Inflammation and Venous Thromboembolism in Hospitalized Patients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Extraction
2.2. Statistical Analysis
2.2.1. Propensity Score Matching and Statistical Analysis
2.2.2. Logistic Regression Using Python and Machine Learning Algorithmic Analysis
3. Results
3.1. Study Population Characteristics
3.2. Inflammatory Markers in Patients with and without VTE
3.3. Disease Severity and Outcome in Patients with and without VTE
3.4. Association of Inflammation Markers and Outcomes in the VTE Group of Patients
3.4.1. Univariate Logistic Regression Analysis. Inflammation and Outcomes in Patients with VTE
3.4.2. Multivariate Logistic Regression Analysis Using Machine Learning (ML)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, L.-L.; Wang, Y.-M.; Wu, Z.-Q.; Xiang, Z.-C.; Guo, L.; Xu, T.; Jiang, Y.-Z.; Xiong, Y.; Li, Y.-J.; Li, X.-W.; et al. Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. Chin. Med. J. 2020, 133, 1015–1024. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 22 July 2023).
- Azer, S.A. COVID-19: Pathophysiology, diagnosis, complications and investigational therapeutics. New Microbes New Infect. 2020, 37, 100738. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-L.; Lu, K.-C.; Chan, C.-Y.; Chao, Y.-C. COVID-19 and the lungs: A review. J. Infect. Public Health 2021, 14, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Jin, X.; Lian, J.-S.; Hu, J.-H.; Gao, J.; Zheng, L.; Zhang, Y.-M.; Hao, S.-R.; Jia, H.-Y.; Cai, H.; Zhang, X.-L.; et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020, 69, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, D.; Sperhake, J.-P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Zeng, H.; Ma, Y.; Zhou, Z.; Liu, W.; Huang, P.; Jiang, M.; Liu, Q.; Chen, P.; Luo, H.; Chen, Y. Spectrum and Clinical Characteristics of Symptomatic and Asymptomatic Coronavirus Disease 2019 (COVID-19) with and without Pneumonia. Front. Med. 2021, 8, 645651. [Google Scholar] [CrossRef]
- Mendiola-Pastrana, I.R.; López-Ortiz, E.; Río de la Loza-Zamora, J.G.; González, J. SARS-CoV-2 Variants and Clinical Outcomes: A Systematic Review. Life 2022, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Hong, R.; Zhao, H.; Wang, Y.; Chen, Y.; Cai, H.; Hu, Y.; Wei, G.; Huang, H. Clinical characterization and risk factors associated with cytokine release syndrome induced by COVID-19 and chimeric antigen receptor T-cell therapy. Bone Marrow Transplant. 2021, 56, 570–580. [Google Scholar] [CrossRef]
- Kox, M.; Waalders, N.J.B.; Kooistra, E.J.; Gerretsen, J.; Pickkers, P. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. JAMA 2020, 324, 1565–1567. [Google Scholar] [CrossRef]
