Multiplexed RT-qPCR Coupled with Whole-Genome Sequencing to Monitor a SARS-CoV-2 Omicron Variant of Concern in a Hospital Laboratory Setting in Latvia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Detection of SARS-CoV-2 RNA
2.3. Detection of SARS-CoV-2 Variants by Multiplexed RT-qPCR
2.4. Whole-Genome Sequencing
2.5. Statistical Analysis
3. Results
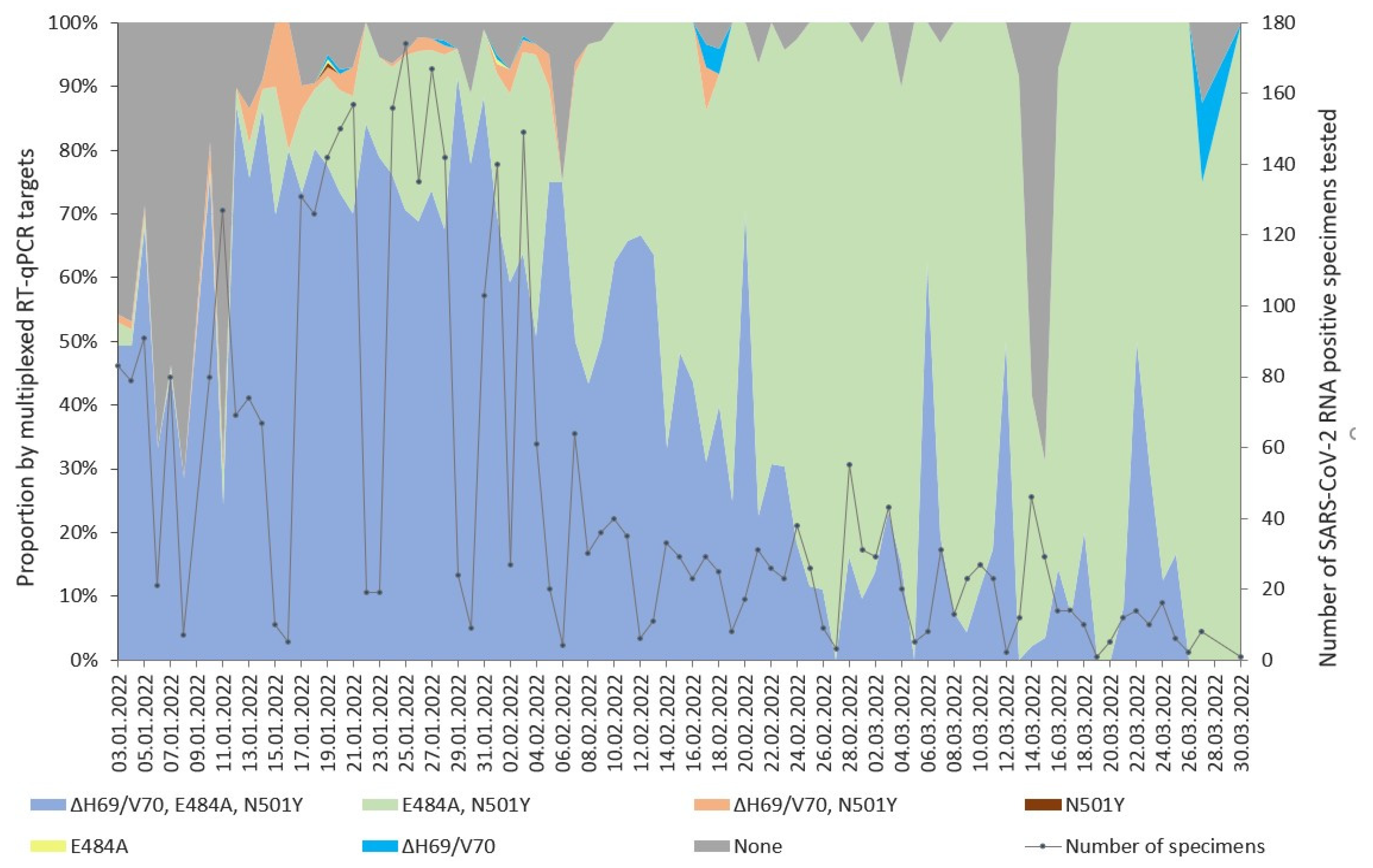
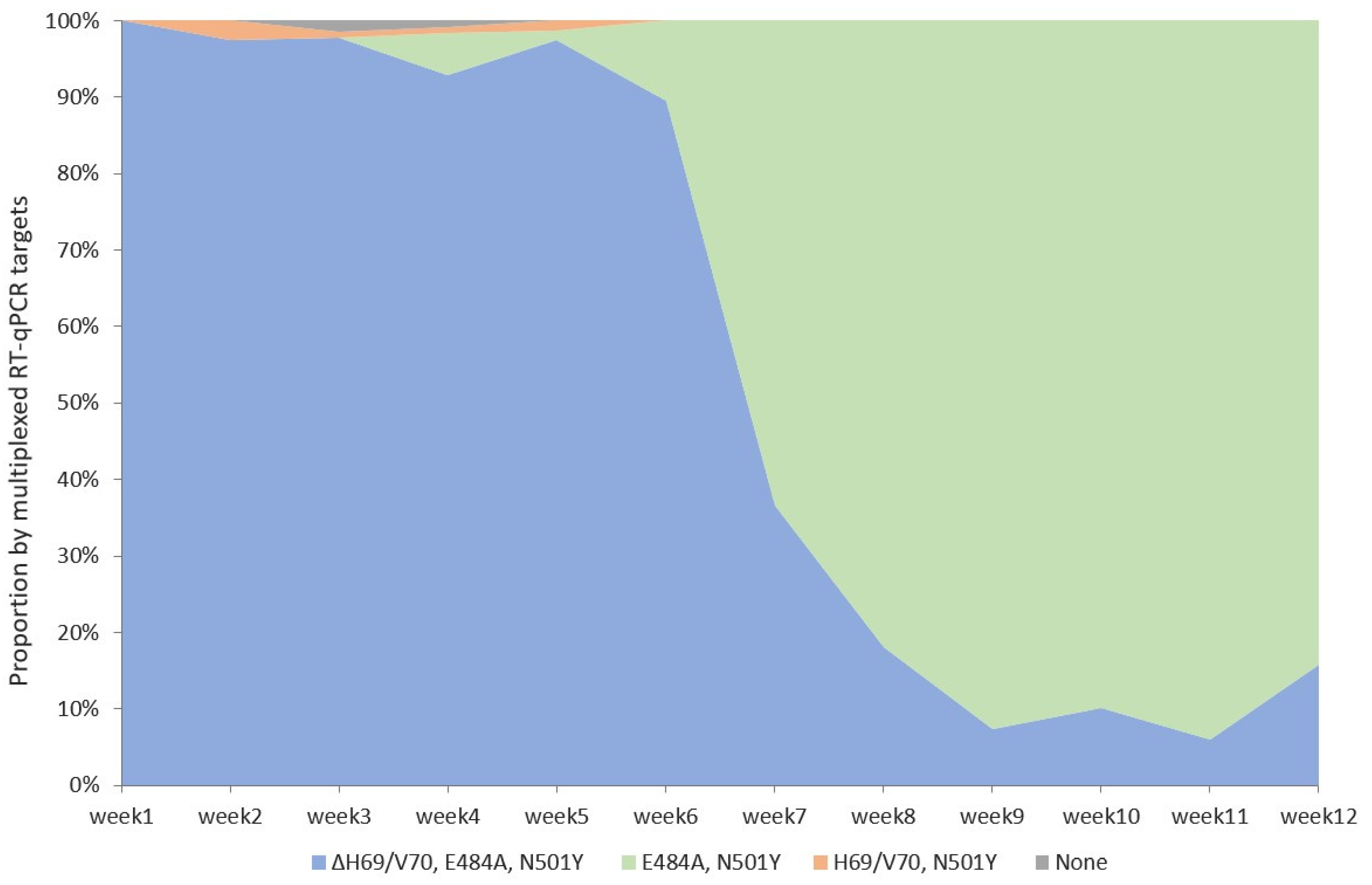
3.1. Detection of SARS-CoV-2 Variants with Multiplexed RT-qPCR
3.2. Whole-Genome Sequencing
3.3. Validation of the Detection of the Three Targeted Omicron VOC Mutations ΔH69/V70, E484A, and N501Y) by Multiplexed RT-qPCR against WGS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; de Silva, T.I.