Agreement between Accelerometer-Assessed and Self-Reported Physical Activity and Sedentary Behavior in Female Breast Cancer Survivors
Abstract
1. Introduction
- -
- To compare the average levels of moderate PA and moderate-to-vigorous physical activity (MVPA) as measured using IPAQ and accelerometry;
- -
- To compare the average sedentary behavior values measured utilizing IPAQ and accelerometry;
- -
- To investigate the associations between PA, sedentary behavior, general adiposity, and adipose tissue distribution.
2. Materials and Methods
2.1. Study Population
2.2. Demographics and Cancer Treatment Variables
2.3. General Adiposity Assessment
2.4. Adipose Tissue Distribution Measurements
2.5. Sedentary Behavior and Physical Activity Assessment
- -
- Low-level activity—individuals who do not meet the criteria for the other two categories, with PA at a level of <600 metabolic equivalents of task (MET)-min/week.
- -
- Moderate-level activity—PA at a level of 600–1500 MET-min/week, or 1500–3000 MET-min/week, although with 1 or 2 days comprising high-intensity exercise.
- -
- High-level activity—1500 MET-min/week, although with at least 3 days comprising high-intensity exercise, at over 3000 MET-min/week [28].
2.6. Statistical Analyses
3. Results
4. Discussion
Strengths and Weaknesses of the Study
5. Conclusions
- -
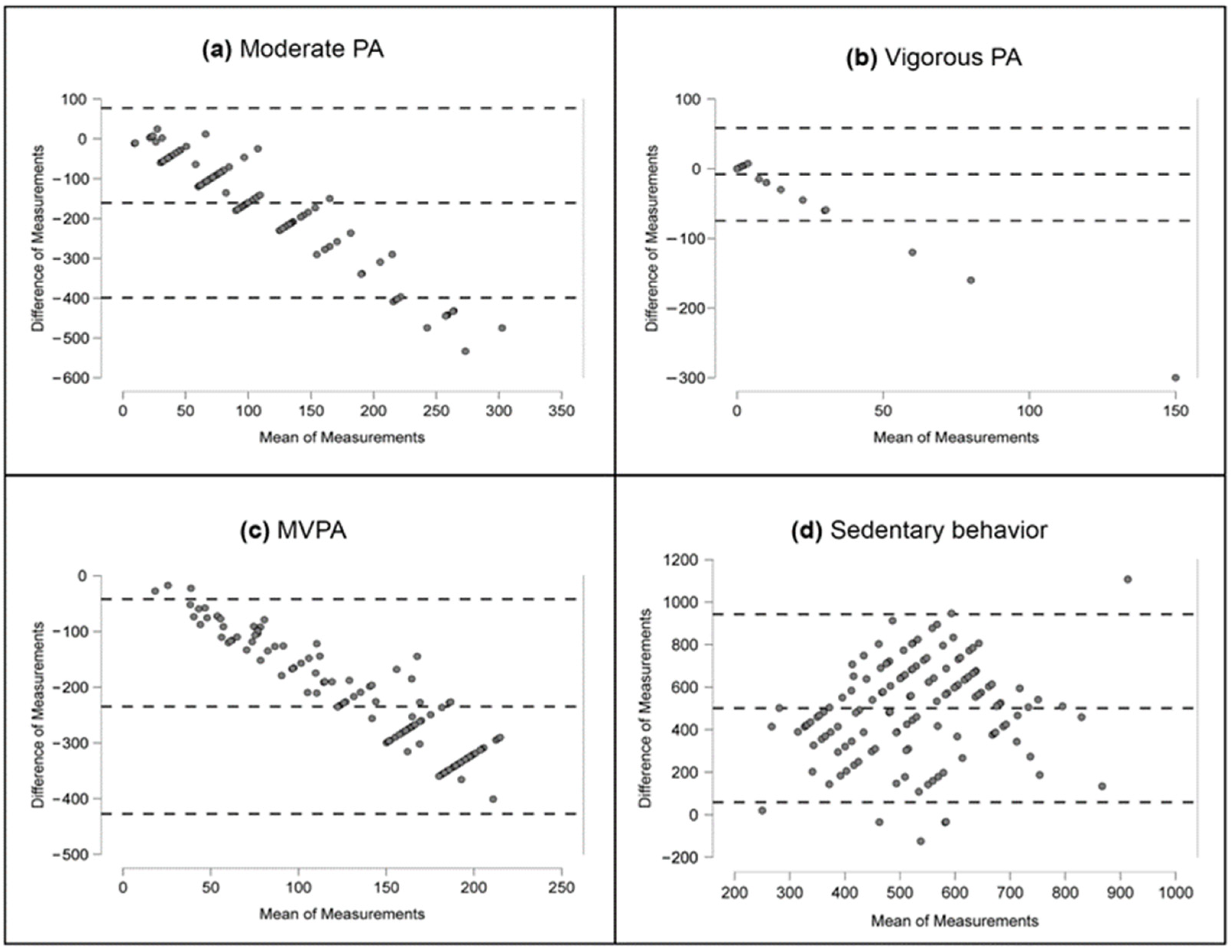
- Across all types of PA, measurement via accelerometry produced significantly lower results than IPAQ, with the average moderate PA and MVPA values being seven times and ten times lower, respectively.
