The Role of the Fatty Liver Index (FLI) in the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review
Abstract
:1. Introduction
2. Material, Methods, and Results
2.1. Part I: The Role of FLI in Diagnostic Management of NAFLD
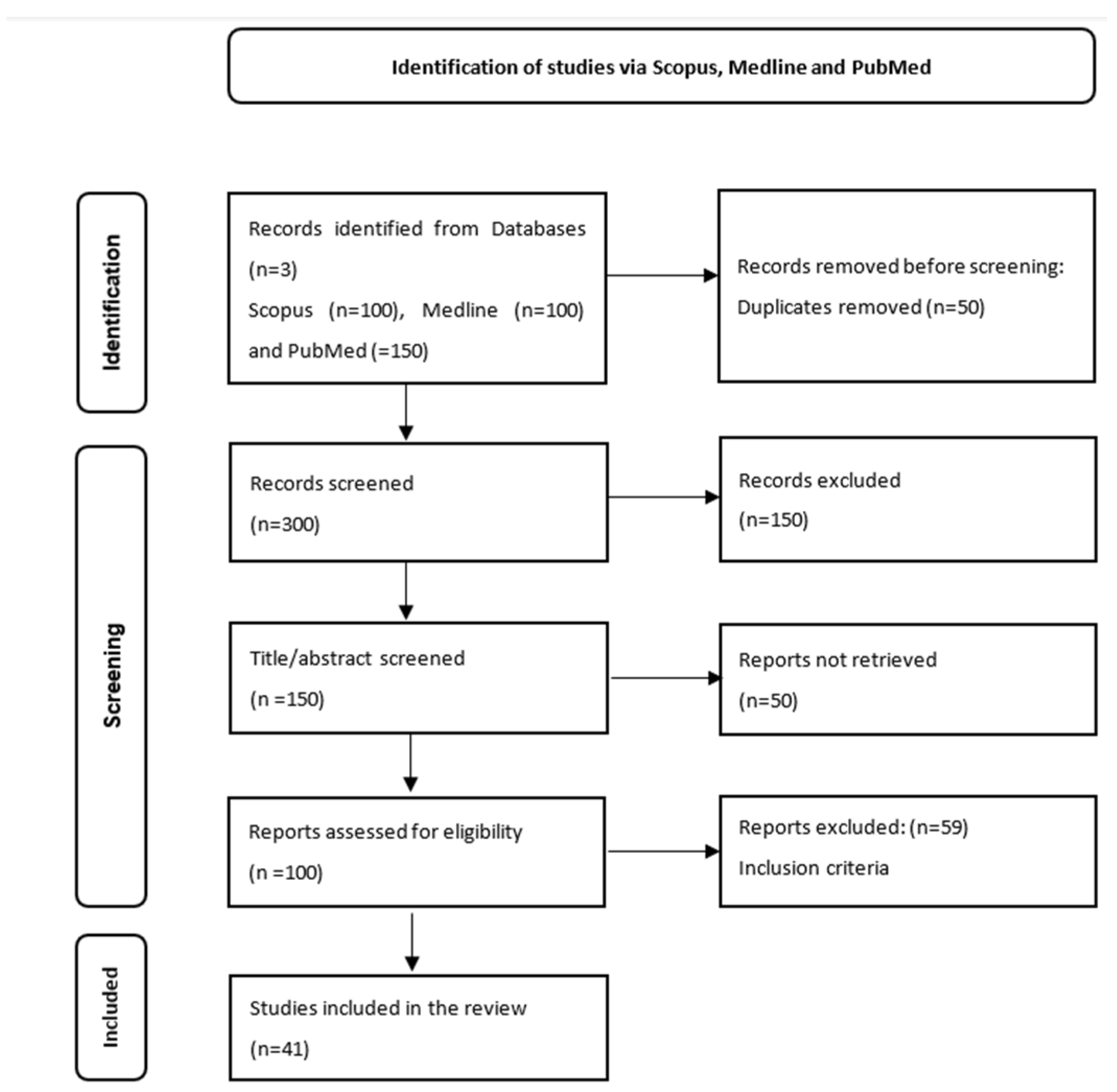
2.1.1. Search Literature
2.1.2. Selection Criteria for Studies
Definition of NAFLD
Diagnosis and Screening of NAFLD
FLI Score
2.1.3. Search Strategy
- i.
- General search terms related to HS: NAFLD, HS and NASH;
- ii.
- Specific search terms related to the diagnostic methods of NAFLD: LBP, AU, CAP, MRI, CT, HSI and FibroTest.
