 2020, 24, 151. [Google Scholar] [CrossRef]
- Yusuff, H.; Zochios, V.; Brodie, D. Thrombosis and coagulopathy in COVID-19 patients rceiving ECMO: A narrative review of current literature. J. Cardiothorac. Vasc. Anesth. 2022, 36 Pt B, 3312–3317. [Google Scholar] [CrossRef]
- Yang, X.; Cai, S.; Luo, Y.; Zhu, F.; Hu, M.; Zhao, Y.; Zheng, R.; Li, X.; Hu, B.; Peng, Z. Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019-Induced Acute Respiratory Distress Syndrome: A Multicenter Descriptive Study. Crit. Care Med. 2020, 48, 1289–1295. [Google Scholar] [CrossRef]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 1121–1131. [Google Scholar] [CrossRef]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Poissy, J.; Goutay, J.; Caplan, M.; Parmentier, E.; Duburcq, T.; Lassalle, F.; Jeanpierre, E.; Rauch, A.; Labreuche, J.; Susen, S. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation 2020, 142, 184–186. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Schmid, E.; Krajewski, S.; Bachmann, D.; Kurz, J.; Wendel, H.P.; Rosenberger, P.; Balkau, B.; Peter, K.; Unertl, K.; Straub, A. The volatile anesthetic sevoflurane inhibits activation of neutrophil granulocytes during simulated extracorporeal circulation. Int. Immunopharmacol. 2012, 14, 202–208. [Google Scholar] [CrossRef]
- Vroman, L.; Adams, A.L.; Fischer, G.C.; Munoz, P.C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 1980, 55, 156–159. [Google Scholar] [CrossRef]
- Turbill, P.; Beugeling, T.; Poot, A.A. Proteins involved in the Vroman effect during exposure of human blood plasma to glass and polyethylene. Biomaterials 1996, 17, 1279–1287. [Google Scholar] [CrossRef]
- Frantzeskaki, F.; Armaganidis, A.; Orfanos, S.E. Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks between Inflammation and Coagulation. Respir. Int. Rev. Thorac. Dis. 2017, 93, 212–225. [Google Scholar] [CrossRef]
- Wilm, J.; Philipp, A.; Müller, T.; Bredthauer, A.; Gleich, O.; Schmid, C.; Lehle, K. Leukocyte Adhesion as an Indicator of Oxygenator Thrombosis During Extracorporeal Membrane Oxygenation Therapy? ASAIO J. 2018, 64, 24–30. [Google Scholar] [CrossRef]
- Fortenberry, J.D.; Bhardwaj, V.; Niemer, P.; Cornish, J.D.; Wright, J.A.; Bland, L. Neutrophil and cytokine activation with neonatal extracorporeal membrane oxygenation. J. Pediatr. 1996, 128, 670–678. [Google Scholar] [CrossRef]
- Abrams, D.; Baldwin, M.R.; Champion, M.; Agerstrand, C.; Eisenberger, A.; Bacchetta, M.; Brodie, D. Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: A cohort study. Intensive Care Med. 2016, 42, 844–852. [Google Scholar] [CrossRef]
- Lukito, P.; Wong, A.; Jing, J.; Arthur, J.F.; Marasco, S.F.; Murphy, D.A.; Bergin, P.J.; Shaw, J.A.; Collecutt, M.; Andrews, R.K.; et al. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J. Thromb. Haemost. JTH 2016, 14, 2253–2260. [Google Scholar] [CrossRef]
- Kalbhenn, J.; Schlagenhauf, A.; Rosenfelder, S.; Schmutz, A.; Zieger, B. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. J. Heart Lung Transplant. 2018, 37, 985–991. [Google Scholar] [CrossRef]
