Prognostic 18F-FDG Radiomic Features in Advanced High-Grade Serous Ovarian Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. PET/CT Imaging Technique
2.3. Radiomic Features Extraction
2.4. Clinical End Points and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Radiomic Parameters and DFS Association
3.3. Radiomic Parameters and OS Association
3.4. Type of Treatment and DFS/OS Association
3.5. Multivariate Analysis: Radiomic Features (GLRLM_RLNU, GLSZM_ZSNU, and Kurtosis), Type of Treatment, and DFS/OS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| OC | Ovarian Cancer |
| HSOC | High-Grade Serous Carcinoma |
| MR | Magnetic Resonance |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| VOI | Volume of interest |
| 18F-FDG | 18F-Fluorodeoxyglucose |
| FIGO | International Federation of Gynecology and Obstetrics |
| PSF | point spread function |
| SUV | standardized uptake value |
| SUVmax | maximum uptake value |
| PERCIST criteria | Positron Emission Tomography Response Criteria in Solid Tumors. |
| SULmax | normalized for lean body weight maximum uptake value |
| DFS | Disease-free Survival |
| OS | Overall Survival |
| HR | Hazard Ratio |
| TLG | total lesion glycolysis |
| GLZLM or GLSZM | Grey Level Zone Length Matrix or Grey Level Size Zone Matrix |
| ZSNU | Zone Size Non Uniformity |
| GLRLM | Gray level Run Length Matrix |
| RLNU | Run Length Non Uniformity |
| NGLDM | Neighbouring grey level dependence |
| GLZLM_ZLNU | Grey level zone length matrix Zone length nonuniformity |
| GLDZM | Grey Level Distance Zone Matrix |
| MTV | Metabolic tumor volume |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.G.; Valdes, Y.R.; Sivapragasam, M.; Barrett, J.W.; Bell, J.C.; Stojdl, D.; DiMattia, G.E.; Shepherd, T.G. Spatial and Temporal Epithelial Ovarian Cancer Cell Heterogeneity Impacts Maraba Virus Oncolytic Potential. BMC Cancer 2017, 17, 594. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the Ovary, Fallopian Tube, and Peritoneum: 2021 Update. Int. J. Gynecol. Obstet. 2021, 155, 61–85. [Google Scholar] [CrossRef]
- Takaya, H.; Nakai, H.; Sakai, K.; Nishio, K.; Murakami, K.; Mandai, M.; Matsumura, N. Intratumor Heterogeneity and Homologous Recombination Deficiency of High-Grade Serous Ovarian Cancer Are Associated with Prognosis and Molecular Subtype and Change in Treatment Course. Gynecol. Oncol. 2020, 156, 415–422. [Google Scholar] [CrossRef]
- Tan, T.Z.; Heong, V.; Ye, J.; Lim, D.; Low, J.; Choolani, M.; Scott, C.; Tan, D.S.P.; Huang, R.Y.J. Decoding Transcriptomic Intra-Tumour Heterogeneity to Guide Personalised Medicine in Ovarian Cancer. J. Pathol. 2019, 247, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Jamal-Hanjani, M.; Quezada, S.A.; Larkin, J.; Swanton, C. Translational Implications of Tumor Heterogeneity. Clin. Cancer Res. 2015, 21, 1258–1266. [Google Scholar] [CrossRef]