- Chen, L.Y.C.; Quach, T.T.T. COVID-19 cytokine storm syndrome: A threshold concept. Lancet Microbe 2021, 2, e49–e50. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. eBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Bruin, S.d.; Bos, L.D.; Roon, M.A.v.; Boer, A.M.T.-d.; Schuurman, A.R.; Koel-Simmelinck, M.J.A.; Bogaard, H.J.; Tuinman, P.R.; Agtmael, M.A.v.; Hamann, J.; et al. Clinical features and prognostic factors in Covid-19: A prospective cohort study. eBioMedicine 2021, 67, 103378. [Google Scholar] [CrossRef]
- Aljohani, F.D.; Khattab, A.; Elbadawy, H.M.; Alhaddad, A.; Alahmadey, Z.; Alahmadi, Y.; Eltahir, H.M.; Matar, H.M.H.; Wanas, H. Prognostic factors for predicting severity and mortality in hospitalized COVID-19 patients. J. Clin. Lab. Anal. 2022, 36, e24216. [Google Scholar] [CrossRef]
- Zong, X.; Gu, Y.; Yu, H.; Li, Z.; Wang, Y. Thrombocytopenia Is Associated with COVID-19 Severity and Outcome: An Updated Meta-Analysis of 5637 Patients with Multiple Outcomes. Lab. Med. 2021, 52, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Lim, M.A.; Yonas, E.; Huang, I.; Nasution, S.A.; Setiati, S.; Alwi, I.; Kuswardhani, R.A.T. Thrombocytopenia as a prognostic marker in COVID-19 patients: Diagnostic test accuracy meta-analysis. Epidemiol. Infect. 2021, 149, E40. [Google Scholar] [CrossRef]
- Barrett, T.J.; Bilaloglu, S.; Cornwell, M.; Burgess, H.M.; Virginio, V.W.; Drenkova, K.; Ibrahim, H.; Yuriditsky, E.; Aphinyanaphongs, Y.; Lifshitz, M.; et al. Platelets contribute to disease severity in COVID-19. J. Thromb. Haemost. 2021, 19, 3139–3153. [Google Scholar] [CrossRef]
- Wu, C.; Lu, W.; Zhang, Y.; Zhang, G.; Shi, X.; Hisada, Y.; Grover, S.P.; Zhang, X.; Li, L.; Xiang, B.; et al. Inflammasome Activation Triggers Blood Clotting and Host Death through Pyroptosis. Immunity 2019, 50, 1401–1411.e4. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zhou, H.; Chao, Y.; Xiong, S.; Zhong, J.; Chai, Z.; Yang, K.; Liu, Z. Bacteria-triggered tumor-specific thrombosis to enable potent photothermal immunotherapy of cancer. Sci. Adv. 2020, 6, eaba3546. [Google Scholar] [CrossRef]
- Angelini, D.E.; Kaatz, S.; Rosovsky, R.P.; Zon, R.L.; Pillai, S.; Robertson, W.E.; Elavalakanar, P.; Patell, R.; Khorana, A. COVID-19 and venous thromboembolism: A narrative review. Res. Pract. Thromb. Haemost. 2022, 6, e12666. [Google Scholar] [CrossRef]
- Poissy, J.; Goutay, J.; Caplan, M.; Parmentier, E.; Duburcq, T.; Lassalle, F.; Jeanpierre, E.; Rauch, A.; Labreuche, J.; Susen, S.; et al. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation 2020, 142, 184–186. [Google Scholar] [CrossRef]