- Harrison, M.J.; Pugsley, W.; Newman, S.; Paschalis, C.; Klinger, L.; Treasure, T.; Aspey, B. Detection of middle cerebral emboli during coronary artery bypass surgery using transcranial Doppler sonography. Stroke 1990, 21, 1512. [Google Scholar] [CrossRef]
- Douda, D.N.; Jackson, R.; Grasemann, H.; Palaniyar, N. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J. Immunol. 2011, 187, 1856–1865. [Google Scholar] [CrossRef]
- Lim, M.Y.; Ataga, K.I.; Key, N.S. Hemostatic abnormalities in sickle cell disease. Curr. Opin. Hematol. 2013, 20, 472–477. [Google Scholar] [CrossRef]
- Zarbock, A.; Singbartl, K.; Ley, K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Investig. 2006, 116, 3211–3219. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T.; ten Cate, H. Tissue factor in infection and severe inflammation. Semin. Thromb. Hemost. 2006, 32, 33–39. [Google Scholar] [CrossRef]
- Kappelmayer, J.; Bernabei, A.; Edmunds, L.H.J.; Edgington, T.S.; Colman, R.W. Tissue factor is expressed on monocytes during simulated extracorporeal circulation. Circ. Res. 1993, 72, 1075–1081. [Google Scholar] [CrossRef]
- Fischer, M.; Sperling, C.; Tengvall, P.; Werner, C. The ability of surface characteristics of materials to trigger leukocyte tissue factor expression. Biomaterials 2010, 31, 2498–2507. [Google Scholar] [CrossRef]
- Plötz, F.B.; van Oeveren, W.; Bartlett, R.H.; Wildevuur, C.R. Blood activation during neonatal extracorporeal life support. J. Thorac. Cardiovasc. Surg. 1993, 105, 823–832. [Google Scholar] [CrossRef]
- Boisclair, M.D.; Lane, D.A.; Philippou, H.; Esnouf, M.P.; Sheikh, S.; Hunt, B.; Smith, K.J. Mechanisms of thrombin generation during surgery and cardiopulmonary bypass. Blood 1993, 82, 3350–3357. [Google Scholar] [CrossRef]
- Wendel, H.P.; Jones, D.W.; Gallimore, M.J. FXII levels, FXIIa-like activities and kallikrein activities in normal subjects and patients undergoing cardiac surgery. Immunopharmacology 1999, 45, 141–144. [Google Scholar] [CrossRef]
- Passmore, M.R.; Fung, Y.L.; Simonova, G.; Foley, S.R.; Diab, S.D.; Dunster, K.R.; Spanevello, M.M.; McDonald, C.I.; Tung, J.-P.; Pecheniuk, N.M.; et al. Evidence of altered haemostasis in an ovine model of venovenous extracorporeal membrane oxygenation support. Crit. Care 2017, 21, 191. [Google Scholar] [CrossRef]
- Davidson, S.J.; Burman, J.F.; Philips, S.M.; Onis, S.J.; Kelleher, A.A.; De Souza, A.C.; Pepper, J.R. Correlation between thrombin potential and bleeding after cardiac surgery in adults. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2003, 14, 175–179. [Google Scholar] [CrossRef]
- Kreyer, S.; Muders, T.; Theuerkauf, N.; Spitzhüttl, J.; Schellhaas, T.; Schewe, J.-C.; Guenther, U.; Wrigge, H.; Putensen, C. Hemorrhage under veno-venous extracorporeal membrane oxygenation in acute respiratory distress syndrome patients: A retrospective data analysis. J. Thorac. Dis. 2017, 9, 5017–5029. [Google Scholar] [CrossRef]