- Ross, J.S.; Ali, S.M.; Wang, K.; Palmer, G.; Yelensky, R.; Lipson, D.; Miller, V.A.; Zajchowski, D.; Shawver, L.K.; Stephens, P.J. Comprehensive Genomic Profiling of Epithelial Ovarian Cancer by next Generation Sequencing-Based Diagnostic Assay Reveals New Routes to Targeted Therapies. Gynecol. Oncol. 2013, 130, 554–559. [Google Scholar] [CrossRef]
- Masoodi, T.; Siraj, S.; Siraj, A.K.; Azam, S.; Qadri, Z.; Parvathareddy, S.K.; Tulbah, A.; Al-Dayel, F.; AlHusaini, H.; AlOmar, O.; et al. Genetic Heterogeneity and Evolutionary History of High-Grade Ovarian Carcinoma and Matched Distant Metastases. Br. J. Cancer 2020, 122, 1219–1230. [Google Scholar] [CrossRef]
- Davnall, F.; Yip, C.S.P.; Ljungqvist, G.; Selmi, M.; Ng, F.; Sanghera, B.; Ganeshan, B.; Miles, K.A.; Cook, G.J.; Goh, V. Assessment of Tumor Heterogeneity: An Emerging Imaging Tool for Clinical Practice? Insights Imaging 2012, 3, 573–589. [Google Scholar] [CrossRef]
- Chicklore, S.; Goh, V.; Siddique, M.; Roy, A.; Marsden, P.K.; Cook, G.J.R. Quantifying Tumour Heterogeneity in 18F-FDG PET/CT Imaging by Texture Analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 133–140. [Google Scholar] [CrossRef]
- Piñeiro-Fiel, M.; Moscoso, A.; Pubul, V.; Ruibal, Á.; Silva-Rodríguez, J.; Aguiar, P. A Systematic Review of Pet Textural Analysis and Radiomics in Cancer. Diagnostics 2021, 11, 380. [Google Scholar] [CrossRef]
- Bailly, C.; Bodet-Milin, C.; Bourgeois, M.; Gouard, S.; Ansquer, C.; Barbaud, M.; Sébille, J.C.; Chérel, M.; Kraeber-Bodéré, F.; Carlier, T. Exploring Tumor Heterogeneity Using PET Imaging: The Big Picture. Cancers 2019, 11, 1282. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Nakajo, M.; Jinguji, M.; Tani, A.; Kikuno, H.; Hirahara, D.; Togami, S.; Kobayashi, H.; Yoshiura, T. Application of a Machine Learning Approach for the Analysis of Clinical and Radiomic Features of Pretreatment [18F]-FDG PET/CT to Predict Prognosis of Patients with Endometrial Cancer. Mol. Imaging Biol. 2021, 23, 756–765. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, F.; Zhang, W.; He, J.; Zhao, Y.; Sun, J. Prognostic Role of Hormone Receptors in Ovarian Cancer: A Systematic Review and Meta-Analysis. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2013, 23, 25–33. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z. Radiomics Analysis of PET and CT Components of 18F-FDG PET/CT Imaging for Prediction of Progression-Free Survival in Advanced High-Grade Serous Ovarian Cancer. Front. Oncol. 2021, 11, 638124. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuze, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. Lifex: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Fornacon-Wood, I.; Mistry, H.; Ackermann, C.J.; Blackhall, F.; McPartlin, A.; Faivre-Finn, C.; Price, G.J.; O’Connor, J.P.B. Reliability and Prognostic Value of Radiomic Features Are Highly Dependent on Choice of Feature Extraction Platform. Eur. Radiol. 2020, 30, 6241–6250. [Google Scholar] [CrossRef] [PubMed]