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 Variant Biology: Immune Escape, Transmission and Fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Gubbay, J.; Hopkins, J.; Patel, S.; Buchan, S.A.; Daneman, N.; Goneau, L.W. S-Gene Target Failure as a Marker of Variant B.1.1.7 Among SARS-CoV-2 Isolates in the Greater Toronto Area, December 2020 to March 2021. JAMA 2021, 325, 2115–2116. [Google Scholar] [CrossRef]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, Á.; et al. Assessing Transmissibility of SARS-CoV-2 Lineage B.1.1.7 in England. Nature 2021, 593, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Nörz, D.; Grunwald, M.; Tang, H.T.; Olearo, F.; Günther, T.; Robitaille, A.; Fischer, N.; Grundhoff, A.; Aepfelbacher, M.; Pfefferle, S.; et al. Rapid Automated Screening for SARS-CoV-2 B.1.617 Lineage Variants (Delta/Kappa) through a Versatile Toolset of qPCR-Based SNP Detection. Diagnostics 2021, 11, 1818. [Google Scholar] [CrossRef]
- Lai, E.; Kennedy, E.B.; Lozach, J.; Hayashibara, K.; Davis-Turak, J.; Becker, D.; Brzoska, P.; Cassens, T.; Diamond, E.; Gandhi, M.; et al. A Method for Variant Agnostic Detection of SARS-CoV-2, Rapid Monitoring of Circulating Variants, and Early Detection of Emergent Variants Such as Omicron. J. Clin. Microbiol. 2022, 60, e00342-22. [Google Scholar] [CrossRef]
- TaqMan SARS-CoV-2 Mutation Research Panel-LV. Available online: https://www.thermofisher.com/tr/en/home/clinical/clinical-genomics/pathogen-detection-solutions/real-time-pcr-research-solutions-sars-cov-2/mutation-panel.html (accessed on 20 February 2023).
- Seegene. More Advanced Solution for COVID-19 and Variants. Available online: https://www.seegene.com/advantages/complete_solution_for_the_covid_19_response (accessed on 1 June 2023).
