Perilipin1 Expression as a Prognostic Factor in Patients with Squamous Cell Carcinoma of the Lung
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinicopathological Data
2.2. Tissue Microarray Construction
2.3. Immunohistochemistry
2.4. PLIN1 Expression
2.5. Statistical Analysis
3. Results
3.1. Clinicopathological Patient Data
3.2. Relationship between Perilipin1 Expression and Clinicopathological Characteristics of Tumor Cells
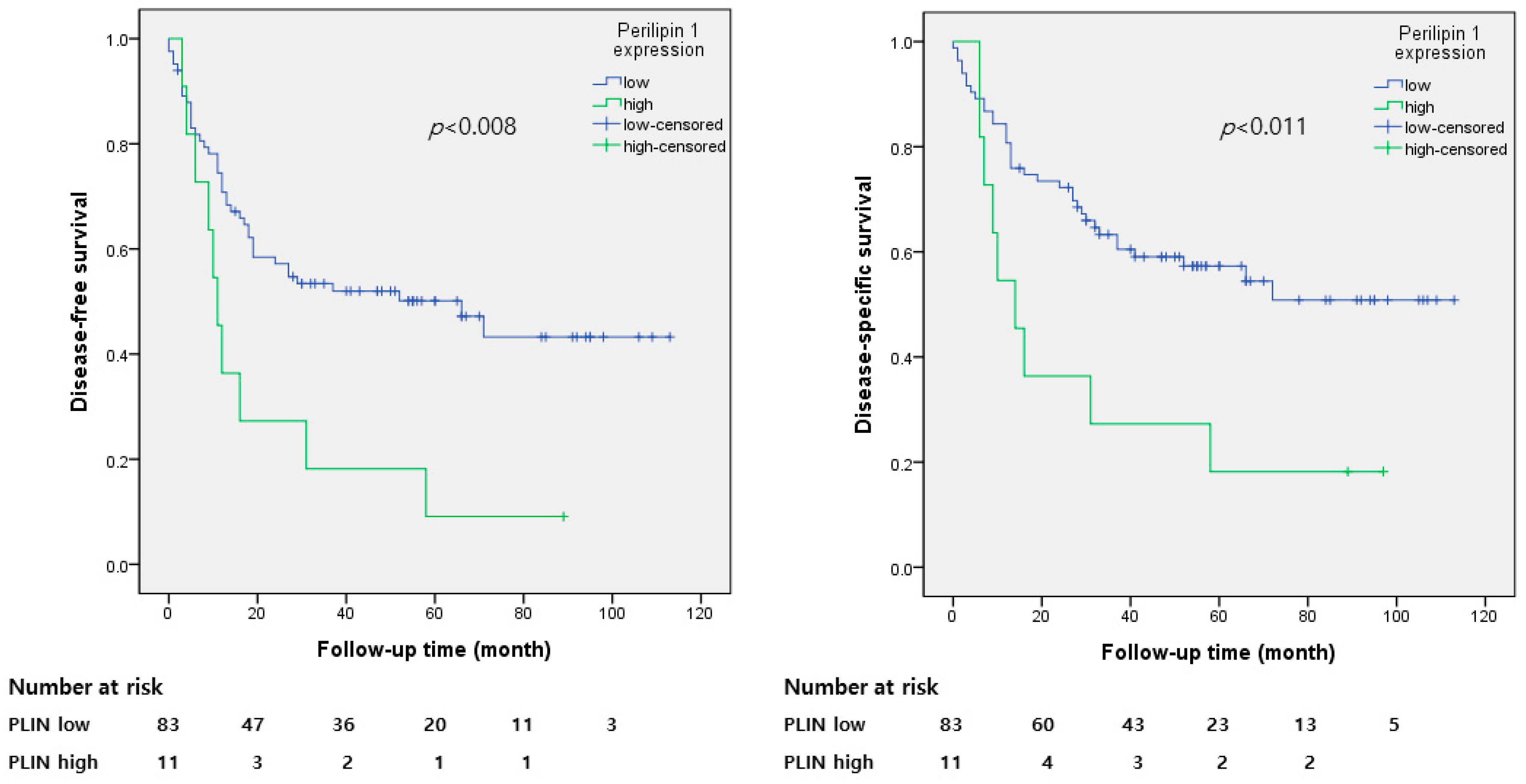
3.3. Perilipin 1 Expression and Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Sabbula, B.R.; Gasalberti, D.P.; Anjum, F. Squamous Cell Lung Cancer; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yang, Q.F.; Wu, D.; Wang, J.; Ba, L.; Tian, C.; Liu, Y.T.; Hu, Y.; Liu, L. Development and validation of an individualized immune prognostic model in stage I–III lung squamous cell carcinoma. Sci. Rep. 2021, 11, 12727. [Google Scholar] [CrossRef]
- Itabe, H.; Yamaguchi, T.; Nimura, S.; Sasabe, N. Perilipins: A diversity of intracellular lipid droplet proteins. Lipids Health Dis. 2017, 16, 83. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, L.; Song, L.; Du, J.; Du, S.; Cui, W.; Liu, C.; Li, F. Roles of perilipins in diseases and cancers. Curr. Genom. 2018, 19, 247–257. [Google Scholar] [CrossRef]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, e20201606. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the wheels of the cancer machine: The role of lipid metabolism in cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, H.; Ren, L.; Lu, Z.; Zheng, Q.; Ding, L.; Xie, H.; Wang, R.; Yu, C.; Lin, Y.; et al. Adding fuel to the fire: The lipid droplet and its associated proteins in cancer progression. Int. J. Biol. Sci. 2022, 18, 6020–6034. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, M.; Zhou, L.; Zhang, Y.; Liu, W.; Qin, W.; He, R.; Lu, Y.; Wang, Y.; Chen, X.Z.; et al. Prognostic significance of PLIN1 expression in human breast cancer. Oncotarget 2016, 7, 54488–54502. [Google Scholar] [CrossRef]
- Wagner, M.; Bjerkvig, R.; Wiig, H.; Dudley, A.C. Loss of adipocyte specification and necrosis augment tumor-associated inflammation. Adipocyte 2013, 2, 176–183. [Google Scholar] [CrossRef]