- -
- The accelerometer-measured sedentary behavior values were nearly triple those measured using IPAQ. These measurement differences were consistent with the magnitude of the average differences observed. The association between moderate PA and both overall adiposity and adipose tissue distribution was negative, regardless of the measurement method used. Conversely, sedentary behavior exhibited a positive correlation with these variables, suggesting an adverse impact of obesity on physical activity levels.
- -
- The IPAQ method was subject to error in the assessment of PA, further increasing with higher objective activity values. However, the relationships with the detrimental effects of a lack of activity maintained the appropriate direction, similar to the accelerometer-based observations.
- -
- The observed limitation of vigorous PA is a consequence of breast cancer treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartman, S.J.; Marinac, C.R.; Bellettiere, J.; Godbole, S.; Natarajan, L.; Patterson, R.E.; Kerr, J. Objectively measured sedentary behavior and quality of life among survivors of early stage breast cancer. Support. Care Cancer 2017, 25, 2495–2503. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, H.; Peng, C. Association of sedentary behavior with the risk of breast cancer in women: Update meta-analysis of observational studies. Ann. Epidemiol. 2015, 25, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Lloyd, G.R.; Awick, E.A.; McAuley, E. Correlates of objectively measured sedentary behavior in breast cancer survivors. Cancer Causes Control 2016, 27, 787–795. [Google Scholar] [CrossRef]
- Nelson, S.H.; Marinac, C.R.; Patterson, R.E.; Nechuta, S.J.; Flatt, S.W.; Caan, B.J.; Kwan, M.L.; Poole, E.M.; Chen, W.Y.; Shu, X.-O.; et al. Impact of very low physical activity, BMI, and comorbidities on mortality among breast cancer survivors. Breast Cancer Res. Treat. 2016, 155, 551–557. [Google Scholar] [CrossRef]
- Phillips, S.M.; Awick, E.A.; Conroy, D.E.; Pellegrini, C.A.; Mailey, E.L.; McAuley, E. Objectively measured physical activity and sedentary behavior and quality of life indicators in survivors of breast cancer. Cancer 2015, 121, 4044–4052. [Google Scholar] [CrossRef] [PubMed]
- Mañas, A.; del Pozo-Cruz, B.; García-García, F.J.; Guadalupe-Grau, A.; Ara, I. Role of objectively measured sedentary behaviour in physical performance, frailty and mortality among older adults: A short systematic review. Eur. J. Sport Sci. 2017, 17, 940–953. [Google Scholar] [CrossRef]
- Włoch, A.; Bocian, A.; Biskup, M.; Krupnik, S.; Opuchlik, A.; Ridan, T.; Zak, M. Effectiveness of specific types of structured physical activities in the rehabilitation of post-mastectomy women: A systematic review. Med. Stud. 2018, 34, 86–92. [Google Scholar] [CrossRef]
- Biskup, M.; Król, H.; Opuchlik, A.; Macek, P.; Włoch, A.; Żak, M. The role of physical activity in maintaining health after mastectomy. Med. Stud. 2015, 31, 146–154. [Google Scholar] [CrossRef]
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T. American Cancer Society guidelines on nutrition and physical activity cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 2012, 6, 30–67. [Google Scholar] [CrossRef]
- Szpunar, J.; Karczmarek-Borowska, B. Physical activity during canser disease. Probl. Appl. Sci. 2018, 8, 185–192. [Google Scholar]