2.1.4. Selection of Articles
2.1.5. Data Extraction
2.1.6. Results
Description of Included Studies
Description of Studies That Appreciated the Diagnostic Role of FLI
Description of Studies That Appreciated the Screening Role of FLI for NAFLD
2.2. Part II: The Role of FLI in Therapeutic Management of NAFLD
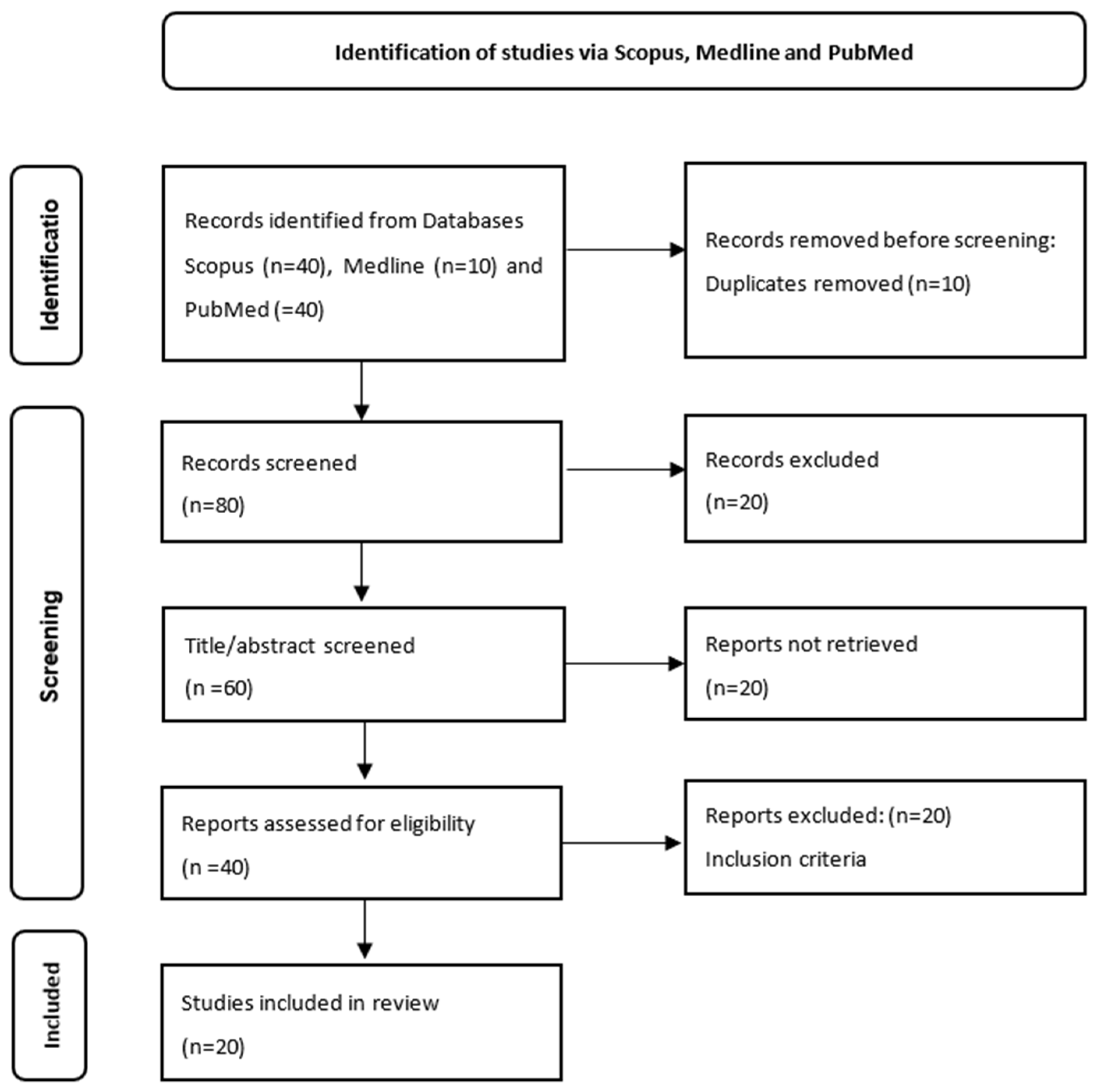
2.2.1. Literature Search, Search Strategy and Eligibility Criteria
- i.
- Disease-specific terms: NAFLD or HS or NASH;
- ii.
- Terms related to the influencing possibilities of NAFLD: diets or lifestyles, or bariatric interventions or HS therapies;
- iii.
- Terms related to other means of evaluation of HS: FLI or AU, CT or MRI, or FibroTest or CAP.
2.2.2. Data Extraction
2.2.3. Synthesis of the Best Evidence: Levels of Evidence
2.2.4. Results
Characteristics of Included Studies
The Role of FLI in Monitoring the Effects of Different Diets on NAFLD
The Role of FLI in Monitoring the Effects of Lifestyle Change on NAFLD
The Role of FLI in Assessing the Effectiveness of Bariatric Interventions on NAFLD
The Role of FLI in Assessing the Effectiveness of Drug Therapies on NAFLD
3. Discussions
4. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Machado, M.V.; Diehl, A.M. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology 2016, 150, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dhyani, M.; Grajo, J.R.; Sirlin, C.; Samir, A.E. Current status of imaging in nonalcoholic fatty liver disease. World J. Hepatol. 2018, 10, 530–542. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S37–S47. [Google Scholar] [CrossRef] [PubMed]
- Manka, P.P.; Kaya, E.; Canbay, A.; Syn, W.K. A Review of the Epidemiology, Pathophysiology, and Efficacy of Anti-diabetic Drugs Used in the Treatment of Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2021, 66, 3676–3688. [Google Scholar] [CrossRef]
- Zhou, J.; Yan, F.; Xu, J.; Lu, Q.; Zhu, X.; Gao, B.; Zhang, H.; Yang, R.; Luo, Y. Diagnosis of steatohepatitis and fibrosis in biopsy-proven nonalcoholic fatty liver diseases: Including two-dimension real-time shear wave elastography and noninvasive fibrotic biomarker scores. Quant. Imaging Med. Surg. 2022, 12, 1800–1814. [Google Scholar] [CrossRef]
- Obika, M.; Noguchi, H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp. Diabetes Res. 2012, 2012, 145754. [Google Scholar] [CrossRef]
- Yang, K.C.; Liao, Y.Y.; Tsui, P.H.; Yeh, C.K. Ultrasound imaging in nonalcoholic liver disease: Current applications and future developments. Quant. Imaging Med. Surg. 2019, 9, 546–551. [Google Scholar] [CrossRef]
- Lee, D.H. Imaging evaluation of non-alcoholic fatty liver disease: Focused on quantification. Clin. Mol. Hepatol. 2017, 23, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.D.; Huang, J.F.; Chen, Q.S.; Lin, G.F.; Zeng, H.X.; Lin, X.F.; Lin, X.J.; Lin, L.; Lin, Q.C. Validation of fatty liver index and hepatic steatosis index for screening of non-alcoholic fatty liver disease in adults with obstructive sleep apnea hypopnea syndrome. Chin. Med. J. 2019, 132, 2670–2676. [Google Scholar] [CrossRef] [PubMed]
- Han, A.L.; Lee, H.K. Comparison of the Diagnostic Performance of Steatosis Indices for Discrimination of CT-Diagnosed Metabolic Dysfunction-Associated Fatty Liver Disease. Metabolites 2022, 12, 664. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Peltonen, M.; Hakkarainen, A.; Sevastianova, K.; Bergholm, R.; Johansson, L.M.; Lundbom, N.; Rissanen, A.; Ridderstråle, M.; Groop, L.; et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009, 137, 865–872. [Google Scholar] [CrossRef]
- Poynard, T.; Ratziu, V.; Naveau, S.; Thabut, D.; Charlotte, F.; Messous, D.; Capron, D.; Abella, A.; Massard, J.; Ngo, Y.; et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp. Hepatol. 2005, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; He, M.; Zhang, Y.; Li, T.; Liu, Y.; Xu, Z.; Chen, W. Validation of simple indexes for nonalcoholic fatty liver disease in western China: A retrospective cross-sectional study. Endocr. J. 2018, 65, 373–381. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Liebe, R.; Esposito, I.; Bock, H.H.; Vom Dahl, S.; Stindt, J.; Baumann, U.; Luedde, T.; Keitel, V. Diagnosis and management of secondary causes of steatohepatitis. J. Hepatol. 2021, 74, 1455–1471. [Google Scholar] [CrossRef]