- Tan, B.K.; Mainbourg, S.; Friggeri, A.; Bertoletti, L.; Douplat, M.; Dargaud, Y.; Grange, C.; Lobbes, H.; Provencher, S.; Lega, J.-C. Arterial and venous thromboembolism in COVID-19: A study-level meta-analysis. Thorax 2021, 76, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; McCarty, M. Thrombotic complications of COVID-19 may reflect an upregulation of endothelial tissue factor expression that is contingent on activation of endosomal NADPH oxidase. Open Heart 2020, 7, e001337. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Fraissé, M.; Logre, E.; Pajot, O.; Mentec, H.; Plantefève, G.; Contou, D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: A French monocenter retrospective study. Crit. Care 2020, 24, 275. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Bozza, P.T. Platelet-leukocyte interactions in COVID-19: Contributions to hypercoagulability, inflammation, and disease severity. Res. Pract. Thromb. Haemost. 2022, 6, e12709. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.B.; Garcia, D.; Crowther, M.; Savage, B.; Peress, S.; Chang, K.; Deitelzweig, S. Frequency of venous thromboembolism in 6513 patients with COVID-19: A retrospective study. Blood Adv. 2020, 4, 5373–5377. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Laukkanen, J.A. Incidence of venous and arterial thromboembolic complications in COVID-19: A systematic review and meta-analysis. Thromb. Res. 2020, 196, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.; García-Sanchez, A.; Rali, P.; Muriel, A.; Bikdeli, B.; Ruiz-Artacho, P.; Mao, R.L.; Rodríguez, C.; Hunt, B.J.; Monreal, M. Incidence of VTE and Bleeding Among Hospitalized Patients with Coronavirus Disease 2019: A Systematic Review and Meta-analysis. CHEST 2021, 159, 1182–1196. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Bonaca, M.P. Studying the coagulopathy of COVID-19. Lancet 2022, 399, 118–119. [Google Scholar] [CrossRef]
- Franquet, T.; Giménez, A.; Prats, R.; Rodríguez-Arias, J.M.; Rodríguez, C. Thrombotic microangiopathy of pulmonary tumors: A vascular cause of tree-in-bud pattern on CT. AJR Am. J. Roentgenol. 2002, 179, 897–899. [Google Scholar] [CrossRef]
- Eddy, R.L.; Sin, D.D. Computed Tomography Vascular Tree-in-Bud: A Novel Prognostic Imaging Biomarker in COVID-19? Am. J. Respir. Crit. Care Med. 2020, 202, 642–644. [Google Scholar] [CrossRef]
- An, Q.; Rahman, S. A Comprehensive Review on Machine Learning in Healthcare Industry: Classification, Restrictions, Opportunities and Challenges. Sensors 2023, 23, 4178. [Google Scholar] [CrossRef]
- Gangloff, C.; Rafi, S.; Bouzille, G.; Soulat, L.; Cuggia, M. Machine learning is the key to diagnose COVID-19: A proof-of-concept study. Sci. Rep. 2021, 11, 7166. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.L.; Abels, E.; McCaffrey, P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod. Pathol. 2021, 34, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Li, Y. Prognostic Assessment of COVID-19 in the Intensive Care Unit by Machine Learning Methods: Model Development and Validation. J. Med. Internet Res. 2020, 22, e23128. [Google Scholar] [CrossRef]