- Orlhac, F.; Soussan, M.; Chouahnia, K.; Martinod, E.; Buvat, I. 18F-FDG PET-Derived Textural Indices Reflect Tissue-Specific Uptake Pattern in Non-Small Cell Lung Cancer. PLoS ONE 2015, 10, e0145063. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Z.; He, L.; Chen, X.; Pan, D.; Ma, Z.; Liang, C.; Tian, J.; Liang, C. Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non-Small Cell Lung Cancer. Radiology 2016, 281, 947–957. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, Z.; Lin, Y.; Jiang, C.; Li, L.; Liu, Z.; Wang, Y.; Dai, C.; Liu, D.; Qin, X.; et al. 18F-FDG-PET-Based Radiomics Signature Predicts MGMT Promoter Methylation Status in Primary Diffuse Glioma. Cancer Imaging 2019, 19, 58. [Google Scholar] [CrossRef]
- Ou, X.; Zhang, J.; Wang, J.; Pang, F.; Wang, Y.; Wei, X.; Ma, X. Radiomics Based on 18F-FDG PET/CT Could Differentiate Breast Carcinoma from Breast Lymphoma Using Machine-Learning Approach: A Preliminary Study. Cancer Med. 2020, 9, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Solari, E.L.; Gafita, A.; Schachoff, S.; Bogdanović, B.; Villagrán Asiares, A.; Amiel, T.; Hui, W.; Rauscher, I.; Visvikis, D.; Maurer, T.; et al. The Added Value of PSMA PET/MR Radiomics for Prostate Cancer Staging. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yuan, Q.; Lv, W.; Xi, S.; Huang, W.; Sun, Z.; Chen, H.; Zhao, L.; Liu, W.; Hu, Y.; et al. Radiomic Signature of 18F Fluorodeoxyglucose PET/CT for Prediction of Gastric Cancer Survival and Chemotherapeutic Benefits. Theranostics 2018, 8, 5915–5928. [Google Scholar] [CrossRef]
- Aksu, A.; Pınar, N.; Şen, K.; Acar, E.; Gamze Kaya, Ç. Evaluating Focal 18F-FDG Uptake in Thyroid Gland with Radiomics. Nucl. Med. Mol. Imaging 2020, 54, 241–248. [Google Scholar] [CrossRef]
- Vargas, H.A.; Veeraraghavan, H.; Micco, M.; Nougaret, S.; Lakhman, Y.; Meier, A.A.; Sosa, R.; Soslow, R.A.; Levine, D.A.; Weigelt, B.; et al. A Novel Representation of Inter-Site Tumour Heterogeneity from Pre-Treatment Computed Tomography Textures Classifies Ovarian Cancers by Clinical Outcome. Eur. Radiol. 2017, 27, 3991–4001. [Google Scholar] [CrossRef]
- Meier, A.; Veeraraghavan, H.; Nougaret, S.; Lakhman, Y.; Sosa, R.; Soslow, R.A.; Sutton, E.J.; Hricak, H.; Sala, E.; Vargas, H.A. Association between CT-Texture-Derived Tumor Heterogeneity, Outcomes, and BRCA Mutation Status in Patients with High-Grade Serous Ovarian Cancer. Abdom. Radiol. 2019, 44, 2040–2047. [Google Scholar] [CrossRef]
- Lu, H.; Arshad, M.; Thornton, A.; Avesani, G.; Cunnea, P.; Curry, E.; Kanavati, F.; Liang, J.; Nixon, K.; Williams, S.T.; et al. A Mathematical-Descriptor of Tumor-Mesoscopic-Structure from Computed-Tomography Images Annotates Prognostic- and Molecular-Phenotypes of Epithelial Ovarian Cancer. Nat. Commun. 2019, 10, 764. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, Y.; Chen, X.; Wu, G.; Liu, X.; Zhang, P.; Bai, Y.; Lu, P.; Yao, W.; Wang, Y.; et al. Magnetic Resonance Imaging Radiomics in Categorizing Ovarian Masses and Predicting Clinical Outcome: A Preliminary Study. Eur. Radiol. 2019, 29, 3358–3371. [Google Scholar] [CrossRef]