- Weiner, L.S.; Takemoto, M.; Godbole, S.; Nelson, S.H.; Natarajan, L.; Sears, D.D.; Hartman, S.J. Breast cancer survivors reduce accelerometer-measured sedentary time in an exercise intervention. J. Cancer Surviv. 2019, 13, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Thraen-Borowski, K.M.; Gennuso, K.P.; Cadmus-Bertram, L. Accelerometer-derived physical activity and sedentary time by cancer type in the United States. PLoS ONE 2017, 12, e0182554. [Google Scholar] [CrossRef] [PubMed]
- Gianoudis, J.; Bailey, C.A.; Daly, R.M. Associations between sedentary behaviour and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos. Int. 2015, 26, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Peddle-Mcintyre, C.J.; Cavalheri, V.; Boyle, T.; Mcveigh, J.A.; Jeffery, E.; Lynch, B.M.; Vallance, J.K. A review of accelerometer-based activity monitoring in cancer survivorship research. Med. Sci. Sports Exerc. 2018, 50, 1790–1801. [Google Scholar] [CrossRef]
- Fjeldsoe, B.S.; Winkler, E.A.; Marshall, A.L.; Eakin, E.G.; Reeves, M.M. Active adults recall their physical activity differently to less active adults: Test-retest reliability and validity of a physical activity survey. Health Promot. J. Aust. 2013, 24, 26–31. [Google Scholar] [CrossRef]
- Cleland, C.L.; Hunter, R.F.; Kee, F.; Cupples, M.E.; Sallis, J.F.; Tully, M.A. Validity of the global physical activity questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health 2014, 14, 1255. [Google Scholar] [CrossRef]
- Dyrstad, S.M.; Hansen, B.H.; Holme, I.M.; Anderssen, S.A. Comparison of self-reported versus accelerometer-measured physical activity. Med. Sci. Sports Exerc. 2014, 46, 99–106. [Google Scholar] [CrossRef]
- Boyle, T.; Lynch, B.M.; Courneya, K.S.; Vallance, J.K. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support. Care Cancer 2015, 23, 1121–1126. [Google Scholar] [CrossRef]
- Lynch, B.M.; Boyle, T.; Winkler, E.; Occleston, J.; Courneya, K.S.; Vallance, J.K. Patterns and correlates of accelerometer-assessed physical activity and sedentary time among colon cancer survivors. Cancer Causes Control. 2016, 27, 59–68. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Delisle Nyström, C.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: A systematic review and practical considerations. Sports Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef] [PubMed]
- Montoye, A.H.; Moore, R.W.; Bowles, H.R.; Korycinski, R.; Pfeiffer, K.A. Reporting accelerometer methods in physical activity intervention studies: A systematic review and recommendations for authors. Br. J. Sports Med. 2016, 52, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Challenge of Obesity in the WHO European Region and the Strategies for Response; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Macek, P.; Terek-Derszniak, M.; Biskup, M.; Krol, H.; Smok-Kalwat, J.; Gozdz, S.; Zak, M. A Two-Year Follow-Up Cohort Study—Improved Clinical Control over CVD Risk Factors through Weight Loss in Middle-Aged and Older Adults. J. Clin. Med. 2020, 9, 2904. [Google Scholar] [CrossRef] [PubMed]
- Macek, P.; Biskup, M.; Terek-Derszniak, M.; Manczuk, M.; Krol, H.; Naszydlowska, E.; Smok-Kalwat, J.; Gozdz, S.; Zak, M. Competing Risks of Cancer and Non-Cancer Mortality When Accompanied by Lifestyle-Related Factors-A Prospective Cohort Study in Middle-Aged and Older Adults. Front. Oncol. 2020, 10, 545078. [Google Scholar] [CrossRef] [PubMed]