- Wendland, P.; Schmitt, V.; Zimmermann, J.; Häger, L.; Göpel, S.; Schenkel-Häger, C.; Kschischo, M. Machine learning models for predicting severe COVID-19 outcomes in hospitals. Inform. Med. Unlocked 2023, 37, 101188. [Google Scholar] [CrossRef]
- Sharifi-Kia, A.; Nahvijou, A.; Sheikhtaheri, A. Machine learning-based mortality prediction models for smoker COVID-19 patients. BMC Med. Inform. Decis. Mak. 2023, 23, 129. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.; Wells, M.; Ouyang, D.; Davis, T.; Kaufman, N.; Cheng, S.; Chugh, S. A Machine Learning Algorithm Predicts Duration of hospitalization in COVID-19 patients. Intell. -Based Med. 2021, 5, 100035. [Google Scholar] [CrossRef]
- Chung, M.; Bernheim, A. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef]
- Fang, Z.G.; Yang, S.Q.; Lv, C.X.; An, S.Y.; Wu, W. Application of a data-driven XGBoost model for the prediction of COVID-19 in the USA: A time-series study. BMJ Open 2022, 12, e056685. [Google Scholar] [CrossRef]
- Freund, Y.; Schapire, R.E. A Decision-Theoretic Generalization of On-Line Learning and an Application to Boosting. J. Comput. Syst. Sci. 1997, 55, 119–139. [Google Scholar] [CrossRef]
- Li, J.Y.; Wang, H.F.; Yin, P.; Li, D.; Wang, D.L.; Peng, P.; Wang, W.H.; Wang, L.; Yuan, X.W.; Xie, J.Y.; et al. Clinical characteristics and risk factors for symptomatic venous thromboembolism in hospitalized COVID-19 patients: A multicenter retrospective study. J. Thromb. Haemost. 2021, 19, 1038–1048. [Google Scholar] [CrossRef]
- Kollias, A.; Kyriakoulis, K.G.; Lagou, S.; Kontopantelis, E.; Stergiou, G.S.; Syrigos, K. Venous thromboembolism in COVID-19: A systematic review and meta-analysis. Vasc. Med. 2021, 26, 415–425. [Google Scholar] [CrossRef]
- Roberts, L.N.; Navaratnam, A.V.; Arya, R.; Briggs, T.W.R.; Gray, W.K. Venous thromboembolism in patients hospitalised with COVID-19 in England. Thromb. Res. 2022, 213, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Poor, H.D. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest 2021, 160, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of Coronavirus Disease 2019. Crit. Care Med. 2020, 48, 1358–1364. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, Z.; Hu, Y. COVID-19 and Venous Thromboembolism: From Pathological Mechanisms to Clinical Management. J. Pers. Med. 2021, 11, 1328. [Google Scholar] [CrossRef]