- Li, H.M.; Gong, J.; Li, R.M.; Bin Xiao, Z.; Qiang, J.W.; Peng, W.J.; Gu, Y.J. Development of MRI-Based Radiomics Model to Predict the Risk of Recurrence in Patients with Advanced High-Grade Serous Ovarian Carcinoma. Am. J. Roentgenol. 2021, 217, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Chitalia, R.; Viswanath, V.; Pantel, A.R.; Peterson, L.M.; Gastounioti, A.; Cohen, E.A.; Muzi, M.; Karp, J.; Mankoff, D.A.; Kontos, D. Functional 4-D Clustering for Characterizing Intratumor Heterogeneity in Dynamic Imaging: Evaluation in FDG PET as a Prognostic Biomarker for Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3990–4001. [Google Scholar] [CrossRef] [PubMed]
- Bashir, U.; Foot, O.; Wise, O.; Siddique, M.M.; Mclean, E.; Bille, A.; Goh, V.; Cook, G.J. Investigating the Histopathologic Correlates of 18F-FDG PET Heterogeneity in Non-Small-Cell Lung Cancer. Nucl. Med. Commun. 2018, 39, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, C.; Zhao, Y.; Hu, S.; Song, S.; Hu, X.; Wang, B.; Gong, C. Heterogeneity Derived from 18F-FDG PET/CT Predicts Immunotherapy Outcome for Metastatic Triple-Negative Breast Cancer Patients. Cancer Med. 2022, 11, 1948–1955. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. Image Biomarker Standardisation Initiative. Radiology 2016, 295, 328–338. [Google Scholar] [CrossRef]
- Kim, M.; Gu, W.; Nakajima, T.; Higuchi, T.; Ogawa, M.; Shimizu, T.; Yamaguchi, T.; Takahashi, A.; Tsushima, Y.; Yokoo, S. Texture Analysis of [18F]-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography for Predicting the Treatment Response of Postoperative Recurrent or Metastatic Oral Squamous Cell Carcinoma Treated with Cetuximab. Ann. Nucl. Med. 2021, 35, 871–880. [Google Scholar] [CrossRef]
- Acar, E.; Turgut, B.; Yiǧit, S.; Kaya, G.C. Comparison of the Volumetric and Radiomics Findings of 18F-FDG PET/CT Images with Immunohistochemical Prognostic Factors in Local/Locally Advanced Breast Cancer. Nucl. Med. Commun. 2019, 40, 764–772. [Google Scholar] [CrossRef]
- Aksu, A.; Vural Topuz, Ö.; Yılmaz, G.; Çapa Kaya, G.; Yılmaz, B. Dual Time Point Imaging of Staging PSMA PET/CT Quantification; Spread and Radiomic Analyses. Ann. Nucl. Med. 2022, 36, 310–318. [Google Scholar] [CrossRef]
- Lue, K.-H.; Wu, Y.-F.; Liu, S.-H.; Hsieh, T.-C.; Chuang, K.-S.; Lin, H.-H.; Chen, Y.-H. Intratumor Heterogeneity Assessed by 18F-FDG PET/CT Predicts Treatment Response and Survival Outcomes in Patients with Hodgkin Lymphoma. Acad. Radiol. 2020, 27, e183–e192. [Google Scholar] [CrossRef]
- Chung, H.H.; Kwon, H.W.; Kang, K.W.; Park, N.-H.; Song, Y.-S.; Chung, J.-K.; Kang, S.-B.; Kim, J.W. Prognostic Value of Preoperative Metabolic Tumor Volume and Total Lesion Glycolysis in Patients with Epithelial Ovarian Cancer. Ann. Surg. Oncol. 2012, 19, 1966–1972. [Google Scholar] [CrossRef]