- Berno, G.; Fabeni, L.; Matusali, G.; Gruber, C.E.M.; Rueca, M.; Giombini, E.; Garbuglia, A.R. SARS-CoV-2 Variants Identification: Overview of Molecular Existing Methods. Pathogens 2022, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Greninger, A.L.; Dien Bard, J.; Colgrove, R.C.; Graf, E.H.; Hanson, K.E.; Hayden, M.K.; Humphries, R.M.; Lowe, C.F.; Miller, M.B.; Pillai, D.R.; et al. Clinical and Infection Prevention Applications of Severe Acute Respiratory Syndrome Coronavirus 2 Genotyping: An Infectious Diseases Society of America/American Society for Microbiology Consensus Review Document. Clin. Infect. Dis. 2022, 74, 1496–1502. [Google Scholar] [CrossRef]
- Walker, A.S.; Vihta, K.-D.; Gethings, O.; Pritchard, E.; Jones, J.; House, T.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; et al. Tracking the Emergence of SARS-CoV-2 Alpha Variant in the United Kingdom. N. Engl. J. Med. 2021, 385, 2582–2585. [Google Scholar] [CrossRef]
- McMillen, T.; Jani, K.; Robilotti, E.V.; Kamboj, M.; Babady, N.E. The Spike Gene Target Failure (SGTF) Genomic Signature Is Highly Accurate for the Identification of Alpha and Omicron SARS-CoV-2 Variants. Sci. Rep. 2022, 12, 18968. [Google Scholar] [CrossRef]
- Caputo, V.; Calvino, G.; Strafella, C.; Termine, A.; Fabrizio, C.; Trastulli, G.; Ingrascì, A.; Peconi, C.; Bardini, S.; Rossini, A.; et al. Tracking the Initial Diffusion of SARS-CoV-2 Omicron Variant in Italy by RT-PCR and Comparison with Alpha and Delta Variants Spreading. Diagnostics 2022, 12, 467. [Google Scholar] [CrossRef]
- Zelyas, N.; Pabbaraju, K.; Croxen, M.A.; Lynch, T.; Buss, E.; Murphy, S.A.; Shokoples, S.; Wong, A.; Kanji, J.N.; Tipples, G. Precision Response to the Rise of the SARS-CoV-2 B.1.1.7 Variant of Concern by Combining Novel PCR Assays and Genome Sequencing for Rapid Variant Detection and Surveillance. Microbiol. Spectr. 2021, 9, e00315-21. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.L.; Chong, Y.M.; Sam, I.-C.; Chan, Y.F. SARS-CoV-2 Multiplex RT-PCR to Detect Variants of Concern (VOCs) in Malaysia, between January to May 2021. J. Virol. Methods 2022, 301, 114462. [Google Scholar] [CrossRef] [PubMed]
- Kami, W.; Kinjo, T.; Arakaki, W.; Oki, H.; Motooka, D.; Nakamura, S.; Fujita, J. Rapid and Simultaneous Identification of Three Mutations by the NovaplexTM SARS-CoV-2 Variants I Assay Kit. J. Clin. Virol. 2021, 141, 104877. [Google Scholar] [CrossRef]
- Carpenter, R.E.; Tamrakar, V.; Chahar, H.; Vine, T.; Sharma, R. Confirming Multiplex RT-qPCR Use in COVID-19 with Next-Generation Sequencing: Strategies for Epidemiological Advantage. Glob. Health Epidemiol. Genom. 2022, 2022, 2270965. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.C.; Machado, R.R.G.; Mitchell, B.M.; McConnell, A.J.; Saada, N.I.; Weaver, S.C.; Ren, P. A Comparison of Seegene Technologies Novaplex SARS-CoV-2 Variants I, II, and IV Assays with Spike Gene Sequencing for Detection of Known Severe Acute Respiratory Syndrome Coronavirus 2 Variants. J. Mol. Diagn. 2022, 24, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Neopane, P.; Nypaver, J.; Shrestha, R.; Beqaj, S.S. SARS-CoV-2 Variants Detection Using TaqMan SARS-CoV-2 Mutation Panel Molecular Genotyping Assays. Infect. Drug Resist. 2021, 14, 4471–4479. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Breban, M.I.; Ott, I.M.; Alpert, T.; Petrone, M.E.; Watkins, A.E.; Kalinich, C.C.; Earnest, R.; Rothman, J.E.; de Jesus, J.G.; et al. Multiplex qPCR Discriminates Variants of Concern to Enhance Global Surveillance of SARS-CoV-2. PLoS Biol. 2021, 19, e3001236. [Google Scholar] [CrossRef]
- Ayadi, W.; Taktak, A.; Gargouri, S.; Smaoui, F.; Chtourou, A.; Skouri-Gargouri, H.; Derbel, R.; Sassi, A.H.; Gargouri, A.; Hammami, A.; et al. Development of a Simple Genotyping Method Based on Indel Mutations to Rapidly Screen SARS-CoV-2 Circulating Variants: Delta, Omicron BA.1 and BA.2. J. Virol. Methods 2022, 307, 114570. [Google Scholar] [CrossRef]
- Wilhelm, A.; Widera, M.; Grikscheit, K.; Toptan, T.; Schenk, B.; Pallas, C.; Metzler, M.; Kohmer, N.; Hoehl, S.; Marschalek, R.; et al. Limited Neutralisation of the SARS-CoV-2 Omicron Subvariants BA.1 and BA.2 by Convalescent and Vaccine Serum and Monoclonal Antibodies. eBioMedicine 2022, 82, 104158. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.-H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable Escape of SARS-CoV-2 Omicron to Antibody Neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron Escapes the Majority of Existing SARS-CoV-2 Neutralizing Antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 Leads to Widespread Escape from Neutralizing Antibody Responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef] [PubMed]
- Commissioner, O. Of the Coronavirus (COVID-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat COVID-19 Due to the Omicron Variant. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron (accessed on 14 April 2023).