- Loo, J.; Spittle, D.A.; Newnham, M. COVID-19, immunothrombosis and venous thromboembolism: Biological mechanisms. Thorax 2021, 76, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zuo, Z.; Yang, D.; Luo, X.; Jiang, L.; Xia, Z.; Xiao, X.; Liu, J.; Ye, M.; Deng, M. Venous thromboembolic events in patients with COVID-19: A systematic review and meta-analysis. Age Ageing 2021, 50, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jehangir, Q.; Li, P.; Gudimella, D.; Mahale, P.; Lin, C.-H.; Apala, D.R.; Krishnamoorthy, G.; Halabi, A.R.; Patel, K.; et al. Venous thromboembolism in COVID-19 patients and prediction model: A multicenter cohort study. BMC Infect. Dis. 2022, 22, 462. [Google Scholar] [CrossRef] [PubMed]
- Iliadi, V.; Konstantinidou, I.; Aftzoglou, K.; Iliadis, S.; Konstantinidis, T.G.; Tsigalou, C. The Emerging Role of Neutrophils in the Pathogenesis of Thrombosis in COVID-19. Int. J. Mol. Sci. 2021, 22, 5368. [Google Scholar] [CrossRef]
- Zuo, Y.; Zuo, M.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Shi, H.; Woodard, W.; Lezak, S.P.; Lugogo, N.L.; Knight, J.S.; et al. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis 2021, 51, 446–453. [Google Scholar] [CrossRef]
- Illg, Z.; Muller, G.; Mueller, M.; Nippert, J.; Allen, B. Analysis of absolute lymphocyte count in patients with COVID-19. Am. J. Emerg. Med. 2021, 46, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; DuPont, A.; Larson, S.; Cash, B.; Farooq, A. Absolute lymphocyte count is a prognostic marker in Covid-19: A retrospective cohort review. Int. J. Lab. Hematol. 2020, 42, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Toori, K.U.; Qureshi, M.A.; Chaudhry, A.; Safdar, M.F. Neutrophil to lymphocyte ratio (NLR) in COVID-19: A cheap prognostic marker in a resource constraint setting. Pak. J. Med. Sci. 2021, 37, 1435–1439. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Lu, R.; Dai, M.; Shen, M.; Zhang, J.; Cui, Y.; Liu, B.; Lin, F.; Chen, L.; et al. Lipid metabolism changes in patients with severe COVID-19. Clin. Chim. Acta Int. J. Clin. Chem. 2021, 517, 66–73. [Google Scholar] [CrossRef]
- Ren, H.; Yang, Y.; Wang, F.; Yan, Y.; Shi, X.; Dong, K.; Yu, X.; Zhang, S. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc. Diabetol. 2020, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Lei, S.; Zhang, Q.; Zhong, Y.; Wu, S. Triglyceride/High-Density Lipoprotein Cholesterol Ratio is Associated with the Mortality of COVID-19: A Retrospective Study in China. Int. J. Gen. Med. 2022, 15, 985–996. [Google Scholar] [CrossRef] [PubMed]

| Total (N = 532) | VTE Group (N = 133) | Non-VTE Group (N = 399) | ||||
|---|---|---|---|---|---|---|
| (n) | (%) | (n) | (%) | (n) | (%) | |
| Demographics | ||||||
| Gender (male/female) | 298/234 | 56.0/44.0 | 76/57 | 57.1/42.9 | 222/177 | 55.6/44.4 |
| Age (mean: years) | 55.4 | - | 60.9 | - | 53.5 | - |