- Wong, V.W.; Adams, L.A.; de Ledinghen, V.; Wong, G.L.; Sookoian, S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Honda, Y.; Yoneda, M.; Imajo, K.; Nakajima, A. Elastography Techniques for the Assessment of Liver Fibrosis in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 4039. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.; Săndulescu, L.; Săftoiu, A. The Role of Elastography in Non-Alcoholic Fatty Liver Disease. Curr. Health. Sci. J. 2020, 46, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Mărginean, C.O.; Meliț, L.E.; Săsăran, M.O. Elastography—A Bona Fide Non-Invasive Method for Assessing Non-Alcoholic Fatty Liver Disease in Children. Appl. Sci. 2021, 11, 3240. [Google Scholar] [CrossRef]
- Reinson, T.; Buchanan, R.M.; Byrne, C.D. Noninvasive serum biomarkers for liver fibrosis in NAFLD: Current and future. Clin. Mol. Hepatol. 2023, 29, S157–S170. [Google Scholar] [CrossRef] [PubMed]
- Vali, Y.; Lee, J.; Boursier, J.; Spijker, R.; Löffler, J.; Verheij, J.; Brosnan, M.J.; Böcskei, Z.; Anstee, Q.M.; LITMUS Systematic Review Team; et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and metaanalysis. J. Hepatol. 2020, 73, 252–262. [Google Scholar] [CrossRef]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef]
- Calès, P.; Boursier, J.; Chaigneau, J.; Lainé, F.; Sandrini, J.; Michalak, S.; Hubert, I.; Dib, N.; Oberti, F.; Bertrais, S.; et al. Diagnosis of different liver fibrosis characteristics by blood tests in non-alcoholic fatty liver disease. Liver Int. 2010, 30, 1346–1354. [Google Scholar] [CrossRef]
- McPherson, S.; Stewart, S.F.; Henderson, E.; Burt, A.D.; Day, C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef]
- Vali, Y.; Lee, J.; Boursier, J.; Spijker, R.; Verheij, J.; Brosnan, M.J.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. FibroTest for evaluating fibrosis in non-alcoholic fatty liver disease patients: A systematic review and meta-analysis. J. Clin. Med. 2021, 10, 2415. [Google Scholar] [CrossRef]
- Gheorghe, G.; Bungau, S.; Ceobanu, G.; Ilie, M.; Bacalbasa, N.; Bratu, O.G.; Vesa, C.M.; Gaman, M.A.; Diaconu, C.C. The non-invasive assessment of hepatic fibrosis. J. Formos. Med. Assoc. 2021, 120, 794–803. [Google Scholar] [CrossRef]
- Brol, M.J.; Drebber, U.; Luetkens, J.A.; Odenthal, M.; Trebicka, J. “The pathogenesis of hepatic fibrosis: Basic facts and clinical challenges”—Assessment of liver fibrosis: A narrative review. Dig. Med. Res. 2022, 5, 24. [Google Scholar] [CrossRef]
- Forlano, D.R.; Sigon, G.; Mullish, B.H.; Yee, M.; Manousou, P. Screening for NAFLD-Current Knowledge and Challenges. Metabolites 2023, 13, 536. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Castera, L.; Wong, V.W.-S. Noninvasive Assessment of Liver Fibrosis in NAFLD. Clin. Gastroenterol. Hepatol. 2023, 21, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mak, L.Y.; Yuen, M.F.; Seto, W.K. Screening strategy for non-alcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S103–S122. [Google Scholar] [CrossRef] [PubMed]
- Kasper, P.; Demir, M.; Steffen, H.M. Screening strategies for non-alcoholic fatty liver disease: A holistic approach is needed. Clin. Mol. Hepatol. 2023, 29, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Staufer, K.; Stauber, R.E. Steatotic Liver Disease: Metabolic Dysfunction, Alcohol, or Both? Biomedicines 2023, 11, 2108. [Google Scholar] [CrossRef]
- Cho, Y.; Lim, S.; Joo, S.K.; Jeong, D.; Kim, J.H.; Bae, J.M.; Park, J.H.; Chang, M.S.; Lee, D.H.; Jung, Y.J.; et al. Nonalcoholic steatohepatitis is associated with a higher risk of advanced colorectal neoplasm. Liver Int. 2019, 39, 1722–1731. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef]
- Dyson, J.K.; Anstee, Q.M.; McPherson, S. Non-alcoholic fatty liver disease: A practical approach to diagnosis and staging. Frontline Gastroenterol. 2014, 5, 211–218. [Google Scholar] [CrossRef]
- Balkau, B.; Lange, C.; Vol, S.; Fumeron, F.; Bonnet, F. Nine-year incident diabetes is predicted by fatty liver indices: The French D.E.S.I.R. study. BMC Gastroenterol. 2010, 10, 56. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Webb, M.; Assy, N.; Blendis, L.; Yeshua, H.; Leshno, M.; Ratziu, V.; Halpern, Z.; Oren, R.; Santo, E. Comparison of fatty liver index with noninvasive methods for steatosis detection and quantification. World J. Gastroenterol. 2013, 19, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Fedchuk, L.; Nascimbeni, F.; Pais, R.; Charlotte, F.; Housset, C.; Ratziu, V. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2014, 40, 1209–1222. [Google Scholar] [CrossRef]