- Biernat, E.; Piątkowska, M. Recommendations of the World Health Organization on leisure physical activity and their implementation among Polish population. Pol. J. Sport. Med. 2013, 29, 255–264. [Google Scholar]
- Macek, P.; Terek-Derszniak, M.; Zak, M.; Biskup, M.; Ciepiela, P.; Krol, H.; Smok-Kalwat, J.; Gozdz, S. WHO recommendations on physical activity versus compliance rate within a specific urban population as assessed through IPAQ survey: A cross-sectional cohort study. BMJ Open 2019, 9, e028334. [Google Scholar] [CrossRef] [PubMed]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef]
- Weiner, L.S.; Nagel, S.; Su, H.I.; Hurst, S.; Levy, S.S.; Arredondo, E.M.; Hekler, E.; Hartman, S.J. A remotely deliv-ered, peer-led intervention to improve physical activity and quality of life in younger breast cancer survivors. J. Behav. Med. 2023, 46, 578–593. [Google Scholar] [CrossRef]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of accelerometer wear and nonwear time classification algorithm. Med. Sci. Sports Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef]
- Murray, J.; Perry, R.; Pontifex, E.; Selva-Nayagam, S.; Bezak, E.; Bennett, H. The impact of breast cancer on fears of exercise and exercise identity. Patient Educ. Couns. 2022, 105, 2443–2449. [Google Scholar] [CrossRef]
- Jammallo, L.S.; Miller, C.L.; Horick, N.K.; Skolny, M.N.; O’Toole, J.; Specht, M.C.; Taghian, A.G. Factors Associated with Fear of Lymphedema after Treatment for Breast Cancer. Oncol. Nurs. Forum 2014, 41, 473–483. [Google Scholar] [CrossRef]
- Lin, H.; Kuo, Y.; Tai, W.; Liu, H. Exercise effects on fatigue in breast cancer survivors after treatments: A systematic review and meta-analysis. Int. J. Nurs. Pract. 2022, 28, 4. [Google Scholar] [CrossRef]
- Papadopoulos, E.; Mina, D.S. Can we HIIT cancer if we attack inflammation? Cancer Causes Control 2018, 29, 7–11. [Google Scholar] [CrossRef]
- Maridaki, M.; Papadopetraki, A.; Karagianni, H.; Koutsilieris, M.; Philippou, A. The Assessment and Relationship Between Quality of Life and Physical Activity Levels in Greek Breast Cancer Female Patients under Chemotherapy. Sports 2020, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.D.; Bae, S.; Kim, H.; Hwang, I.G.; Kim, S.M.; Han, D.H. The Relationship between Physical Activity Intensity and Mental Health Status in Patients with Breast Cancer. J. Korean Med. Sci. 2017, 32, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Colley, R.C.; Butler, G.; Garriguet, D.; Prince, S.A.; Roberts, K.C. Comparison of self-reported and accelerometer-measured physical activity in Canadian adults. Health Rep. 2018, 29, 3–15. [Google Scholar] [PubMed]
- Ortiz, A.; Tirado, M.; Hughes, D.C.; Gonzalez, V.; Song, J.J.; Mama, S.K.; Basen-Engquist, K. Relationship between physical activity, disability, and physical fitness profile in sedentary Latina breast cancer survivors. Physiother. Theory Pract. 2018, 10, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Bluethmann, S.M.; Vernon, S.W.; Gabriel, K.P.; Murphy, C.C.; Bartholomew, L.K. Taking the next step: A systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res. Treat. 2015, 149, 331–342. [Google Scholar] [CrossRef]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst. Rev. 2018, 1, CD011292. [Google Scholar] [CrossRef]