- Granja, T.; Hohenstein, K.; Schüssel, P.; Fischer, C.; Prüfer, T.; Schibilsky, D.; Wendel, H.P.; Jaschonek, K.; Serna-Higuita, L.; Schlensak, C.; et al. Multi-Modal Characterization of the Coagulopathy Associated With Extracorporeal Membrane Oxygenation. Crit. Care Med. 2020, 48, e400–e408. [Google Scholar] [CrossRef]
- Kalbhenn, J.; Schmidt, R.; Nakamura, L.; Schelling, J.; Rosenfelder, S.; Zieger, B. Early diagnosis of acquired von Willebrand Syndrome (AVWS) is elementary for clinical practice in patients treated with ECMO therapy. J. Atheroscler. Thromb. 2015, 22, 265–271. [Google Scholar] [CrossRef]
- McVeen, R.V.; Lorch, V.; Carroll, R.C.; Goldberg, L.; Keszler, M.; Podlasek, S.; Stewart, D.L. Changes in fibrinolytic factors in newborns during extracorporeal membrane oxygenation (ECMO). Am. J. Hematol. 1991, 38, 254–255. [Google Scholar] [CrossRef]
- Da, Q.; Teruya, M.; Guchhait, P.; Teruya, J.; Olson, J.S.; Cruz, M.A. Free hemoglobin increases von Willebrand factor-mediated platelet adhesion in vitro: Implications for circulatory devices. Blood 2015, 126, 2338–2341. [Google Scholar] [CrossRef]
- Van Der Meijden, P.E.J.; Van Schilfgaarde, M.; Van Oerle, R.; Renné, T.; ten Cate, H.; Spronk, H.M.H. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. JTH 2012, 10, 1355–1362. [Google Scholar] [CrossRef]
- Omar, H.R.; Mirsaeidi, M.; Socias, S.; Sprenker, C.; Caldeira, C.; Camporesi, E.M.; Mangar, D. Plasma Free Hemoglobin Is an Independent Predictor of Mortality among Patients on Extracorporeal Membrane Oxygenation Support. PLoS ONE 2015, 10, e0124034. [Google Scholar] [CrossRef]
- Hunt, B.J.; Parratt, R.N.; Segal, H.C.; Sheikh, S.; Kallis, P.; Yacoub, M. Activation of coagulation and fibrinolysis during cardiothoracic operations. Ann. Thorac. Surg. 1998, 65, 712–718. [Google Scholar] [CrossRef]
- Helms, J.; Curtiaud, A.; Severac, F.; Merdji, H.; Angles-Cano, E. Dynamic longitudinal increase in D-dimers: An early predictor of bleeding complications in ECMO. Intensive Care Med. 2023, 49, 1416–1417. [Google Scholar] [CrossRef]
- Gorog, D.A.; Price, S.; Sibbing, D.; Baumbach, A.; Capodanno, D.; Gigante, B.; Halvorsen, S.; Huber, K.; Lettino, M.; Leonardi, S.; et al. Antithrombotic therapy in patients with acute coronary syndrome complicated by cardiogenic shock or out-of-hospital cardiac arrest: A joint position paper from the European Society of Cardiology (ESC) Working Group on Thrombosis, in association with the Acute Cardiovascular Care Association (ACCA) and European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 125–140. [Google Scholar] [CrossRef]
- Vandenbriele, C.; Vanassche, T.; Price, S. Why we need safer anticoagulant strategies for patients on short-term percutaneous mechanical circulatory support. Intensive Care Med. 2020, 46, 771–774. [Google Scholar] [CrossRef]
- Baird, C.W.; Zurakowski, D.; Robinson, B.; Gandhi, S.; Burdis-Koch, L.; Tamblyn, J.; Munoz, R.; Fortich, K.; Pigula, F.A. Anticoagulation and pediatric extracorporeal membrane oxygenation: Impact of activated clotting time and heparin dose on survival. Ann. Thorac. Surg. 2007, 83, 912–919; discussion 919–920. [Google Scholar] [CrossRef]