- Kahl, S.; Straßburger, K.; Nowotny, B.; Livingstone, R.; Klüppelholz, B.; Keßel, K.; Hwang, J.-H.; Giani, G.; Hoffmann, B.; Pacini, G.; et al. Comparison of Liver Fat Indices for the Diagnosis of Hepatic Steatosis and Insulin Resistance. PLoS ONE 2014, 9, e94059. [Google Scholar] [CrossRef]
- Goulart, A.C.; Oliveira, I.R.S.; Alencar, A.P.; Santos, M.S.C.; dos Santos, I.S.; Martines, B.M.R.; Meireles, D.P.; Martines, J.A.; Misciagna, G.; Benseñor, I.M.; et al. Diagnostic accuracy of a noninvasive hepatic ultrasound score for non-alcoholic fatty liver disease (NAFLD) in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Sao Paulo Med. J. 2015, 133, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.F.; Yki-Järvinen, H.; Bian, H.; Lin, H.D.; Yan, H.M.; Chang, X.X.; Zhou, Y.; Gao, X. Influence of Ethnicity on the Accuracy of Non-Invasive Scores Predicting Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0160526. [Google Scholar] [CrossRef]
- Motamed, N.; Sohrabi, M.; Ajdarkosh, H.; Hemmasi, G.; Maadi, M.; Sayeedian, F.S.; Pirzad, R.; Abedi, K.; Aghapour, S.; Fallahnezhad, M.; et al. Fatty liver indexvswaist circumference for predicting non-alcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 3023–3030. [Google Scholar] [CrossRef]
- Dehnavi, Z.; Razmpour, F.; Belghaisi Naseri, M.; Nematy, M.; Alamdaran, S.A.; Vatanparast, H.A.; Azimi Nezhad, M.; Abbasi, B.; Ganji, A. Fatty Liver Index (FLI) in Predicting Non-alcoholic Fatty Liver Disease (NAFLD). Hepat. Mon. 2018, 18, e63227. [Google Scholar] [CrossRef]
- Lind, L.; Johansson, L.; Ahlström, H.; Eriksson, J.W.; Larsson, A.; Risérus, U.; Kullberg, J.; Oscarsson, J. Comparison of four non-alcoholic fatty liver disease detection scores in a Caucasian population. World J. Hepatol. 2020, 12, 149–159. [Google Scholar] [CrossRef]
- Chen, L.W.; Huang, P.R.; Chien, C.H.; Lin, C.L.; Chien, R.N. A community-based study on the application of fatty liver index in screening subjects with nonalcoholic fatty liver disease. J. Formos. Med. Assoc. 2020, 119, 173–181. [Google Scholar] [CrossRef]
- Jung, T.Y.; Kim, M.S.; Hong, H.P.; Kang, K.A.; Jun, D.W. Comparative Assessment and External Validation of Hepatic Steatosis Formulae in a Community-Based Setting. J. Clin. Med. 2020, 9, 2851. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, Y.; Yu, W.; Xu, Z.; Xin, Y.; Zhao, Z.; Liu, S.; Lv, K. Diagnostic accuracy assessment of molecular prediction model for the risk of NAFLD based on MRI-PDFF diagnosed Chinese Han population. BMC Gastroenterol. 2021, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Donghia, R.; Guerra, V.; Procino, F.; Lampignano, L.; Castellana, F.; Zupo, R.; Sardone, R.; De Pergola, G.; Romanelli, F.; et al. Performance of Fatty Liver Index in Identifying Non-Alcoholic Fatty Liver Disease in Population Studies. A Meta-Analysis. J. Clin. Med. 2021, 10, 1877. [Google Scholar] [CrossRef] [PubMed]
- Preciado-Puga, M.C.; Ruiz-Noa, Y.; Garcia-Ramirez, J.R.; Jordan-Perez, B.; Garnelo-Cabañas, S.; Lazo de la Vega-Monroy, M.L.; Gutierrez-Aguirre, K.I.; Ibarra-Reynoso, L.R. Non-invasive diagnosis of non-alcoholic fatty liver disease using an algorithm combining clinical indexes and ultrasonographic measures. Ann. Hepatol. 2021, 21, 100264. [Google Scholar] [CrossRef]
- Borges-Canha, M.; Neves, J.; Silva, M.; Mendonça, F.; Moreno, T.; Ribeiro, S.; Correa, J.; Vale, C.; Gonçalves, J.; Urbano Ferreira, H.; et al. Waist-to-Hip Ratio and Inflammatory Parameters Are Associated with Risk of Non-Alcoholic Fatty Liver Disease in Patients with Morbid Obesity. Biomedicines 2022, 10, 2416. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Ye, J.; Li, X.; Lin, Y.; Feng, S.; Liao, B.; Wang, W.; Gong, X.; Zhong, B. Discrepancies between Nonalcoholic and Metabolic-associated Fatty Liver Disease by Multiple Steatosis Assessment. J. Clin. Transl. Hepatol. 2022, 10, 1013–1026. [Google Scholar] [CrossRef]
- de Silva, M.H.A.D.; Hewawasam, R.P.; Kulatunge, C.R.; Chamika, R.M.A. The accuracy of fatty liver index for the screening of overweight and obese children for non-alcoholic fatty liver disease in resource limited settings. BMC Pediatr. 2022, 22, 511. [Google Scholar] [CrossRef]
- Ali, M.A.E.H.; Kamal, G.M.; Younis, M.A.A.; Zaghloul, A.M. Diagnostic performance of serum steatosis biomarkers in Prediction of Non Alcoholic Fatty Liver Disease in Adult Asymptomatic Egyptians. SVU-Int. J. Med. Sci. 2022, 5, 51–63. [Google Scholar] [CrossRef]