- Short, C.E.; James, E.L.; Stacey, F.; Plotnikoff, R.C. A qualitative synthesis of trials promoting physical activity behaviour change among post-treatment breast cancer survivors. J. Cancer Surviv. 2013, 7, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, S.; Schmidt, M.E.; Steindorf, K. Long-term effects of exercise interventions on physical activity in breast cancer patients: A systematic review and meta-analysis of randomized controlled trials. Support. Care Cancer 2023, 31, 130. [Google Scholar] [CrossRef] [PubMed]
- Grimmett, C.; Corbett, T.; Brunet, J.; Shepherd, J.; Pinto, B.M.; May, C.R.; Foster, C. Systematic review and meta-analysis of maintenance of physical activity behaviour change in cancer survivors. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 37. [Google Scholar] [CrossRef]
- Pinto, B.; Dunsiger, S.; Stein, K. Does a peer-led exercise intervention affect sedentary behavior among breast cancer survivors? Psycho-Oncology 2017, 26, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Siddique, J.; de Chavez, P.J.; Craft, L.L.; Freedson, P.; Spring, B. The effect of changes in physical activity on sedentary behavior: Results from a randomized lifestyle intervention trial. Am. J. Health Promot. 2017, 31, 287–295. [Google Scholar] [CrossRef]
- Silva, D.T.C.; Vanderlei, L.C.M.; Palma, M.R.; Ribeiro, F.E.; Tebar, W.R.; Tosello, G.T.; Christofaro, D.G.D. Association between Different Domains of Physical Activity and Body Adiposity Indicators in Breast Cancer Survivors. Clin. Breast Cancer 2022, 22, E438–E443. [Google Scholar] [CrossRef]
- Ruiz-Casado, A.; Alvarez-Bustos, A.; de Pedro, C.; Mendez-Otero, M.; Romero-Elias, M. Cancer-related Fatigue in Breast Cancer Survivors: A Review. Clin. Breast Cancer 2021, 21, 10–25. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Pekarek, L.; Guijarro, L.G.; Castellanos, A.J.; Sanchez-Trujillo, L.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; et al. Physical Activity as an Imperative Support in Breast Cancer Management. Cancers 2021, 13, 55. [Google Scholar] [CrossRef]
- Shaikh, H.; Bradhurst, P.; Xin Ma, L.; Yee Cindy Tan, S.; Egger, S.J.; Vardy, J.L. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst. Rev. 2020, 12, CD012110. [Google Scholar] [CrossRef]
- James, F.R.; Wootton, S.; Jackson, A.; Wiseman, M.; Copson, E.R.; Cutress, R.I. Obesity in breast cancer—What is the risk factor? Eur. J. Cancer 2015, 51, 705–720. [Google Scholar] [CrossRef]
- Lee, K.; Kruper, L.; Dieli-Conwright, C.M.; Mortimer, J.E. The Impact of Obesity on Breast Cancer Diagnosis and Treatment. Curr. Oncol. Rep. 2019, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Abar, L.; Cariolou, M.; Nanu, N.; Greenwood, D.C.; Bandera, E.V.; McTiernan, A.; Norat, T. World Cancer Research Fund International: Continuous Update Project—Systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control 2019, 30, 1183–1200. [Google Scholar] [CrossRef] [PubMed]