- Lee, J.W.; Cho, A.; Lee, J.-H.; Yun, M.; Lee, J.D.; Kim, Y.T.; Kang, W.J. The Role of Metabolic Tumor Volume and Total Lesion Glycolysis on 18F-FDG PET/CT in the Prognosis of Epithelial Ovarian Cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Feng, Z.; Jiang, Z.; Wen, H.; Xu, J.; Pan, H.; Deng, Y.; Zhang, L.; Ju, X.; Chen, X.; et al. Prognostic Predictive Value of Preoperative Intratumoral 2-Deoxy-2-(18F)Fluoro- d -Glucose Uptake Heterogeneity in Patients with High-Grade Serous Ovarian Cancer. Nucl. Med. Commun. 2018, 39, 928–935. [Google Scholar] [CrossRef]
- Soo Kim, B.; Kim, S.-J. Prognostic Value of Intratumoral Heterogeneity of Preoperative 18F-FDG PET/CT in Pancreatic Cancer. Hell. J. Nucl. Med. 2021, 24, 172–177. [Google Scholar]
- Moazemi, S.; Erle, A.; Lütje, S.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. Estimating the Potential of Radiomics Features and Radiomics Signature from Pretherapeutic PSMA-PET-CT Scans and Clinical Data for Prediction of Overall Survival When Treated with 177Lu-PSMA. Diagnostics 2021, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, X.L.; Pu, L.T.; Zhou, R.F.; Ou, X.J.; Tian, R. Prediction of Overall Survival and Progression-Free Survival by the 18F-FDG PET/CT Radiomic Features in Patients with Primary Gastric Diffuse Large B-Cell Lymphoma. Contrast Media Mol. Imaging 2019, 2019, 5963607. [Google Scholar] [CrossRef] [PubMed]
- Traverso, A.; Wee, L.; Dekker, A.; Gillies, R. Repeatability and Reproducibility of Radiomic Features: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1143–1158. [Google Scholar] [CrossRef]
- Eertink, J.J.; Pfaehler, E.A.G.; Wiegers, S.E.; Lugtenburg, P.J.; Hoekstra, O.S.; Zijlstra, J.M.; de Vet, H.C.W.; Boellaard, R. Quantitative Radiomics Features in Diffuse Large B-Cell Lymphoma: Does Segmentation Method Matter? J. Nucl. Med. 2022, 63, 389–395. [Google Scholar] [CrossRef]


| Matrix | Index |
|---|---|
| First order features | |
| Morphological | Approximate Volume |
| MORPHOLOGICAL_Compacity | |
| MORPHOLOGICAL_Compactness2 | |
| MORPHOLOGICAL_Centre OF Mass Shift | |
| Intensity-based | Total Lesion Glycolysis |
| Variance | |
| Kurtosis | |
| Minimum Grey Level | |
| Histogram Uniformity | |
| Higher order features | |
| Gray-Level Cooccurrence Matrix (GLCM) | GLCM_Joint Maximum |
| GLCM_Inverse Difference Moment | |
| GLCM_Inverse Variance | |
| GLCM_Correlation | |
| GLCM_Cluster Tendency | |
| GLCM_Cluster Shade | |
| Neighborhood grey tone difference (NGTDM) | Coarseness |
| Contrast | |
| Busyness | |
| Gray level Run-Length Matrix (GLRLM) | Long Runs Emphasis |
| Run Length Non Uniformity | |
| Grey-Level Zone Length Matrix (GLZLM) or Grey-Level Size Zone Matrix (GLSZM) | Large Zone High Grey Level Emphasis |
| Zone Size Non Uniformity | |
| Normalised Zone Size Non Uniformity |
| Characteristic | Patients |
|---|---|
| Total patients | 36 |
| Mean age, years (range) | 60 (42–84) |
| FIGO stage | |
| III | 12 (33.3%) |
| IV | 24 (66.7%) |
| Histology | |
| High-grade serous carcinoma | 36 |