| BMI (mean: kg/m2) | 29.1 | - | 30.1 | - | 29.1 | - |
| Vaccination | 104 | 21.0 | 14 | 11.3 | 90 | 24.2 |
| Comorbidities—risk factors | ||||||
| AH | 136 | 25.6 | 66 | 49.6 | 70 | 17.5 |
| Dyslipidemia | 83 | 15.6 | 48 | 36.1 | 35 | 8.8 |
| DM | 39 | 7.3 | 24 | 18.0 | 15 | 3.8 |
| CAD | 40 | 7.5 | 15 | 11.3 | 25 | 6.3 |
| Thyroid disease | 61 | 12.2 | 10 | 7.9 | 51 | 13.5 |
| Pulmonary disease | 21 | 3.9 | 6 | 4.5 | 15 | 3.8 |
| Autoimmune disease | 33 | 6.2 | 6 | 4.5 | 27 | 6.8 |
| CKD | 13 | 2.4 | 2 | 1.5 | 11 | 2.8 |
| Cancer | 26 | 4.9 | 6 | 4.5 | 20 | 5.0 |
| Dementia | 12 | 2.4 | 2 | 1.6 | 10 | 2.5 |
| Smoking | 15 | 2.8 | 15 | 11.3 | 0 | 0 |
| Morbid obesity | 46 | 8.6 | 11 | 8.3 | 35 | 8.8 |
| Groups |
Total
(n = 574) |
VTE Group
(n =144) |
Non-VTE
(n =430) |
Mann–Whitney
Test | ||||
|---|---|---|---|---|---|---|---|---|
| Variables | Units | Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation | p-Value * |
| Neutrophils (count) | #/μL | 5355 | 3321 | 5664 | 3691 | 5252 | 3186 | 0.45 |
| Lymphocytes (count) | #/μL | 1219 | 2081 | 1452 | 3992 | 1141 | 657 | 0.93 |
| Platelet count | #/μL | 209,814 | 82,627 | 212,267 | 91,746 | 208,986 | 79,423 | 0.89 |
| Fibrinogen | mg/dL | 529.3 | 147.1 | 565.2 | 119.6 | 516.8 | 153.8 | 0.01 |
| D-dimers | μg/mL | 1.3 | 2.4 | 1.7 | 3.4 | 1.1 | 2.0 | 0.03 |
| aPTT | sec | 34.1 | 10.9 | 35.2 | 15.8 | 33.6 | 8.4 | 0.44 |
| Ferritin | ng/mL | 453.7 | 536.9 | 507.4 | 629.9 | 434.8 | 499.8 | 0.22 |
| LDH | IU/L | 325.4 | 132.2 | 346.0 | 128.1 | 318.5 | 133.0 | 0.01 |
| IL-6 | pg/mL | 51.2 | 132.4 | 51.3 | 90.7 | 51.1 | 146.3 | 0.91 |
| Procalcitonin | ng/mL | 0.3 | 1.2 | 0.4 | 1.4 | 0.2 | 1.2 | <0.01 |
| CRP | mg/L | 69.1 | 71.9 | 81.3 | 76.6 | 65.0 | 69.8 | <0.01 |
| T-C | mg/dL | 150.4 | 41.1 | 152.4 | 38.1 | 149.8 | 42.0 | 0.33 |
| TRG | mg/dL | 113.7 | 63.4 | 123.4 | 60.6 | 110.5 | 64.0 | <0.01 |
| HDL-C | mg/dL | 35.9 | 9.8 | 34.3 | 8.53 | 36.4 | 10.1 | 0.13 |
| LDL-C | mg/dL | 89.8 | 33.9 | 96.0 | 36.1 | 88.0 | 33.0 | 0.09 |
| Novel markers | ||||||||
|
Neut/Lymph
ratio | - | 6.2 | 6.1 | 6.2 | 5.7 | 6.2 | 6.3 | 0.43 |
|
Neut/HDL-C
ratio | - | 162.4 | 172.7 | 167.9 | 113.1 | 160.7 | 187.5 | 0.39 |
|
Lymph/HDL-C
ratio | - | 36.4 | 91.8 | 54.3 | 185.2 | 30.9 | 19.1 | 0.25 |
|
TRG/HDL-C
ratio | - | 3.4 | 2.3 | 3.7 | 2.0 | 3.3 | 2.4 | 0.01 |
|
CRP/HDL-C
ratio | - | 2.1 | 2.4 | 2.3 | 2.0 | 2.1 | 2.5 | 0.05 |
| TyG index | - | 8.7 | 0.6 | 8.8 | 0.6 | 8.6 | 0.5 | 0.10 |
| Groups |
Total
(n = 532) |
VTE Group
(n =133) |
Non-VTE Group
(n =399) |
Mann–Whitney
Test | ||||
|---|---|---|---|---|---|---|---|---|
| Variables | Units | Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation | p-Value * |
| Duration of symptoms | days | 7.0 | 4.6 | 8.1 | 5.8 | 6.6 | 4.1 | 0.02 |