- Paxton, R.J.; Garner, W.; Dean, L.T.; Logan, G.; Allen-Watts, K. Health Behaviors and Lifestyle Interventions in African American Breast Cancer Survivors: A Review. Front. Oncol. 2019, 9, 3. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Basen-Engquist, K.; Bea, J. Weight Management and Physical Activity for Breast Cancer Prevention and Control. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e22–e33. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Dodd, K.W.; Steeves, J.; McClain, J.; Alfano, C.M.; McAuley, E. Physical activity and sedentary behavior in breast cancer survivors: New insight into activity patterns and potential intervention targets. Gynecol. Oncol. 2015, 138, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Makari-Judson, G.; Braun, B.; Jerry, D.J.; Mertens, W.C. Weight gain following breast cancer diagnosis: Implication and proposed mechanisms. World J. Clin. Oncol. 2014, 5, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Au, C.C.; Benito-Martin, A.; Ladumor, H.; Oshchepkova, S.; Moges, R.; Brown, K.A. Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression. J. Steroid Biochem. Mol. Biol. 2019, 189, 161–170. [Google Scholar] [CrossRef]
- Martel, S.; Poletto, E.; Ferreira, A.R.; Lambertini, M.; Sottotetti, F.; Bertolini, I.; Montemurro, F.; Bernardo, A.; Risi, E.; Zanardi, E.; et al. Impact of body mass index on the clinical outcomes of patients with HER2-positive metastatic breast cancer. Breast 2018, 37, 142–147. [Google Scholar] [CrossRef]
- Sini, V.; Lunardi, G.; Cirillo, M.; Turazza, M.; Bighin, C.; Giraudi, S.; Levaggi, A.; Piccioli, P.; Bisagni, G.; Gnoni, R.; et al. Body mass index and circulating oestrone sulphate in women treated with adjuvant letrozole. Br. J. Cancer 2014, 110, 1133–1138. [Google Scholar] [CrossRef][Green Version]
- Coughlin, S.S.; Caplan, L.S.; Stone, R. Use of consumer wearable devices to promote physical activity among breast, prostate, and colorectal cancer survivors: A review of health intervention studies. J. Cancer Surviv. 2020, 14, 386–392. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 242–274. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, C.S.; Ligibel, J.A.; Are, M.; Baker, K.S.; Broderick, G.; Demark-Wahnefried, W.; Friedman, D.L.; Goldman, M.; Jones, L.W.; King, A.; et al. NCCN Guidelines insights: Survivorship, version 1.2016. J. Natl. Compr. Cancer Netw. 2016, 14, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Fong, A.J.; Faulkner, G.; Jones, J.M.; Sabiston, C.M. A qualitative analysis of oncology clinicians’ perceptions and barriers for physical activity counseling in breast cancer survivors. Support. Care Cancer 2018, 26, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M.; Walsh, D.; Furlong, B.; Moyna, N.; McCaffrey, N.; Boran, L.; Smyth, S.; Woods, C. Healthcare professionals’ knowledge and practice of physical activity promotion in cancer care: Challenges and solutions. Eur. J. Cancer Care 2018, 27, 12795. [Google Scholar] [CrossRef]
- Kędzierawski, P.; Macek, P.; Góźdź, S. Following the requirements of Breast Cancer Centre-bettering outcomes of the treatment of the patients with a breast cancer. Med. Stud. 2020, 36, 167–171. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Rosenblatt, D.N.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Aiello, E.; McTiernan, A. Weight loss in breast cancer patient management. J. Clin. Oncol. 2002, 20, 1128–1143. [Google Scholar] [CrossRef]
- Sweegers, M.G.; Boyle, T.; Vallance, J.K.; Chinapaw, M.J.; Brug, J.; Aaronson, N.K.; D’silva, A.; Kampshoff, C.S.; Lynch, B.M.; Nollet, F.; et al. Which cancer survivors are at risk for a physically inactive and sedentary lifestyle? Results from pooled accelerometer data of 1447 cancer survivors. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 66. [Google Scholar] [CrossRef]