- Kim, H.N.; Jeon, H.J.; Choi, H.G.; Kwon, I.S.; Rou, W.S.; Lee, J.E.; Lee, T.H.; Kim, S.H.; Lee, B.S.; Shin, K.S.; et al. CT-based Hounsfield unit values reflect the degree of steatohepatitis in patients with low-grade fatty liver disease. BMC Gastroenterol. 2023, 23, 77. [Google Scholar] [CrossRef]
- Mertens, J.; Weyler, J.; Dirinck, E.; Vonghia, L.; Kwanten, W.J.; Mortelmans, L.; Peleman, C.; Chotkoe, S.; Spinhoven, M.; Vanhevel, F.; et al. Prevalence, risk factors and diagnostic accuracy of non-invasive tests for NAFLD in people with type 1 diabetes. JHEP Rep. 2023, 5, 100753. [Google Scholar] [CrossRef]
- Kamari, N.; Fateh, H.L.; Darbandi, M.; Najafi, F.; Moradi, M.; Pasdar, Y. Fatty liver index relationship with biomarkers and lifestyle: Result from RaNCD cohort study. BMC Gastroenterol. 2023, 23, 172. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, K.; Chang, J.; Choi, S.; Kim, S.M.; Son, J.S.; Lee, G.; Kim, W.; Park, S.M. Development of a simple nonalcoholic fatty liver disease scoring system indicative of metabolic risks and insulin resistance. Ann. Transl. Med. 2020, 8, 1414. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.B. Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: Current and future developments. Clin. Mol. Hepatol. 2023, 29, S150–S156. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Babazono, A.; Maeda, T.; Imatoh, T.; Une, H. Evaluation of the fatty liver index as a predictor for the development of diabetes among insurance beneficiaries with prediabetes. J. Diabetes Investig. 2015, 6, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, P.; Zhang, R.; Zhang, M.; Li, Y.; Huang, M.; Ji, X.; Jiang, Y.; Wang, C.; Li, R.; et al. Both WHR and FLI as Better Algorithms for Both Lean and Overweight/Obese NAFLD in a Chinese Population. J. Clin. Gastroenterol. 2018, 53, e253–e260. [Google Scholar] [CrossRef]
- Klisic, A.; Isakovic, A.; Kocic, G.; Kavaric, N.; Jovanovic, M.; Zvrko, E.; Skerovic, V.; Ninic, A. Relationship between Oxidative Stress, Inflammation and Dyslipidemia with Fatty Liver Index in Patients with Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2018, 126, 371–378. [Google Scholar] [CrossRef]
- Khang, A.R.; Lee, H.W.; Yi, D.; Kang, Y.H.; Son, S.M. The fatty liver index, a simple and useful predictor of metabolic syndrome: Analysis of the Korea National Health and Nutrition Examination Survey 2010–2011. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 181–190. [Google Scholar] [CrossRef]
- Hsu, C.L.; Wu, F.Z.; Lin, K.H.; Chen, Y.H.; Wu, P.C.; Chen, Y.H.; Chen, C.S.; Wang, W.H.; Mar, G.Y.; Yu, H.C. Role of Fatty Liver Index and Metabolic Factors in the Prediction of Nonalcoholic Fatty Liver Disease in a Lean Population Receiving Health Checkup. Clin. Transl. Gastroenterol. 2019, 10, e00042. [Google Scholar] [CrossRef]
- Motamed, N.; Faraji, A.H.; Khonsari, M.R.; Maadi, M.; Tameshkel, F.S.; Keyvani, H.; Ajdarkosh, H.; Karbalaie Niya, M.H.; Rezaie, N.; Zamani, F. Fatty liver index (FLI) and prediction of new cases of non-alcoholic fatty liver disease: A population-based study of northern Iran. Clin. Nutr. 2020, 39, 468–474. [Google Scholar] [CrossRef]
- Busquets-Cortés, C.; Bennasar-Veny, M.; López-González, A.-A.; Fresneda, S.; Aguiló, A.; Yanez, A. Fatty liver index and progression to type 2 diabetes: A 5-year longitudinal study in Spanish workers with pre-diabetes. BMJ Open 2021, 11, e045498. [Google Scholar] [CrossRef]
- Fresneda, S.; Abbate, M.; Busquets-Cortés, C.; López-González, A.; Fuster-Parra, P.; Bennasar-Veny, M.; Yáñez, A.M. Sex and age differences in the association of fatty liver index-defined non-alcoholic fatty liver disease with cardiometabolic risk factors: A cross-sectional study. Biol. Sex Differ. 2022, 13, 64. [Google Scholar] [CrossRef]
- DiStefano, J.K.; Gerhard, G.S. NAFLD in normal weight individuals. Diabetol. Metab. Syndr. 2022, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, W.; Yang, L.; Pan, W.; Xu, K.; Chen, X.; Li, Q.; Zhang, Y.; Chen, G.; Wen, J.; et al. First-degree family history of diabetes is associated with nonalcoholic fatty liver disease independent of glucose metabolic status. J. Diabetes Its Complicat. 2022, 36, 108083. [Google Scholar] [CrossRef]
- Han, E.; Han, K.D.; Lee, Y.; Kim, K.S.; Hong, S.; Park, J.H.; Park, C.Y. Fatty Liver & Diabetes Statistics in Korea: Nationwide Data 2009 to 2017. Metab. J. 2023, 47, 347–355. [Google Scholar] [CrossRef]