- Kitahara, H.; Shoji, F.; Akamine, T.; Kinoshita, F.; Haratake, N.; Takenaka, T.; Tagawa, T.; Sonoda, T.; Shimokawa, M.; Maehara, Y.; et al. Preoperative prognostic nutritional index level is associated with tumour-infiltrating lymphocyte status in patients with surgically resected lung squamous cell carcinoma. Eur. J. Cardiothorac. Surg. 2021, 60, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeck, G.; Mendez-Gimenez, L.; Fernandez-Formoso, J.A.; Fernandez, S.; Rodriguez, A. Regulation of adipocyte lipolysis. Nutr. Res. Rev. 2014, 27, 63–93. [Google Scholar] [CrossRef] [PubMed]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Acta Mol. Cell Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef]
- Westhoff, C.C.; Mrozinski, J.; Riedel, I.; Heid, H.W.; Moll, R. Perilipin 1 is a highly specific marker for adipocytic differentiation in sarcomas with intermediate sensitivity. J. Cancer Res. Clin. Oncol. 2017, 143, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Ordovas, J.M. Update on perilipin polymorphisms and obesity. Nutr. Rev. 2012, 70, 611–621. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Fu, M.; Li, M.D.; Zhang, K.; Zhang, B.; Wang, S.; Liu, Y.; Ni, W.; Ong, Q.; Mi, J.; et al. O-GlcNAc transferase inhibits visceral fat lipolysis and promotes diet-induced obesity. Nat. Commun. 2020, 11, 181. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Pissas, G.; Arampatzis, S.; Sparopoulou, T.; Galaktidou, G.; Stefanidis, I. Perilipin-1 in hemodialyzed patients: Association with history of coronary heart disease and lipid profile. TherApher Dial. 2012, 16, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.M.; Dhir, R.; Mahadev, K.; Comerford, M.; Chalasani, N.P.; Ahima, R.S. Perilipin Staining Distinguishes between Steatosis and Nonalcoholic Steatohepatitis in Adults and Children. Clin. Gastroenterol. Hepatol. 2017, 15, 145–147. [Google Scholar] [CrossRef]
- Zhou, J.; Cui, S.; He, Q.; Guo, Y.; Pan, X.; Zhang, P.; Huang, N.; Ge, C.; Wang, G.; Gonzalez, F.J.; et al. SUMOylation inhibitors synergize with FXR agonists in combating liver fibrosis. Nat. Commun. 2020, 11, 240. [Google Scholar] [CrossRef]