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human Neutralizing Antibodies Elicited by SARS-CoV-2 Infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A Neutralizing Human Antibody Binds to the N-Terminal Domain of the Spike Protein of SARS-CoV-2. Science 2020, 369, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid Epidemic Expansion of the SARS-CoV-2 Omicron Variant in Southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef]
- Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 2 February 2023).
- Martin, D.P.; Weaver, S.; Tegally, H.; San, J.E.; Shank, S.D.; Wilkinson, E.; Lucaci, A.G.; Giandhari, J.; Naidoo, S.; Pillay, Y.; et al. The Emergence and Ongoing Convergent Evolution of the SARS-CoV-2 N501Y Lineages. Cell 2021, 184, 5189–5200.e7. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Zhou, J.; Ye, Q. The Global Epidemic of the SARS-CoV-2 Delta Variant, Key Spike Mutations and Immune Escape. Front. Immunol. 2021, 12, 751778. [Google Scholar] [CrossRef]
- Moustafa, A.M.; Bianco, C.; Denu, L.; Ahmed, A.; Coffin, S.E.; Neide, B.; Everett, J.; Reddy, S.; Rabut, E.; Deseignora, J.; et al. Comparative Analysis of Emerging B.1.1.7+E484K SARS-CoV-2 Isolates. Open Forum. Infect. Dis. 2021, 8, ofab300. [Google Scholar] [CrossRef]
- Yang, W.-T.; Huang, W.-H.; Liao, T.-L.; Hsiao, T.-H.; Chuang, H.-N.; Liu, P.-Y. SARS-CoV-2 E484K Mutation Narrative Review: Epidemiology, Immune Escape, Clinical Implications, and Future Considerations. Infect. Drug Resist. 2022, 15, 373–385. [Google Scholar] [CrossRef]
- Methods for the Detection and Characterisation of SARS-CoV-2 Variants-Second Update. Available online: https://www.ecdc.europa.eu/en/publications-data/methods-detection-and-characterisation-sars-cov-2-variants-second-update (accessed on 20 February 2023).
- Nextstrain. Available online: https://docs.nextstrain.org/projects/nextclade/en/stable/user/algorithm/07-quality-control.html (accessed on 10 January 2023).
- SARS-CoV-2 Variants of Concern as of 12 January 2023. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 24 January 2023).
- WHO. WHO Weekly Epidemiological Update on COVID-19. Edition 158, 1 September 2023. Available online: https://www.who.int/publications (accessed on 15 September 2023).
- Focosi, D.; Quiroga, R.; McConnell, S.; Johnson, M.C.; Casadevall, A. Convergent Evolution in SARS-CoV-2 Spike Creates a Variant Soup from Which New COVID-19 Waves Emerge. Int. J. Mol. Sci. 2023, 24, 2264. [Google Scholar] [CrossRef]
- Available online: https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2_variant_mutations_conferring_reduced_susceptibility_to_antiviral_drugs_and_monoclonal_antibodies.pdf (accessed on 17 August 2023).