- McMichael, A.B.V.; Ryerson, L.M.; Ratano, D.; Fan, E.; Faraoni, D.; Annich, G.M. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J. 2022, 68, 303–310. [Google Scholar] [CrossRef]
- Basu, D.; Gallus, A.; Hirsh, J.; Cade, J. A prospective study of the value of monitoring heparin treatment with the activated partial thromboplastin time. N. Engl. J. Med. 1972, 287, 324–327. [Google Scholar] [CrossRef]
- Aubron, C.; DePuydt, J.; Belon, F.; Bailey, M.; Schmidt, M.; Sheldrake, J.; Murphy, D.; Scheinkestel, C.; Cooper, D.J.; Capellier, G.; et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann. Intensive Care 2016, 6, 97. [Google Scholar] [CrossRef]
- Arnouk, S.; Altshuler, D.; Lewis, T.C.; Merchan, C.; Smith, D.E., 3rd; Toy, B.; Zakhary, B.; Papadopoulos, J. Evaluation of Anti-Xa and Activated Partial Thromboplastin Time Monitoring of Heparin in Adult Patients Receiving Extracorporeal Membrane Oxygenation Support. ASAIO J. 2020, 66, 300–306. [Google Scholar] [CrossRef]
- Raghunathan, V.; Liu, P.; Kohs, T.C.L.; Amirsoltani, R.; Oakes, M.; McCarty, O.J.T.; Olson, S.R.; Zonies, D.; Shatzel, J.J. Heparin Resistance Is Common in Patients Undergoing Extracorporeal Membrane Oxygenation but Is Not Associated with Worse Clinical Outcomes. ASAIO J. 2021, 67, 899–906. [Google Scholar] [CrossRef]
- Boer, C.; Meesters, M.I.; Milojevic, M.; Benedetto, U.; Bolliger, D.; von Heymann, C.; Jeppsson, A.; Koster, A.; Osnabrugge, R.L.; Ranucci, M.; et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2018, 32, 88–120. [Google Scholar] [CrossRef]
- Nair, P.; Hoechter, D.J.; Buscher, H.; Venkatesh, K.; Whittam, S.; Joseph, J.; Jansz, P. Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. J. Cardiothorac. Vasc. Anesth. 2015, 29, 288–296. [Google Scholar] [CrossRef]
- Durila, M.; Smetak, T.; Hedvicak, P.; Berousek, J. Extracorporeal membrane oxygenation-induced fibrinolysis detected by rotational thromboelastometry and treated by oxygenator exchange. Perfusion 2019, 34, 330–333. [Google Scholar] [CrossRef]
- Hellmann, C.; Schmutz, A.; Kalbhenn, J. Bleeding during veno-venous ECMO cannot reliably be predicted by rotational thrombelastometry (ROTEMTM). Perfusion 2018, 33, 289–296. [Google Scholar] [CrossRef]
- Panigada, M.; E Iapichino, G.; Brioni, M.; Panarello, G.; Protti, A.; Grasselli, G.; Occhipinti, G.; Novembrino, C.; Consonni, D.; Arcadipane, A.; et al. Thromboelastography-based anticoagulation management during extracorporeal membrane oxygenation: A safety and feasibility pilot study. Ann. Intensive Care 2018, 8, 7. [Google Scholar] [CrossRef]
- Bareille, M.; Hardy, M.; Douxfils, J.; Roullet, S.; Lasne, D.; Levy, J.H.; Stépanian, A.; Susen, S.; Frère, C.; Lecompte, T.; et al. Viscoelastometric Testing to Assess Hemostasis of COVID-19: A Systematic Review. J. Clin. Med. 2021, 10, 1740. [Google Scholar] [CrossRef]