- Keadle, S.K.; Conroy, D.E.; Buman, M.P.; Dunstan, D.W.; Matthews, C.E. Targeting reductions in sitting time to increase physical activity and improve health. Med. Sci. Sports Exerc. 2017, 49, 1572–1582. [Google Scholar] [CrossRef]
- Scholes, S.; Bridges, S.; Ng Fat, L.; Mindell, J.S. Comparison of the Physical Activity and Sedentary Behaviour Assessment Questionnaire and the Short-Form International Physical Activity Questionnaire: An Analysis of Health Survey for England Data. PLoS ONE 2016, 11, e0151647. [Google Scholar] [CrossRef] [PubMed]
- de Moraes Ferrari, G.L.; Kovalskys, I.; Fisberg, M.; Gómez, G.; Rigotti, A.; Sanabria, L.Y.C.; García, M.C.Y.; Torres, R.G.P.; Herrera-Cuenca, M.; Zimberg, I.Z.; et al. Study Group Comparison of self-report versus accelerometer–measured physical activity and sedentary behaviors and their association with body composition in Latin American countries. PLoS ONE 2020, 15, e0232420. [Google Scholar] [CrossRef]
| Characteristic | n (%) |
|---|---|
| Mastectomy side | |
| Left side | 70 (51.9) |
| Right side | 48 (35.6) |
| Both side | 17 (12.6) |
| Lymphadenectomy | |
| No | 82 (60.7) |
| Yes | 53 (39.3) |
| RTH | |
| No | 66 (48.9) |
| Yes | 69 (51.1) |
| CHTH | |
| No | 62 (45.9) |
| Yes | 73 (54.1) |
| Area of residence | |
| Rural | 50 (37.0) |
| Urban | 85 (63.0) |
| Marital status | |
| In a relationship | 90 (66.7) |
| Single | 45 (33.3) |
| Education | |
| Higher level | 115 (85.2) |
| Lower level | 20 (14.8) |
| Occupational status | |
| Professionally active | 40 (29.6) |
| Professionally inactive | 95 (70.4) |
| Comorbidities | |
| No | 38 (28.2) |
| Yes | 97 (71.9) |
| Characteristic | Mean (SD) | Median (IQR) | Min–Max |
|---|---|---|---|
| Age (years) | 63.2 (10.0) | 65.0 (14.0) | 40.0–85.0 |
| BMI (kg/m2) | 27.5 (5.0) | 26.8 (7.2) | 18.0–46.7 |
| WC (cm) | 94.9 (13.2) | 94.0 (19.0) | 62.0–139.0 |
| WHR | 0.9 (0.1) | 0.9 (0.1) | 0.7–1.0 |
| WHtR | 0.6 (0.1) | 0.6 (0.1) | 0.4–0.9 |
| Accelerometer (min/day) | |||
| Moderate PA | 23.2 (18.6) | 19.6 (21.9) | 0.1–95.3 |
| Vigorous PA | 0.2 (0.9) | 0.0 (0.0) | 0.0–7.5 |
| MVPA | 23.6 (18.8) | 19.6 (22.4) | 0.1–95.3 |
| Sedentary behaviors | 780.8 (185.8) | 820.8 (317.8) | 260.0–1466.8 |
| Light PA | 299.6 (76.2) | 303.5 (98.8) | 93.4–477.4 |
| Steps (number) | 6177.7 (2269.1) | 6083.6 (3145.6) | 919.6–13911.3 |
| Energy expenditure | 2344.7 (1226.0) | 2153.7 (1458.0) | 247.9–6153.3 |
| IPAQ (min/day) | |||
| Moderate PA | 183.9 (124.7) | 150.0 (120.0) | 15.0–540.0 |
| Moderate PA (MET) | 4925.3 (3650.3) | 3360.0 (4800.0) | 60.0–15,120.0 |
| Vigorous PA | 8.2 (34.0) | 0.0 (0.0) | 0.0–300.0 |
| Vigorous PA (MET) | 136.9 (497.8) | 0.0 (0.0) | 0.0–2880.0 |
| MVPA | 258.2 (100.2) | 300.0 (180.0) | 32.1–411.4 |
| Sedentary behaviors | 279.9 (151.1) | 240.0 (180.0) | 30.0–800.0 |
| Sum of MET | 6670.8 (2594.1) | 7518.0 (4746.0) | 753.0–12,078.0 |
| Follow-up | 7.7 (7.3) | 4.0 (10.0) | 1.0–41.0 |
| Characteristic | Bias and Limits | Point Value | Lower 95% CI | Upper 95% CI | p |
|---|---|---|---|---|---|
| Moderate PA | Mean difference + 1.96 SD | 77.9 | 42.0 | 113.7 | <0.001 |
| Mean difference | −160.7 | −181.4 | −140.0 | ||
| Mean difference − 1.96 SD | −399.3 | −435.1 | −363.4 | ||