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming Antibody Evasion Properties of Rising SARS-CoV-2 BQ and XBB Subvariants. Cell 2023, 186, 279–286.e8. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.; Peacock, T.P.; Harvey, W.T.; Hughes, J.; Wright, D.W.; Willett, B.J.; Thomson, E.; Gupta, R.K.; Peacock, S.J.; Robertson, D.L.; et al. SARS-CoV-2 Variant Evasion of Monoclonal Antibodies Based on in Vitro Studies. Nat. Rev. Microbiol. 2023, 21, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Lineage. S:N501Y Mutation Report. Available online: https://outbreak.info/situation-reports?pango&muts=S:N501Y (accessed on 7 August 2023).
- Lineage. S:DEL24/26 Mutation Report. Available online: https://outbreak.info/situation-reports?pango&muts=S:DEL24/26 (accessed on 7 August 2023).
- Stanhope, B.J.; Peterson, B.; Knight, B.; Decadiz, R.N.; Pan, R.; Davis, P.; Fraser, A.; Nuth, M.; vanWestrienen, J.; Wendlandt, E.; et al. Development, Testing and Validation of a SARS-CoV-2 Multiplex Panel for Detection of the Five Major Variants of Concern on a Portable PCR Platform. Front. Public Health 2022, 10, 1042647. [Google Scholar] [PubMed]
- Peterson, S.W.; Lidder, R.; Daigle, J.; Wonitowy, Q.; Dueck, C.; Nagasawa, A.; Mulvey, M.R.; Mangat, C.S. RT-qPCR Detection of SARS-CoV-2 Mutations S 69–70 Del, S N501Y and N D3L Associated with Variants of Concern in Canadian Wastewater Samples. Sci. Total Environ. 2022, 810, 151283. [Google Scholar] [CrossRef]
- Yolshin, N.D.; Komissarov, A.B.; Varchenko, K.V.; Musaeva, T.D.; Fadeev, A.V.; Lioznov, D.A. Detection of the Omicron SARS-CoV-2 Lineage and Its BA.1 Variant with Multiplex RT-qPCR. Int. J. Mol. Sci. 2022, 23, 16153. [Google Scholar] [CrossRef]
- Lineage. N:DEL31/33 Mutation Report. Available online: https://outbreak.info/situation-reports?pango&muts=N:DEL31/33 (accessed on 7 August 2023).
- Borillo, G.A.; Kagan, R.M.; Marlowe, E.M. Rapid and Accurate Identification of SARS-CoV-2 Variants Using Real Time PCR Assays. Front. Cell. Infect. Microbiol. 2022, 12, 894613. [Google Scholar] [CrossRef]
- Kannan, S.R.; Spratt, A.N.; Sharma, K.; Goyal, R.; Sönnerborg, A.; Apparsundaram, S.; Lorson, C.L.; Byrareddy, S.N.; Singh, K. Complex Mutation Pattern of Omicron BA.2: Evading Antibodies without Losing Receptor Interactions. Int. J. Mol. Sci. 2022, 23, 5534. [Google Scholar] [CrossRef]
- Wang, H.; Miller, J.A.; Verghese, M.; Sibai, M.; Solis, D.; Mfuh, K.O.; Jiang, B.; Iwai, N.; Mar, M.; Huang, C.; et al. Multiplex SARS-CoV-2 Genotyping Reverse Transcriptase PCR for Population-Level Variant Screening and Epidemiologic Surveillance. J. Clin. Microbiol. 2021, 59, e0085921. [Google Scholar] [CrossRef]
| n | Detection of Targets, Multiplexed RT-qPCR | Presence of Targets, WGS | n | ||
|---|---|---|---|---|---|