- Arachchillage, D.R.J.; Laffan, M.; Khanna, S.; Vandenbriele, C.; Kamani, F.; Passariello, M.; Rosenberg, A.; Aw, T.C.; Banya, W.; Ledot, S.; et al. Frequency of Thrombocytopenia and Heparin-Induced Thrombocytopenia in Patients Receiving Extracorporeal Membrane Oxygenation Compared With Cardiopulmonary Bypass and the Limited Sensitivity of Pretest Probability Score. Crit. Care Med. 2020, 48, e371–e379. [Google Scholar] [CrossRef]
- Arachchillage, D.J.; Rajakaruna, I.; Scott, I.; Gaspar, M.; Odho, Z.; Banya, W.; Vlachou, A.; Isgro, G.; Cagova, L.; Wade, J.; et al. Impact of major bleeding and thrombosis on 180-day survival in patients with severe COVID-19 supported with veno-venous extracorporeal membrane oxygenation in the United Kingdom: A multicentre observational study. Br. J. Haematol. 2022, 196, 566–576. [Google Scholar] [CrossRef]
- Ranucci, M.; Colella, D.; Baryshnikova, E.; Di Dedda, U. Effect of preoperative P2Y12 and thrombin platelet receptor inhibition on bleeding after cardiac surgery. Br. J. Anaesth. 2014, 113, 970–976. [Google Scholar] [CrossRef]
- Van Poucke, S.; Stevens, K.; Kicken, C.; Simons, A.; Marcus, A.; Lancé, M. Platelet Function During Hypothermia in Experimental Mock Circulation. Artif. Organs 2016, 40, 288–293. [Google Scholar] [CrossRef]
- Sniderman, J.; Monagle, P.; Annich, G.M.; MacLaren, G. Hematologic concerns in extracorporeal membrane oxygenation. Res. Pract. Thromb. Haemost. 2020, 4, 455–468. [Google Scholar] [CrossRef]
- Balle, C.M.; Jeppesen, A.N.; Christensen, S.; Hvas, A.-M. Platelet Function During Extracorporeal Membrane Oxygenation in Adult Patients: A Systematic Review. Front. Cardiovasc. Med. 2018, 5, 157. [Google Scholar] [CrossRef]
- Panigada, M.; Spinelli, E.; De Falco, S.; Consonni, D.; Novembrino, C.; Boscolo Anzoletti, M.; Panarello, G.; Occhipinti, G.; Dos Santos, C.C.; Pesenti, A.; et al. The relationship between antithrombin administration and inflammation during veno-venous ECMO. Sci. Rep. 2022, 12, 14284. [Google Scholar] [CrossRef]
- Lorusso, R.; Gelsomino, S.; Parise, O.; Di Mauro, M.; Barili, F.; Geskes, G.; Vizzardi, E.; Rycus, P.T.; Muellenbach, R.; Mueller, T.; et al. Neurologic Injury in Adults Supported With Veno-Venous Extracorporeal Membrane Oxygenation for Respiratory Failure: Findings from the Extracorporeal Life Support Organization Database. Crit. Care Med. 2017, 45, 1389–1397. [Google Scholar] [CrossRef]
- Fletcher-Sandersjöö, A.; Bartek, J.J.; Thelin, E.P.; Eriksson, A.; Elmi-Terander, A.; Broman, M.; Bellander, B.-M. Predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: An observational cohort study. J. Intensive Care 2017, 5, 27. [Google Scholar] [CrossRef]
- Kurihara, C.; Walter, J.M.; Karim, A.; Thakkar, S.; Saine, M.; Odell, D.D.; Kim, S.; Tomic, R.; Wunderink, R.G.; Budinger, G.R.S.; et al. Feasibility of Venovenous Extracorporeal Membrane Oxygenation without Systemic Anticoagulation. Ann. Thorac. Surg. 2020, 110, 1209–1215. [Google Scholar] [CrossRef]
- Wood, K.L.; Ayers, B.; Gosev, I.; Kumar, N.; Melvin, A.L.; Barrus, B.; Prasad, S. Venoarterial-Extracorporeal Membrane Oxygenation Without Routine Systemic Anticoagulation Decreases Adverse Events. Ann. Thorac. Surg. 2020, 109, 1458–1466. [Google Scholar] [CrossRef]