| PFR | - | 281.4 | 115.6 | 263.2 | 100.9 | 287.7 | 119.8 | 0.02 |
| CTBoD | % | 52.6 | 24.8 | 61.1 | 19.6 | 48.0 | 26.1 | <0.01 |
| Days to death | days | 23.8 | 16.5 | 26.3 | 17.8 | 21.4 | 15.2 | 0.49 |
| Days of hospitalization | days | 11.1 | 9.2 | 14.2 | 10.5 | 10.1 | 8.4 | <0.01 |
| Outcomes | - | (n) | (%) | (n) | (%) | (n) | (%) | - |
| LoS (>7) | days | 307 | 58.1 | 98 | 73.7 | 209 | 52.4 | - |
| Intubation | - | 34 | 6.4 | 15 | 11.3 | 19 | 4.8 | - |
| Death | - | 34 | 6.4 | 17 | 12.8 | 17 | 4.3 | - |
| VTE Group | LoS > 7 Days | Intubation | Death | |||
|---|---|---|---|---|---|---|
| OR | p-Value | OR | p-Value | OR | p-Value | |
| Age | 1.01 | 0.38 | 1.03 | 0.06 | 1.05 | 0.01 |
| BMI | 1.03 | 0.57 | 0.85 | 0.16 | 0.85 | 0.16 |
| Duration of symptoms | 1.06 | 0.14 | 1.07 | 0.11 | 1.05 | 0.19 |
| Neutrophil count | 1.00 | 0.87 | 1.00 | 0.26 | 1.00 | 0.28 |
| Lymphocyte count | 1.00 | 0.85 | 1.00 | 0.59 | 1.00 | 0.54 |
| Platelet count | 1.00 | 0.96 | 1.00 | 0.89 | 1.00 | 0.99 |
| Leukocytosis | 0.65 | 0.40 | 0.79 | 0.77 | 0.67 | 0.61 |
| Lymphocytopenia | 3.08 | 0.01 | 3.81 | 0.02 | 3.35 | 0.03 |
| Thrombocytopenia | 1.38 | 0.52 | 0.22 | 0.15 | 0.20 | 0.13 |
| Neut/Lymph ratio | 1.08 | 0.10 | 1.10 | 0.01 | 1.09 | 0.01 |
| Neut/Lymph ratio > 3.1 | 0.73 | 0.52 | 5.33 | 0.11 | 6.24 | 0.08 |
| Fibrinogen | 1.00 | 0.99 | 1.00 | 0.48 | 1.00 | 0.41 |
| Fibrinogen > 600 | 0.90 | 0.87 | 1.26 | 0.75 | 1.66 | 0.46 |
| D-dimers | 0.96 | 0.51 | 1.07 | 0.28 | 1.06 | 0.30 |
| D-dimers > 2 | 0.32 | 0.04 | 2.93 | 0.10 | 2.63 | 0.14 |
| LDH | 1.00 | 0.23 | 1.00 | 0.06 | 1.00 | 0.14 |
| LDH > 230 | 1.11 | 0.84 | 0.56 | 0.42 | 0.20 | 0.20 |
| aPTT | 1.01 | 0.64 | 0.99 | 0.97 | 0.99 | 0.78 |
| IL-6 | 0.99 | 0.12 | 1.00 | 0.56 | 1.00 | 0.18 |
| IL-6 > 24 | 1.48 | 0.41 | 0.71 | 0.64 | 0.70 | 0.60 |
| Ferritin | 1.00 | 0.60 | 1.00 | 0.17 | 1.00 | 0.24 |
| Ferritin > 335 | 0.94 | 0.90 | 0.79 | 0.72 | 0.82 | 0.74 |
| Procalcitonin | 0.88 | 0.45 | 0.89 | 0.79 | 0.97 | 0.93 |
| Procalcitonin > 0.5 | 0.82 | 0.70 | 1.29 | 0.70 | 1.66 | 0.42 |
| CRP | 1.00 | 0.32 | 1.00 | 0.62 | 1.00 | 0.55 |
| CRP > 100 | 1.18 | 0.70 | 0.75 | 0.65 | 0.87 | 0.82 |
| PFR | 0.99 | 0.13 | 0.99 | 0.05 | 0.99 | 0.12 |
| PFR < 150 | 4.16 | 0.06 | 3.04 | 0.10 | 2.32 | 0.19 |
| PFR < 300 | 1.10 | 0.80 | 3.64 | 0.10 | 2.68 | 0.14 |
| CTBoD > 50% | 5.65 | <0.01 | 12.29 | 0.01 | 13.48 | 0.01 |
| Troponin | 0.99 | 0.42 | 1.01 | 0.06 | 1.00 | 0.07 |
| TRG/HDL-C ratio | 1.28 | 0.14 | 1.24 | 0.11 | 1.12 | 0.39 |
| TRG/HDL-C ratio > 2.5 | 2.38 | 0.11 | 3.11 | 0.29 | 0.93 | 0.93 |
| CRP/HDL-C ratio | 1.08 | 0.52 | 0.79 | 0.29 | 0.80 | 0.27 |
| TyG index | 1.18 | 0.69 | 2.55 | 0.06 | 1.77 | 0.22 |