- Alhinai, A.; Patel, K.; Fonseca, V.A.; Sebastiani, G. Non-invasive diagnosis of nonalcoholic fatty liver disease in patients with type 2 diabetes. J. Diabetes Its Complicat. 2021, 35, 107978. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, A.; Maeda, S.; Fukuda, Y. Fatty liver index as a predictive marker for the development of diabetes: A retrospective cohort study using Japanese health check-up data. PLoS ONE 2021, 16, e0257352. [Google Scholar] [CrossRef] [PubMed]
- Arias-Fernández, M.; Fresneda, S.; Abbate, M.; Torres-Carballo, M.; Huguet-Torres, A.; Sánchez-Rodríguez, C.; Bennasar-Veny, M.; Yañez, A.M.; Busquets-Cortés, C. Fatty Liver Disease in Patients with Prediabetes and Overweight or Obesity. Metabolites 2023, 13, 531. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.; Westerink, J.; Kaasjager, K.H.A.H.; de Valk, H.W. Prevalence of Nonalcoholic Fatty Liver Disease (NAFLD) in Patients With Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2020, 105, 3842–3853. [Google Scholar] [CrossRef]
- Ciardullo, S.; Monti, T.; Sala, I.; Grassi, G.; Mancia, G.; Perseghin, G. Nonalcoholic Fatty Liver Disease and Advanced Fibrosis in US Adults Across Blood Pressure Categories. Hypertension 2020, 76, 562–568. [Google Scholar] [CrossRef]
- Lee, C.H.; Han, K.D.; Kim, D.H.; Kwak, M.S. The Repeatedly Elevated Fatty Liver Index Is Associated With Increased Mortality: A Population-Based Cohort Study. Front. Endocrinol. 2021, 12, 638615. [Google Scholar] [CrossRef]
- Chung, T.H.; Kim, J.K.; Kim, J.H.; Lee, Y.J. Fatty Liver Index as a Simple and Useful Predictor for 10-year Cardiovascular Disease Risks Determined by Framingham Risk Score in the General Korean Population. J. Gastrointestin. Liver Dis. 2021, 30, 221–226. [Google Scholar] [CrossRef]
- Kanerva, N.; Sandboge, S.; Kaartinen, N.E.; Männistö, S.; Eriksson, J.G. Higher fructose intake is inversely associated with risk of nonalcoholic fatty liver disease in older Finnish adults. Am. J. Clin. Nutr. 2014, 100, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Lee, K.J.; Lim, J.S.; Lee, M.Y.; Park, H.J.; Kim, M.Y.; Kim, J.W.; Chung, C.H.; Shin, J.Y.; Kim, H.-S.; et al. High Dietary Sodium Intake Assessed by Estimated 24-h Urinary Sodium Excretion Is Associated with NAFLD and Hepatic Fibrosis. PLoS ONE 2015, 10, e0143222. [Google Scholar] [CrossRef]
- Cantero, I.; Abete, I.; Monreal, J.; Martinez, J.; Zulet, M. Fruit Fiber Consumption Specifically Improves Liver Health Status in Obese Subjects under Energy Restriction. Nutrients 2017, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Cantoral, A.; Contreras-Manzano, A.; Luna-Villa, L.; Batis, C.; Roldán-Valadez, E.; Ettinger, A.; Mercado, A.; Peterson, K.; Téllez-Rojo, M.; Rivera, J. Dietary Sources of Fructose and Its Association with Fatty Liver in Mexican Young Adults. Nutrients 2019, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Repetto, E.; Guja, C.; Hardy, E.; Han, J.; Jabbour, S.A.; Ferrannini, E. Exenatide and dapagliflozin combination improves markers of liver steatosis and fibrosis in patients with type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 393–403. [Google Scholar] [CrossRef]
- De Nucci, S.; Rinaldi, R.; Di Chito, M.; Donghia, R.; Giannuzzi, V.; Shahini, E.; Cozzolongo, R.; Pesole, P.L.; Coletta, S.; De Pergola, G.; et al. The Replacement of Only One Portion of Starchy Carbohydrates with Green Leafy Vegetables Regresses Mid and Advanced Stages of NAFLD: Results from a Prospective Pilot Study. Nutrients 2023, 15, 2289. [Google Scholar] [CrossRef]
- Mirmiran, P.; Amirhamidi, Z.; Ejtahed, H.S.; Bahadoran, Z.; Azizi, F. Relationship between Diet and Non-alcoholic Fatty Liver Disease: A Review Article. Iran. J. Public Health 2017, 46, 1007–1017. [Google Scholar]
- Naomi, N.D.; Ngo, J.; Brouwer-Brolsma, E.M.; Buso, M.E.C.; Soedamah-Muthu, S.S.; Pérez-Rodrigo, C.; Harrold, J.A.; Halford, J.C.G.; Raben, A.; Geleijnse, J.M.; et al. Sugar-sweetened beverages, low/no-calorie beverages, fruit juice and non-alcoholic fatty liver disease defined by fatty liver index: The SWEET project. Nutr. Diabetes 2023, 13, 6. [Google Scholar] [CrossRef]
- Darbandi, M.; Hamzeh, B.; Ayenepour, A.; Rezaeian, S.; Najafi, F.; Shakiba, E.; Pasdar, Y. Anti-inflammatory diet consumption reduced fatty liver indices. Sci. Rep. 2021, 11, 22601. [Google Scholar] [CrossRef]