- Savage, D.B. Perilipin 1 Antibodies in Patients with Acquired Generalized Lipodystrophy. Diabetes 2023, 72, 16–18. [Google Scholar] [CrossRef]
- Straub, B.K.; Witzel, H.R.; Pawella, L.M.; Renner, M.; Eiteneuer, E.; Hashani, M.; Schirmacher, P.; Roth, W.; Mechtersheimer, G. Perilipin 1 Expression Differentiates Liposarcoma from Other Types of Soft Tissue Sarcoma. Am. J. Pathol. 2019, 189, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, N.; Jalava, N.; Chen, Y.; Ivaska, K.K. Perilipin-1 immunostaining improves semi-automated digital quantitation of bone marrow adipocytes in histological bone sections. Adipocyte 2023, 12, 2252711. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Romero, C.; Carreon-Burciaga, R.; Gonzalez-Gonzalez, R.; Villarroel-Dorrego, M.; Molina-Frechero, N.; Bologna-Molina, R. Perilipin 1 and adipophilin immunoexpression suggests the presence of lipid droplets in tooth germ, ameloblastoma, and ameloblastic carcinoma. J. Oral Pathol. Med. 2021, 50, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Deneo-Pellegrini, H.; Ronco, A.L.; De Stefani, E. Meat consumption and risk of squamous cell carcinoma of the lung: A case-control study in Uruguayan men. Nutr. Cancer 2015, 67, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.K.; Cross, A.J.; Consonni, D.; Randi, G.; Bagnardi, V.; Bertazzi, P.A.; Caporaso, N.E.; Sinha, R.; Subar, A.F.; Landi, M.T. Intakes of red meat, processed meat, and meat mutagens increase lung cancer risk. Cancer Res. 2009, 69, 932–939. [Google Scholar] [CrossRef] [PubMed]
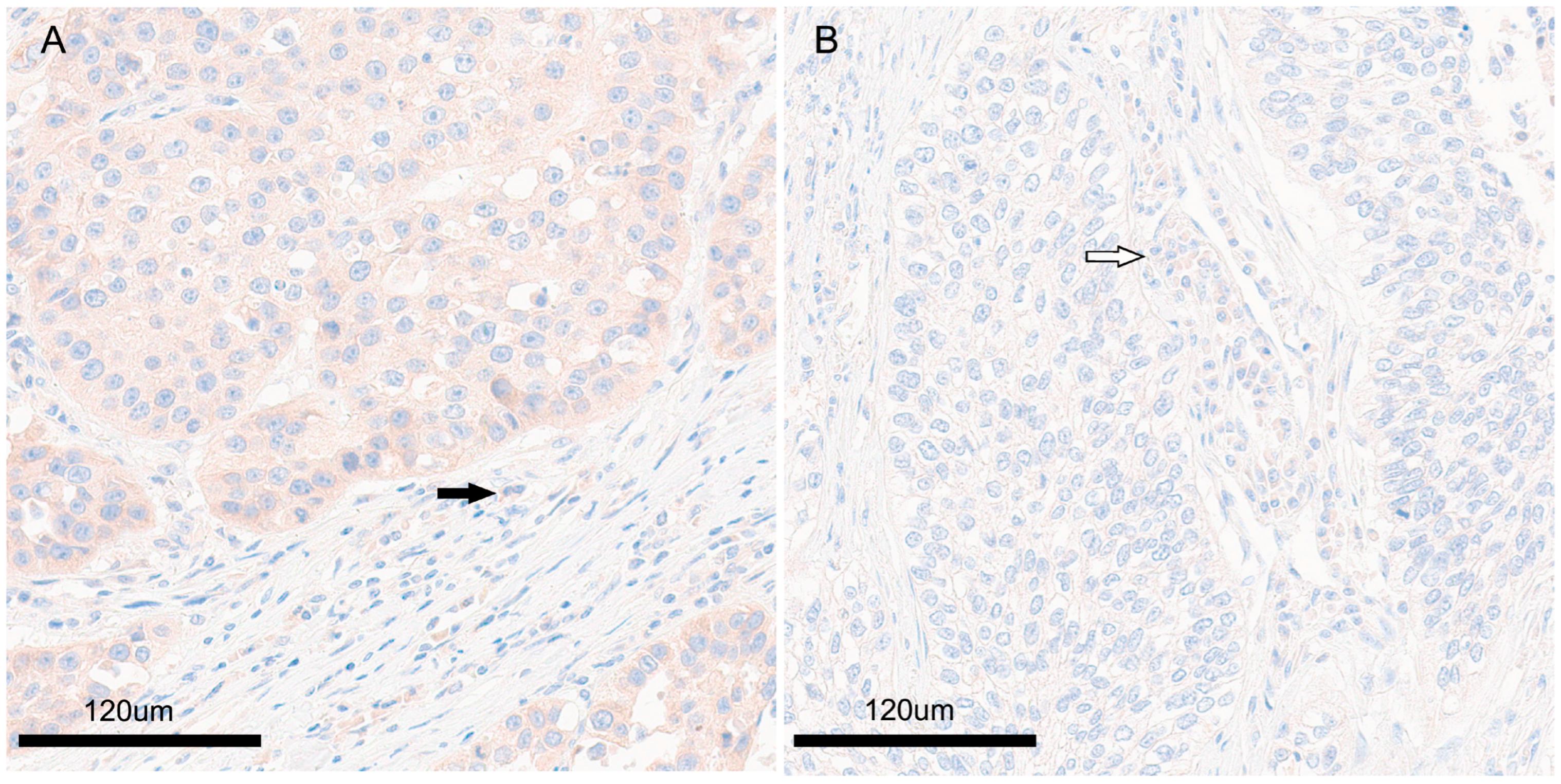
| Clinicopathological Data of the Patients | Number (%) (n = 94) | |
|---|---|---|
| Mean age, yr | 65.6 | |
| Male gender | 90 | (95.7) |
| Surgery | ||
| Lobectomy | 77 | (81.9) |
| Bilobectomy or sleeve | 3 | (3.2) |
| Pneumonectomy | 14 | (14.9) |
| T stage | ||
| T1a | 0 | (0) |
| T1b | 11 | (11.7) |
| T1c | 15 | (16.0) |
| T2a | 33 | (35.1) |
| T2b | 11 | (11.7) |
| T3 | 17 | (18.1) |
| T4 | 7 | (7.4) |
| N stage | ||
| n0 | 57 | (60.6) |
| n1 | 34 | (36.2) |
| n2 | 3 | (3.2) |
| Differentiation | ||
| W/D | 14 | (14.9) |
| M/D | 58 | (61.7) |
| P/D | 22 | (23.4) |
| Smoker, ex-smoker | 70 | (74.5) |
| Lymph node metastasis | ||
| n0 | 57 | (60.6) |