| 2 | ΔH69/V70, E484A, N501Y | Omicron, BA.1 | - | Delta, B.1.617.2 Delta, AY.121 | 1 1 |
| 5 | E484A, N501Y | Omicron, BA.2 | ΔH69/V70, E484A, N501Y ΔH69/V70, E484A, N501Y ΔH69/V70, E484A, N501Y | Omicron, BA.1.17.2 Omicron, BA.1.1 Omicron, BA.2 * | 1 2 2 |
| 1 | ΔH69/V70, N501Y | Other | ΔH69/V70, E484A, N501Y | Omicron, BA.1.17.2 | 1 |
| 18 | - | Delta | ΔH69/V70, E484A, N501Y ΔH69/V70, E484A, N501Y ΔH69/V70, E484A, N501Y ΔH69/V70, E484A, N501Y ΔH69/V70, E484A, N501Y ΔH69/V70, E484A, N501Y ΔH69/V70, E484A, N501Y | Omicron, BA.1 Omicron, BA.1.1 Omicron, BA.1.10 Omicron, BA.1.15 Omicron, BA.1.15.1 Omicron, BA.1.17 Omicron, BA.1.17.2 | 6 4 1 1 1 1 4 |
| Measures | Target | ||
|---|---|---|---|
| ΔH69/V70 | E484A | N501Y | |
| True positives (TP) | 686 | 933 | 942 |
| True negatives (TN) | 255 | 10 | 2 |
| False positives (FP) | 21 | 19 | 18 |
| False negatives (FN) | 2 | 2 | 2 |
| Correct classification rate (CCR), % (95% CI) | 97.6 (96.7–98.6) | 97.8 (96.9–98.7) | 97.9 (97.0–98.8) |
| Sensitivity, % (95% CI) | 97.0 (96.0–98.1) | 98.0 (97.1–98.9) | 98.1 (97.3–99.0) |
| Specificity, % (95% CI) | 99.2 (98.7–99.8) | 83.3 (81.0–85.7) | 50.0 (46.8–53.2) |
| Positive predictive value (PPV), % (95% CI) | 99.7 (99.4–100) | 99.8 (99.5–100) | 99.8 (99.5–100) |
| Negative predictive value (NPV), % (95% CI) | 92.4 (90.7–94.1) | 34.5 (31.5–37.5) | 10.0 (8.1–11.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedre-Otomere, B.; Kampenusa, I.; Trofimova, J.; Bodrenko, J.; Vangravs, R.; Skenders, G.; Nikisins, S.; Savicka, O. Multiplexed RT-qPCR Coupled with Whole-Genome Sequencing to Monitor a SARS-CoV-2 Omicron Variant of Concern in a Hospital Laboratory Setting in Latvia. Diagnostics 2023, 13, 3467. https://doi.org/10.3390/diagnostics13223467
Niedre-Otomere B, Kampenusa I, Trofimova J, Bodrenko J, Vangravs R, Skenders G, Nikisins S, Savicka O. Multiplexed RT-qPCR Coupled with Whole-Genome Sequencing to Monitor a SARS-CoV-2 Omicron Variant of Concern in a Hospital Laboratory Setting in Latvia. Diagnostics. 2023; 13(22):3467. https://doi.org/10.3390/diagnostics13223467
Chicago/Turabian StyleNiedre-Otomere, Baiba, Inara Kampenusa, Julija Trofimova, Jevgenijs Bodrenko, Reinis Vangravs, Girts Skenders, Sergejs Nikisins, and Oksana Savicka. 2023. "Multiplexed RT-qPCR Coupled with Whole-Genome Sequencing to Monitor a SARS-CoV-2 Omicron Variant of Concern in a Hospital Laboratory Setting in Latvia" Diagnostics 13, no. 22: 3467. https://doi.org/10.3390/diagnostics13223467
APA StyleNiedre-Otomere, B., Kampenusa, I., Trofimova, J., Bodrenko, J., Vangravs, R., Skenders, G., Nikisins, S., & Savicka, O. (2023). Multiplexed RT-qPCR Coupled with Whole-Genome Sequencing to Monitor a SARS-CoV-2 Omicron Variant of Concern in a Hospital Laboratory Setting in Latvia. Diagnostics, 13(22), 3467. https://doi.org/10.3390/diagnostics13223467