- Son, D.H.; Kwon, Y.J.; Lee, H.S.; Kim, H.M.; Lee, J.W. Effects of a Calorie-Restricted Mediterranean-Style Diet on Plasma Lipids in Hypercholesterolemic South Korean Patients. Nutrients 2021, 13, 3393. [Google Scholar] [CrossRef]
- Drinda, S.; Grundler, F.; Neumann, T.; Lehmann, T.; Steckhan, N.; Michalsen, A.; Wilhelmi de Toledo, F. Effects of Periodic Fasting on Fatty Liver Index-A Prospective Observational Study. Nutrients 2019, 11, 2601. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Bertoli, S.; Bedogni, G.; Vignati, L.; Pellizzari, M.; Battezzati, A. Association between Mediterranean Diet and Fatty Liver in Women with Overweight and Obesity. Nutrients 2022, 14, 3771. [Google Scholar] [CrossRef] [PubMed]
- Khalatbari-Soltani, S.; Imamura, F.; Brage, S.; De Lucia Rolfe, E.; Griffin, S.J.; Wareham, N.J.; Marques-Vidal, P.; Forouhi, N.G. The association between adherence to the Mediterranean diet and hepatic steatosis: Cross-sectional analysis of two independent studies, the UK Fenland Study and the Swiss CoLaus Study. BMC Med. 2019, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Gelli, C.; Tarocchi, M.; Abenavoli, L.; Di Renzo, L.; Galli, A.; De Lorenzo, A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3150–3162. [Google Scholar] [CrossRef]
- Lampignano, L.; Donghia, R.; Sila, A.; Bortone, I.; Tatoli, R.; De Nucci, S.; Castellana, F.; Zupo, R.; Tirelli, S.; Giannoccaro, V.; et al. Mediterranean Diet and Fatty Liver Risk in a Population of Overweight Older Italians: A Propensity Score-Matched Case-Cohort Study. Nutrients 2022, 14, 258. [Google Scholar] [CrossRef]
- Freer, C.L.; George, E.S.; Tan, S.-Y.; Abbott, G.; Dunstan, D.W.; Daly, R.M. Effect of progressive resistance training with weight loss compared with weight loss alone on the fatty liver index in older adults with type 2 diabetes: Secondary analysis of a 12-month randomized controlled trial. BMJ Open Diabetes Res. Care 2022, 10, e002950. [Google Scholar] [CrossRef]
- Coll, E.E.; Lolo, C.V.; Galán, P.D.; Valero, J.A.G.; Bacchiddu, S.; Tomás, C.Q.; Irigoyen, D.; Gunnard, K.; Comamala, A.J.-C. Bariatric and metabolic endoscopy in the handling of fatty liver disease. A new emerging approach? Rev. Española Enferm. Dig. 2019, 111, 283–292. [Google Scholar] [CrossRef]
- Kurinami, N.; Sugiyama, S.; Yoshida, A.; Hieshima, K.; Miyamoto, F.; Kajiwara, K.; Jinnouch, K.; Jinnouchi, T.; Jinnouchi, H. Dapagliflozin significantly reduced liver fat accumulation associated with a decrease in abdominal subcutaneous fat in patients with inadequately controlled type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2018, 142, 254–263. [Google Scholar] [CrossRef]
- Cho, K.Y.; Nakamura, A.; Omori, K.; Takase, T.; Miya, A.; Yamamoto, K.; Nomoto, H.; Kameda, H.; Taneda, S.; Kurihara, Y.; et al. Favorable effect of sodium-glucose cotransporter 2 inhibitor, dapagliflozin, on non-alcoholic fatty liver disease compared with pioglitazone. J. Diabetes Investig. 2021, 12, 1272–1277. [Google Scholar] [CrossRef]
- Seino, H. Efficacy and Safety of Luseogliflozin in Patients with Type 2 Diabetes Complicated by Hepatic Dysfunction: A Single-Site, Single-Arm, Open-Label, Exploratory Trial. Diabetes Ther. 2021, 12, 863–877. [Google Scholar] [CrossRef]
- Kobyliak, N.; Abenavoli, L.; Falalyeyeva, T.; Beregova, T. Efficacy of Probiotics and Smectite in Rats with Non-Alcoholic Fatty. Liver Dis. Ann. Hepatol. 2018, 17, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Long, M.T.; Gandhi, S.; Loomba, R. Advances in non-invasive biomarkers for the diagnosis and monitoring of non-alcoholic fatty liver disease. Metabolism 2020, 111, 154259. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, S.; Scamporrino, A.; Filippello, A.; Di Pino, A.; Scicali, R.; Malaguarnera, R.; Purrello, F.; Piro, S. Clinical and Molecular Biomarkers for Diagnosis and Staging of NAFLD. Int. J. Mol. Sci. 2021, 22, 11905. [Google Scholar] [CrossRef]
- Piazzolla, V.A.; Mangia, A. Noninvasive Diagnosis of NAFLD and NASH. Cells 2020, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.; Lee, T.P. Diagnosis and Evaluation of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Including Noninvasive Biomarkers and Transient Elastography. Clin. Liver Dis. 2018, 22, 73–92. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Fukusato, T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 15539–15548. [Google Scholar] [CrossRef]