| n1 | 34 | (36.2) |
| n2 | 3 | (3.2) |
| Perilipin1 | ||
| Low | 83 | (88.3) |
| High | 11 | (11.7) |
| Factor | Perilipin1 | p-Value | ||
|---|---|---|---|---|
| Subgroup | Low | High | ||
| Age | <65 Years | 31 | 3 | 0.513 |
| ≥65 years | 52 | 8 | ||
| Sex | Male | 80 | 10 | 0.397 |
| Female | 3 | 1 | ||
| Smoking | Non-smoker | 22 | 2 | 0.552 |
| Smoker | 61 | 9 | ||
| Surgery | Lobectomy | 68 | 9 | 0.993 |
| Bilobectomy or Pneumonectomy | 15 | 2 | ||
| Histologic differentiation | W/D | 11 | 3 | 0.176 |
| M/D | 54 | 4 | ||
| P/D | 18 | 4 | ||
| Tumor stage | <2 | 24 | 2 | 0.455 |
| ≥2 | 59 | 9 | ||
| Lymph node metastasis | Absent | 52 | 5 | 0.273 |
| Present | 31 | 6 | ||
| Distant metastasis | Absent | 82 | 11 | 0.714 |
| Present | 1 | 0 | ||
| TNM | I | 32 | 3 | 0.467 |
| II, III, IV | 51 | 8 | ||
| Variables | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| DFS | DSS | DFS | DSS | |||||
| HR | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||
| Age, yr | 1.386 | 0.271 | 1.158 | 0.638 | ||||
| (<65 vs. ≥65) | (0.775–2.479) | (0.628–2.135) | ||||||
| Sex | 0.802 | 0.760 | 0.388 | 0.35 | ||||
| (male vs. female) | (0.195–3.298) | (0.053–2.823) | ||||||
| T stage | 1.205 | 0.562 | 1.512 | 0.25 | ||||
| (<2 vs. ≥2) | (0.642–2.261) | (0.748–3.057) | ||||||
| N stage | 1.033 | 0.910 | 1.12 | 0.711 | ||||
| (N0 vs. N1,2,3) | (0.590–1.807) | (0.616–2.034) | ||||||
| Smoking | 0.666 | 0.179 | 0.693 | 0.257 | ||||
| (non vs. current, ex-smoker) | (0.369–1.205) | (0.367–1.307) | ||||||
| Surgery | 1.509 | 0.216 | 1.432 | 0.319 | ||||
| (lobectomy vs. bilobectomy, pneumonectomy) | (0.786–2.895) | (0.707–2.903) | ||||||
| SCC differentiation | 2.263 | 0.006 | 2.188 | 0.017 | 2.098 | 0.013 | 1.952 | 0.037 |
| (W/D, M/D vs. P/D) | (1.267–4.039) | (1.183–4.045) | (1.167–3.774) | (1.040–3.664) | ||||
| Perilipin1 | 2.454 | 0.011 | 2.503 | 0.014 | 2.178 | 0.03 | 2.116 | 0.051 |
| (low vs. high) | (1.225–4.919) | (1.200–5.221) | (1.078–4.399) | (0.996–4.497) | ||||
| No. of Patient | Age | Sex | Smoking | Surgery | TNM Stage_8th | Pathologic Differentiation | Period of DFS (Month) | Recur | Period of DSS (Month) | Death for Lung Cancer | Chemo Tx. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | male | current, ex-smoker | lobectomy | IA3 | P/D | 3 | yes | 6 | yes | yes |