| 8.7 < TyG < 9.1 | 1.85 | 0.35 | 0.76 | 0.82 | 0.35 | 0.37 |
| TyG > 9.1 | 0.70 | 0.61 | 6.57 | 0.09 | 6.57 | 0.09 |
| TyG > 9.1 (compared to <8.7) | 1.29 | 0.66 | 5.00 | 0.06 | 2.35 | 0.22 |
| VTE Group | Intubation | VTE Group | Death | ||
|---|---|---|---|---|---|
| OR | p-Value | OR | p-Value | ||
| Gender | 1.15 | 0.89 | Gender | 1.33 | 0.69 |
| Age | 1.05 | 0.15 | Age | 1.06 | 0.07 |
| BMI | 0.75 | 0.02 | BMI | 1.01 | 0.89 |
| Duration of symptoms | 1.08 | 0.24 | Duration of symptoms | 1.74 | 0.26 |
| Lymphocytopenia | 4.12 | 0.19 | Lymphocytopenia | 4.19 | 0.08 |
| Neut/Lymph ratio > 3.1 | 1.10 | 0.28 | Neut/Lymp ratio | 1.31 | 0.82 |
| D-dimers > 2 | 19.15 | 0.01 | D-dimers > 2 | 0.52 | 0.94 |
| Procalcitonin > 0.5 | 1.01 | 0.06 | CRP | 5.95 | 0.01 |
| PFR < 300 | 0.99 | 0.57 | PFR | 1.70 | 0.49 |
| CTBoD > 50% | 3.86 | 0.30 | CTBoD > 50% | 6.31 | 0.05 |
| TyG > 9.1 | 0.06 | 0.06 | TRG/HDL-C > 2.5 | 0.91 | 0.91 |
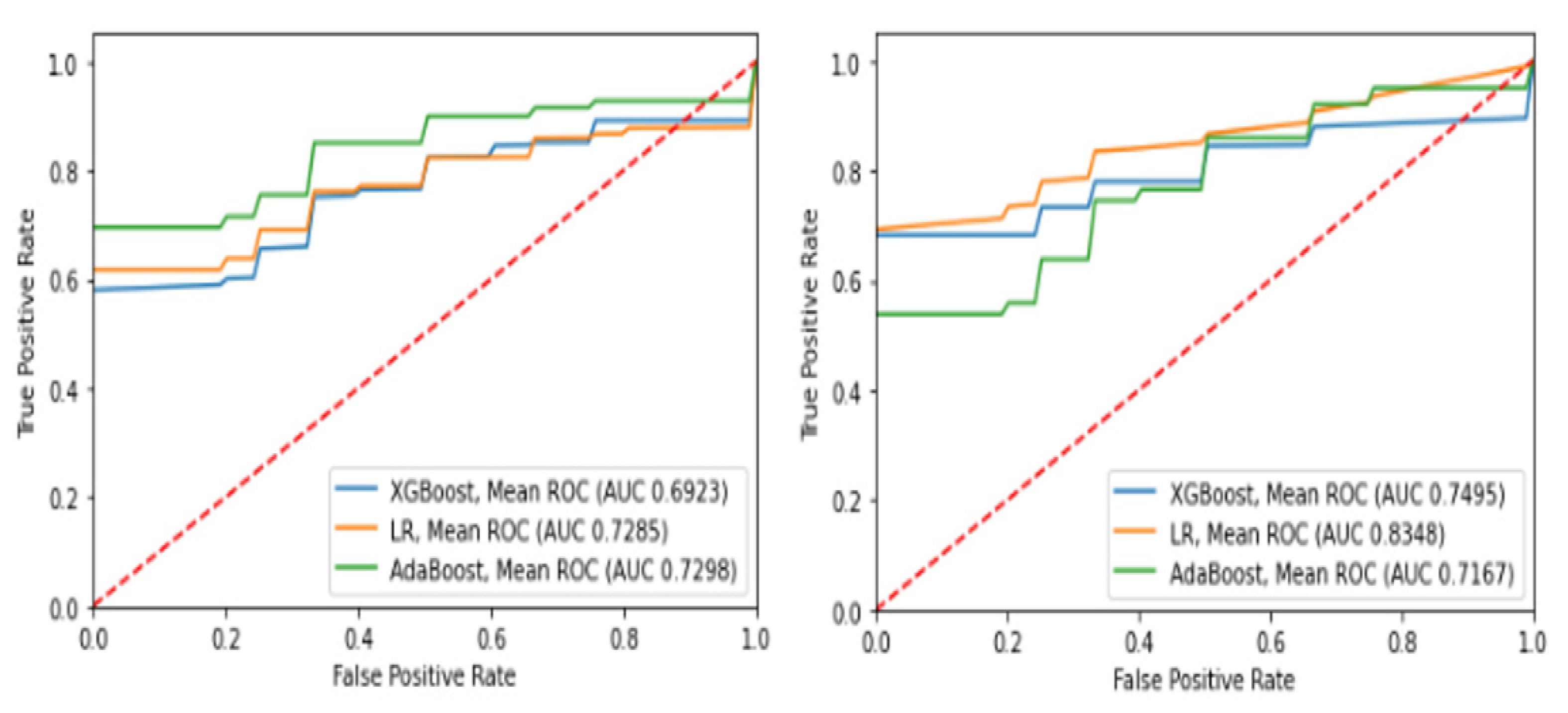
| Performance Metrics | Intubation | Death | ||||
|---|---|---|---|---|---|---|
| XGBoost | LR | AdaBoost | XGBoost | LR | AdaBoost | |
| Accuracy | 0.73 | 0.82 | 0.70 | 0.69 | 0.72 | 0.71 |
| Sensitivity | 0.73 | 0.80 | 0.66 | 0.71 | 0.70 | 0.75 |
| Specificity | 0.76 | 0.86 | 0.76 | 0.67 | 0.75 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liontos, A.; Biros, D.; Matzaras, R.; Tsarapatsani, K.-H.; Kolios, N.-G.; Zarachi, A.; Tatsis, K.; Pappa, C.; Nasiou, M.; Pargana, E.; et al. Inflammation and Venous Thromboembolism in Hospitalized Patients with COVID-19. Diagnostics 2023, 13, 3477. https://doi.org/10.3390/diagnostics13223477
Liontos A, Biros D, Matzaras R, Tsarapatsani K-H, Kolios N-G, Zarachi A, Tatsis K, Pappa C, Nasiou M, Pargana E, et al. Inflammation and Venous Thromboembolism in Hospitalized Patients with COVID-19. Diagnostics. 2023; 13(22):3477. https://doi.org/10.3390/diagnostics13223477
Chicago/Turabian StyleLiontos, Angelos, Dimitrios Biros, Rafail Matzaras, Konstantina-Helen Tsarapatsani, Nikolaos-Gavriel Kolios, Athina Zarachi, Konstantinos Tatsis, Christiana Pappa, Maria Nasiou, Eleni Pargana, and et al. 2023. "Inflammation and Venous Thromboembolism in Hospitalized Patients with COVID-19" Diagnostics 13, no. 22: 3477. https://doi.org/10.3390/diagnostics13223477
APA StyleLiontos, A., Biros, D., Matzaras, R., Tsarapatsani, K.-H., Kolios, N.-G., Zarachi, A., Tatsis, K., Pappa, C., Nasiou, M., Pargana, E., Tsiakas, I., Lymperatou, D., Filippas-Ntekouan, S., Athanasiou, L., Samanidou, V., Konstantopoulou, R., Vagias, I., Panteli, A., Milionis, H., & Christaki, E. (2023). Inflammation and Venous Thromboembolism in Hospitalized Patients with COVID-19. Diagnostics, 13(22), 3477. https://doi.org/10.3390/diagnostics13223477