- Byun, J.-H.; Jang, I.-S.; Kim, J.W.; Koh, E.-H. Establishing the heparin therapeutic range using aPTT and anti-Xa measurements for monitoring unfractionated heparin therapy. Blood Res. 2016, 51, 171–174. [Google Scholar] [CrossRef]
- Aubron, C.; McQuilten, Z.; Bailey, M.; Board, J.; Buhr, H.; Cartwright, B.; Dennis, M.; Hodgson, C.; Forrest, P.; McIlroy, D.; et al. Low-Dose Versus Therapeutic Anticoagulation in Patients on Extracorporeal Membrane Oxygenation: A Pilot Randomized Trial. Crit. Care Med. 2019, 47, e563–e571. [Google Scholar] [CrossRef]
- Song, K.; Kim, J.B. Safety of low-dose anticoagulation in extracorporeal membrane oxygenation using the Permanent Life Support System: A retrospective observational study. J. Yeungnam Med. Sci. 2023, 40, 276–282. [Google Scholar] [CrossRef]
- Lv, X.; Deng, M.; Wang, L.; Dong, Y.; Chen, L.; Dai, X. Low vs standardized dose anticoagulation regimens for extracorporeal membrane oxygenation: A meta-analysis. PLoS ONE 2021, 16, e0249854. [Google Scholar] [CrossRef]
- Rajsic, S.; Treml, B.; Jadzic, D.; Breitkopf, R.; Oberleitner, C.; Bachler, M.; Bösch, J.; Bukumiric, Z. aPTT-guided anticoagulation monitoring during ECMO support: A systematic review and meta-analysis. J. Crit. Care 2023, 77, 154332. [Google Scholar] [CrossRef]
- Olson, S.R.; Murphree, C.R.; Zonies, D.; Meyer, A.D.; Mccarty, O.J.T.; Deloughery, T.G.; Shatzel, J.J. Thrombosis and Bleeding in Extracorporeal Membrane Oxygenation (ECMO) Without Anticoagulation: A Systematic Review. ASAIO J. 2021, 67, 290–296. [Google Scholar] [CrossRef]
- Esper, S.A.; Welsby, I.J.; Subramaniam, K.; John Wallisch, W.; Levy, J.H.; Waters, J.H.; Triulzi, D.J.; Hayanga, J.W.A.; Schears, G.J. Adult extracorporeal membrane oxygenation: An international survey of transfusion and anticoagulation techniques. Vox Sang. 2017, 112, 443–452. [Google Scholar] [CrossRef]
- Dornia, C.; Philipp, A.; Bauer, S.; Stroszczynski, C.; Schreyer, A.G.; Müller, T.; Koehl, G.E.; Lehle, K. D-dimers Are a Predictor of Clot Volume Inside Membrane Oxygenators During Extracorporeal Membrane Oxygenation. Artif. Organs 2015, 39, 782–787. [Google Scholar] [CrossRef]
- Favaloro, E.J. Utility of the platelet function analyser (PFA-100/200) for exclusion or detection of von Willebrand disease: A study 22 years in the making. Thromb. Res. 2020, 188, 17–24. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, S.; Hilton, T.; Tang, L.; Cruz, M.; Hernandez, R.; Moake, J.L.; Tian, Q.; Frazier, O.H.; Dong, J.-F.; et al. Quantification of Von Willebrand Factor Cleavage by adamts-13 in Patients Supported by Left Ventricular Assist Devices. ASAIO J. 2017, 63, 849–853. [Google Scholar] [CrossRef]
- Gorog, D.A.; Lip, G.Y.H. Impaired Spontaneous/Endogenous Fibrinolytic Status as New Cardiovascular Risk Factor?: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 1366–1375. [Google Scholar] [CrossRef]
- Spinthakis, N.; Gue, Y.; Farag, M.; Ren, G.; Srinivasan, M.; Baydoun, A.; Gorog, D.A. Impaired endogenous fibrinolysis at high shear using a point-of-care test in STEMI is associated with alterations in clot architecture. J. Thromb. Thrombolysis 2019, 47, 392–395. [Google Scholar] [CrossRef]