| 2 | 68 | female | current, ex-smoker | lobectomy | IIB | P/D | 11 | yes | 97 | no | NI |
| 3 | 72 | male | current, ex-smoker | lobectomy | IIB | M/D | 4 | yes | 7 | yes | no |
| 4 | 71 | male | current, ex-smoker | pneumonectomy | IIIA | M/D | 9 | yes | 9 | yes | no |
| 5 | 67 | male | Nonsmoker | bilobectomy or sleeve op | IIB | W/D | 6 | yes | 6 | yes | yes |
| 6 | 68 | male | current, ex-smoker | lobectomy | IIB | P/D | 12 | yes | 14 | yes | no |
| 7 | 63 | male | current, ex-smoker | lobectomy | IIA | W/D | 58 | yes | 58 | yes | no |
| 8 | 62 | male | current, ex-smoker | lobectomy | IIB | M/D | 16 | yes | 16 | yes | no |
| 9 | 69 | male | current, ex-smoker | lobectomy | IB | M/D | 89 | no | 89 | no | no |
| 10 | 67 | male | current, ex-smoker | lobectomy | IB | P/D | 31 | yes | 31 | yes | no |
| 11 | 60 | male | Nonsmoker | lobectomy | IIB | W/D | 10 | yes | 10 | yes | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.H.; Lee, J.H.; Lee, J.S.; Kim, D.C.; Yang, J.W.; An, H.J.; Na, J.M.; Jung, W.J.; Song, D.H. Perilipin1 Expression as a Prognostic Factor in Patients with Squamous Cell Carcinoma of the Lung. Diagnostics 2023, 13, 3475. https://doi.org/10.3390/diagnostics13223475
Kim MH, Lee JH, Lee JS, Kim DC, Yang JW, An HJ, Na JM, Jung WJ, Song DH. Perilipin1 Expression as a Prognostic Factor in Patients with Squamous Cell Carcinoma of the Lung. Diagnostics. 2023; 13(22):3475. https://doi.org/10.3390/diagnostics13223475
Chicago/Turabian StyleKim, Min Hye, Jeong Hee Lee, Jong Sil Lee, Dong Chul Kim, Jung Wook Yang, Hyo Jung An, Ji Min Na, Wook Jae Jung, and Dae Hyun Song. 2023. "Perilipin1 Expression as a Prognostic Factor in Patients with Squamous Cell Carcinoma of the Lung" Diagnostics 13, no. 22: 3475. https://doi.org/10.3390/diagnostics13223475
APA StyleKim, M. H., Lee, J. H., Lee, J. S., Kim, D. C., Yang, J. W., An, H. J., Na, J. M., Jung, W. J., & Song, D. H. (2023). Perilipin1 Expression as a Prognostic Factor in Patients with Squamous Cell Carcinoma of the Lung. Diagnostics, 13(22), 3475. https://doi.org/10.3390/diagnostics13223475