| Vigorous PA | Mean difference + 1.96 SD | 58.6 | 48.6 | 68.6 | <0.05 |
| Mean difference | −8.0 | −13.8 | −2.3 | ||
| Mean difference − 1.96 SD | −74.7 | −84.7 | −64.6 | ||
| MVPA | Mean difference + 1.96 SD | −41.9 | −70.9 | −12.9 | <0.001 |
| Mean difference | −234.6 | −251.4 | −217.9 | ||
| Mean difference − 1.96 SD | −427.4 | −456.4 | −398.4 | ||
| Sedentary behavior | Mean difference + 1.96 SD | 943.0 | 876.5 | 1009.5 | <0.001 |
| Mean difference | 500.9 | 462.5 | 539.3 | ||
| Mean difference − 1.96 SD | 58.8 | −7.7 | 125.3 |
| Characteristic | Accelerometer | IPAQ | ||
|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | |
| MVPA | ||||
| BMI (kg/m2) | −0.35 (−0.90, 0.19) | 0.2195 | −1.00 (−4.47, 2.47) | 0.5728 |
| WC (cm) | −0.24 (−0.45, −0.04) | 0.0207 | −1.17 (−2.49, 0.16) | 0.0851 |
| WHR | −47.53 (−92.28, −2.77) | 0.0377 | −337.12 (−619.34, −54.89) | 0.0206 |
| WHtR | −42.63 (−73.49, −11.79) | 0.0091 | −169.68 (−370.66, 31.31) | 0.0993 |
| Sedentary behaviors | ||||
| BMI (kg/m2) | 2.72 (−3.36, 8.81) | 0.3794 | 4.74 (−0.54, 10.01) | 0.0789 |
| WC (cm) | 0.54 (−1.80, 2.88) | 0.6514 | 1.86 (−0.10, 3.81) | 0.0619 |
| WHR | −85.90 (−591.02, 419.21) | 0.0205 | 220.90 (−223.97, 665.77) | 0.3300 |
| WHtR | 79.67 (−275.78, 435.12) | 0.6619 | 245.98 (−57.34, 549.30) | 0.1105 |
| Characteristic | Accelerometer | IPAQ | ||
|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | |
| MVPA | ||||
| BMI (kg/m2) | −0.01 (−0.60, 0.57) | 0.9616 | −0.58 (−4.50, 3.34) | 0.7712 |
| WC (cm) | −0.09 (−0.31, 0.13) | 0.4434 | −0.96 (−2.43, 0.51) | 0.1984 |
| WHR | −42.46 (−87.31, 2.38) | 0.0549 | −300.87 (−607.11, 5.37) | 0.0549 |
| WHtR | −10.78 (−45.34, 23.76) | 0.5496 | −115.05 (−350.78, 120.67) | 0.3382 |
| Sedentary behaviors | ||||
| BMI (kg/m2) | 3.50 (−3.25, 10.26) | 0.3113 | 6.55 (1.26, 11.84) | 0.0166 |
| WC (cm) | 0.93 (−1.68, 3.54) | 0.4876 | 2.46 (0.34, 4.59) | 0.0236 |
| WHR | 6.89 (−542.37, 528.60) | 0.0549 | 293.40 (−139.26, 726.07) | 0.1859 |
| WHtR | 164.52 (−241.60, 570.63) | 0.4316 | 379.35 (58.48, 700.21) | 0.0214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biskup, M.; Macek, P.; Terek-Derszniak, M.; Zak, M.; Krol, H.; Falana, K.; Gozdz, S. Agreement between Accelerometer-Assessed and Self-Reported Physical Activity and Sedentary Behavior in Female Breast Cancer Survivors. Diagnostics 2023, 13, 3447. https://doi.org/10.3390/diagnostics13223447
Biskup M, Macek P, Terek-Derszniak M, Zak M, Krol H, Falana K, Gozdz S. Agreement between Accelerometer-Assessed and Self-Reported Physical Activity and Sedentary Behavior in Female Breast Cancer Survivors. Diagnostics. 2023; 13(22):3447. https://doi.org/10.3390/diagnostics13223447
Chicago/Turabian StyleBiskup, Malgorzata, Pawel Macek, Malgorzata Terek-Derszniak, Marek Zak, Halina Krol, Krzysztof Falana, and Stanislaw Gozdz. 2023. "Agreement between Accelerometer-Assessed and Self-Reported Physical Activity and Sedentary Behavior in Female Breast Cancer Survivors" Diagnostics 13, no. 22: 3447. https://doi.org/10.3390/diagnostics13223447
APA StyleBiskup, M., Macek, P., Terek-Derszniak, M., Zak, M., Krol, H., Falana, K., & Gozdz, S. (2023). Agreement between Accelerometer-Assessed and Self-Reported Physical Activity and Sedentary Behavior in Female Breast Cancer Survivors. Diagnostics, 13(22), 3447. https://doi.org/10.3390/diagnostics13223447