- Wexels, F.; Dahl, O.E.; Pripp, A.H.; Seljeflot, I. Thrombin Generation in Patients With Suspected Venous Thromboembolism. Clin. Appl. Thromb. /Hemost. 2017, 23, 416–421. [Google Scholar] [CrossRef]
- Mazzeffi, M.; Strauss, E.; Meyer, M.; Hasan, S.; Judd, M.; Abuelkasem, E.; Chow, J.; Nandwani, V.; McCarthy, P.; Tanaka, K. Coagulation Factor Levels and Underlying Thrombin Generation Patterns in Adult Extracorporeal Membrane Oxygenation Patients. Anesth. Analg. 2019, 129, 659–666. [Google Scholar] [CrossRef]
- Scaravilli, V.; Fumagalli, J.; Rosso, L.; Polli, F.; Panigada, M.; Abbruzzese, C.; Crotti, S.; Lissoni, A.; Nosotti, M.; Pesenti, A.; et al. Heparin-Free Lung Transplantation on Venovenous Extracorporeal Membrane Oxygenation Bridge. ASAIO J. 2021, 67, e191–e197. [Google Scholar] [CrossRef]
| Test | Advantages | Disadvantages | Normal Range |
|---|---|---|---|
| ACT |
|
| 70–180 s |
| aPTT |
|
| 21–35 s |
| Anti-Xa |
|
| <0.1 U/mL |
| Viscoelastic assays |
|
| * CT: 38–79 s CFT:34–159 s A10: 43–65 mm MCF: 50–72 mm LI 60: ≥60% |
| Test | Goals | Management |
|---|---|---|
| aPTT | 1.5–2 patient’s baseline | Heparin range change 10–20% |
| Anti-Xa | 0.3–0.7 U/Ml | Heparin range change 10–20% |
| Hemoglobin | >7–9 g/dL | RBC 10 mL/kg |
| Fibrinogen | >1.5–2 g/L | Cryoprecipitate 1 unit/5 kg |
| Platelets | ≥100,000 × 109/L in a bleeding patient ≥50,000 × 109/L in a bleeding patient | Platelets 10 mL/kg |
| Antithrombin (AT) | >50–80%, if anticoagulation goals cannot be achieved by maximum UFH dose | Antithrombin concentrate Desired AT − Current AT × weight (kg)/1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frantzeskaki, F.; Konstantonis, D.; Rizos, M.; Kitsinelis, V.; Skyllas, G.; Renieris, I.; Doumani, M.; Kolias, V.; Kefalidi, E.; Angouras, D.; et al. Extracorporeal Membrane Oxygenation (ECMO)-Associated Coagulopathy in Adults. Diagnostics 2023, 13, 3496. https://doi.org/10.3390/diagnostics13233496
Frantzeskaki F, Konstantonis D, Rizos M, Kitsinelis V, Skyllas G, Renieris I, Doumani M, Kolias V, Kefalidi E, Angouras D, et al. Extracorporeal Membrane Oxygenation (ECMO)-Associated Coagulopathy in Adults. Diagnostics. 2023; 13(23):3496. https://doi.org/10.3390/diagnostics13233496
Chicago/Turabian StyleFrantzeskaki, Frantzeska, Dimitrios Konstantonis, Michail Rizos, Vasileios Kitsinelis, Georgios Skyllas, Ioannis Renieris, Maria Doumani, Vasileios Kolias, Eirini Kefalidi, Dimitrios Angouras, and et al. 2023. "Extracorporeal Membrane Oxygenation (ECMO)-Associated Coagulopathy in Adults" Diagnostics 13, no. 23: 3496. https://doi.org/10.3390/diagnostics13233496
APA StyleFrantzeskaki, F., Konstantonis, D., Rizos, M., Kitsinelis, V., Skyllas, G., Renieris, I., Doumani, M., Kolias, V., Kefalidi, E., Angouras, D., Tsantes, A., & Tsangaris, I. (2023). Extracorporeal Membrane Oxygenation (ECMO)-Associated Coagulopathy in Adults. Diagnostics, 13(23), 3496. https://doi.org/10.3390/diagnostics13233496