| Type of treatment | |
| Neoadjuvant chemotherapy + interval debulking surgery | 20 (55.6%) |
| Primary cytoreductive surgery + adjuvant chemotherapy | 8 (22.2%) |
| Chemotherapy only | 8 (22.2%) |
| Chemotherapy | |
| Carboplatin + paclitaxel with Bevacizumab + Bevacizumab as maintenance | 6 (16.6%) |
| Carboplatin + paclitaxel + Bevacizumab as maintenance | 13 (36.1%) |
| Carboplatin + paclitaxel without Bevacizumab | 3 (8.3%) |
| Carboplatin + paclitaxel with Bevacizumab without maintenance | 14 (38.9%) |
| PARPi | 1 (2.7%) |
| Mean Follow-up months | 31.19 |
| Mean DFS, months | 19.6 ± 11 |
| Mean OS, months | 37.1 ± 20.3 |
| n = 36 | MTV | TLG | _Kurtosis | GLSZM_ZSNU | GLRLM_RLNU |
|---|---|---|---|---|---|
| Mean | 1025.7 | 3828.1 | 4.3 | 1275.7 | 11,100.4 |
| Median | 756.8 | 2885.5 | 1.3 | 1059.2 | 8017.6 |
| Max | 4016.2 | 13,023.0 | 58.7 | 5265.0 | 42,719.5 |
| Min | 19.2 | 154.0 | −0.8 | 60.0 | 194.9 |
| SD | 973.8 | 3383.4 | 10.1 | 1225.7 | 10,107.9 |
| Feature | ROC Cut Off | Se | Sp | Mean DFS/OS (Months) | p | HR (CI 95%) | p | |
|---|---|---|---|---|---|---|---|---|
| Group Superior to Cut-Off | Group Inferior to Cut-Off | |||||||
| DSF analysis | ||||||||
| GLRLM_RLNU | 7388.3 | 0.73 | 0.60 | 19.7 | 31.7 | 0.035 * | 0.402 | 0.041 * |
| GLSZM_ZSNU | 1103.9 | 0.50 | 0.60 | 21.6 | 26.9 | 0.206 | - | - |
| OS analysis | ||||||||
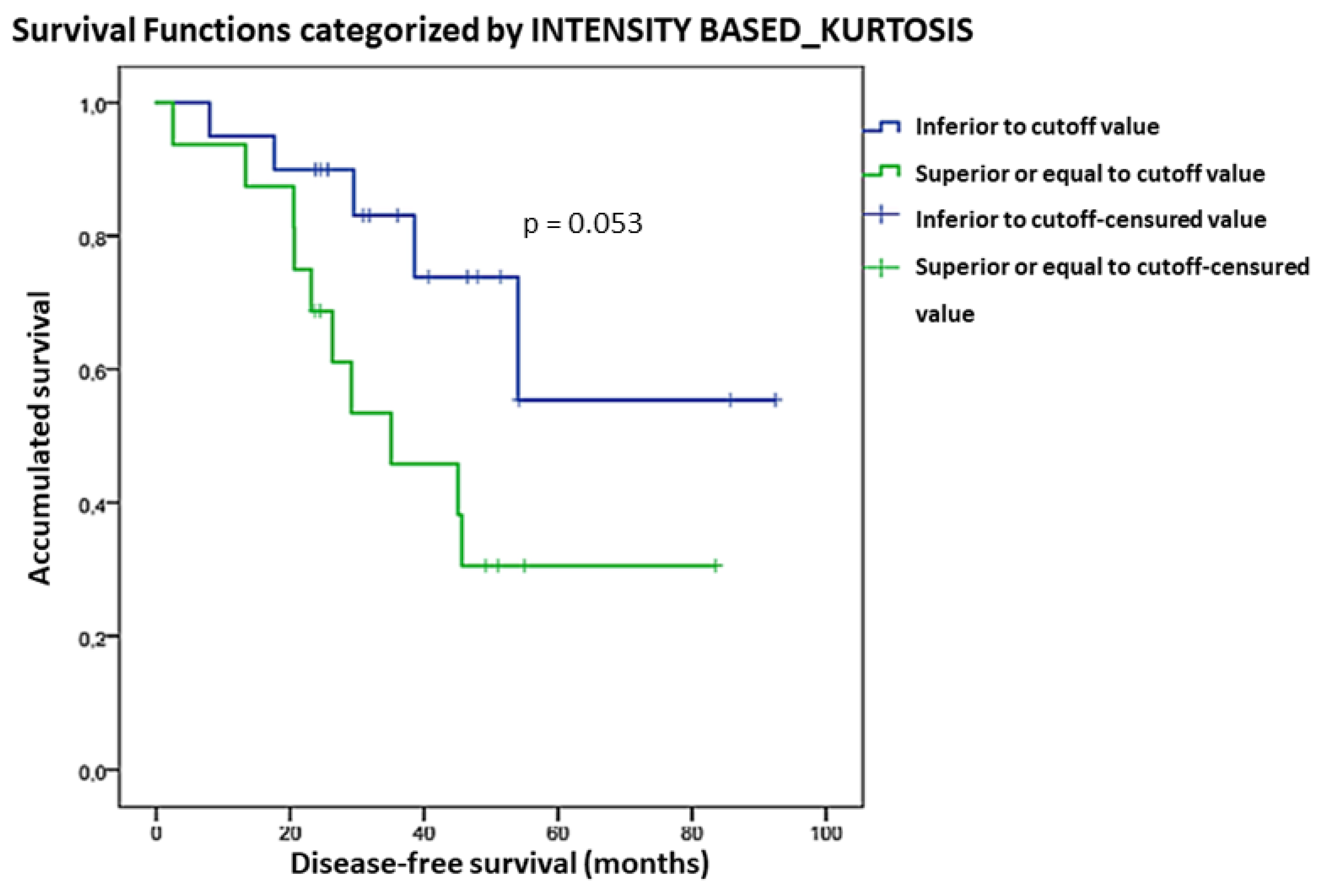
| _Kurtosis | 1.8 | 0.66 | 0.71 | 44.3 | 68 | 0.053 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Travaglio Morales, D.; Huerga Cabrerizo, C.; Losantos García, I.; Coronado Poggio, M.; Cordero García, J.M.; Llobet, E.L.; Monachello Araujo, D.; Rizkallal Monzón, S.; Domínguez Gadea, L. Prognostic 18F-FDG Radiomic Features in Advanced High-Grade Serous Ovarian Cancer. Diagnostics 2023, 13, 3394. https://doi.org/10.3390/diagnostics13223394
Travaglio Morales D, Huerga Cabrerizo C, Losantos García I, Coronado Poggio M, Cordero García JM, Llobet EL, Monachello Araujo D, Rizkallal Monzón S, Domínguez Gadea L. Prognostic 18F-FDG Radiomic Features in Advanced High-Grade Serous Ovarian Cancer. Diagnostics. 2023; 13(22):3394. https://doi.org/10.3390/diagnostics13223394
Chicago/Turabian StyleTravaglio Morales, Daniela, Carlos Huerga Cabrerizo, Itsaso Losantos García, Mónica Coronado Poggio, José Manuel Cordero García, Elena López Llobet, Domenico Monachello Araujo, Sebastián Rizkallal Monzón, and Luis Domínguez Gadea. 2023. "Prognostic 18F-FDG Radiomic Features in Advanced High-Grade Serous Ovarian Cancer" Diagnostics 13, no. 22: 3394. https://doi.org/10.3390/diagnostics13223394
APA StyleTravaglio Morales, D., Huerga Cabrerizo, C., Losantos García, I., Coronado Poggio, M., Cordero García, J. M., Llobet, E. L., Monachello Araujo, D., Rizkallal Monzón, S., & Domínguez Gadea, L. (2023). Prognostic 18F-FDG Radiomic Features in Advanced High-Grade Serous Ovarian Cancer. Diagnostics, 13(22), 3394. https://doi.org/10.3390/diagnostics13223394