- Spengler, E.K.; Loomba, R. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mayo Clin. Proc. 2015, 90, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Pirmoazen, A.M.; Khurana, A.; El Kaffas, A.; Kamaya, A. Quantitative ultrasound approaches for diagnosis and monitoring hepatic steatosis in nonalcoholic fatty liver disease. Theranostics 2020, 10, 4277–4289. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef]
- Reeder, S.B.; Cruite, I.; Hamilton, G.; Sirlin, C.B. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J. Magn. Reson. Imaging 2011, 34, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Orlic, L.; Franjic, N.; Hauser, G.; Stimac, D.; Milic, S. Transient elastography (FibroScan®) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease-Where do we stand? World J. Gastroenterol. 2016, 22, 7236–7251. [Google Scholar] [CrossRef] [PubMed]
- Sasso, M.; Beaugrand, M.; de Ledinghen, V.; Douvin, C.; Marcellin, P.; Poupon, R.; Sandrin, L.; Miette, V. Controlled attenuation parameter (CAP): A novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med. Biol. 2010, 36, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.; Wang, Y.; Bai, S.; Wei, H.; Zhou, Y.; Fan, J.; Qiao, L. Diagnostic Accuracy of Controlled Attenuation Parameter (CAP) as a Non-Invasive Test for Steatosis in Suspected Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. BMC Gastroenterol. 2019, 19, 51. [Google Scholar] [CrossRef]
- Ferraioli, G.; Soares Monteiro, L.B. Ultrasound-based techniques for the diagnosis of liver steatosis. World J. Gastroenterol. 2019, 25, 6053–6062. [Google Scholar] [CrossRef]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef]
- Duvnjak, M.; Blažević, N. Diagnostic Approach. In Gastrointestinal Complications of Diabetes: A Comprehensive Guide, 1st ed.; Duvnjak, M., Smirčić-Duvnjak, L., Eds.; Humana Press: Cham, Switzerland, 2018; pp. 317–329. [Google Scholar]
- Lee, Y.-H.; Cho, Y.; Lee, B.-W.; Park, C.-Y.; Lee, D.H.; Cha, B.-S.; Rhee, E.-J. Nonalcoholic fatty liver disease in diabetes. Part I: Epidemiology and diagnosis. Diabetes Metab. J. 2019, 43, 31–45. [Google Scholar] [CrossRef]
- Chartampilas, E. Imaging of nonalcoholic fatty liver disease and its clinical utility. Hormones 2018, 17, 69–81. [Google Scholar] [CrossRef]
- Park, C.C.; Nguyen, P.; Hernandez, C.; Bettencourt, R.; Ramirez, K.S.; Fortney, L.E.; Hooker, J.; Sy, E.; Alquiraish, M.H.; Valasek, M.A.; et al. Magnetic resonance elastography vs. transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. J. Hepatol. 2017, 66, S233. [Google Scholar] [CrossRef]
- Jia, S.; Zhao, Y.; Liu, J.; Guo, X.; Chen, M.; Zhou, S.; Zhou, J. Magnetic Resonance Imaging-Proton Density Fat Fraction vs. Transient Elastography-Controlled Attenuation Parameter in Diagnosing Non-alcoholic Fatty Liver Disease in Children and Adolescents: A Meta-Analysis of Diagnostic Accuracy. Front. Pediatr. 2022, 9, 784221. [Google Scholar] [CrossRef]
- Fennoun, H.; El Mansouri, S.; Tahiri, M.; Haraj, N.E.; El Aziz, S.; Haddad, F.; Hliwa, W.; Badr, W.; Asma, C. Interest of hepatic steatosis index (HSI) in screening for metabolic steatopathy in patients with type 2 diabetes. Pan. Ait. Med. J. 2020, 37, 270. [Google Scholar] [CrossRef]
- Vidal-González, D.; Uribe, M.; Montalvo-Javé, E.E.; Nuño-Lámbarri, N. Assessment of non-alcoholic fatty liver disease by non-invasive methods: Present and future perspectives. Rev. Med. Del Hosp. Gen. Mex. 2020, 83, 135–143. [Google Scholar] [CrossRef]
- Bhatt, V.; Koneru, K.; Kakrani, A.; Edara, M.; Reddy, V.; Jawade, P. A study of non-alcoholic fatty liver disease-liver fat score in overweight and obese individuals. J. Fam. Med. Prim. Care 2022, 11, 4368–4374. [Google Scholar] [CrossRef] [PubMed]
- Wargny, M.; Smati, S.; Pichelin, M.; Bigot-Corbel, E.; Authier, C.; Dierry, V.; Zaïr, Y.; Jacquin, V.; Hadjadj, S.; Boursier, J.; et al. Fatty liver index is a strong predictor of changes in glycemic status in people with prediabetes: The IT-DIAB study. PLoS ONE 2019, 14, e0221524. [Google Scholar] [CrossRef]
- Luis, D.; Primo, D.; Izaola, O.; Lopez, J.J. Effects of a Short-Term Meal Replacement Hypocaloric Diet in Subjects with Obesity and High Fatty Liver Index. Nutrients 2022, 14, 5353. [Google Scholar] [CrossRef]
- Mascaró, C.M.; Bouzas, C.; Tur, J.A. Association between Non-Alcoholic Fatty Liver Disease and Mediterranean Lifestyle: A Systematic Review. Nutrients 2021, 14, 49. [Google Scholar] [CrossRef]
- Toman, D.; Vavra, P.; Jelinek, P.; Ostruszka, P.; Ihnat, P.; Foltys, A.; Pelikan, A.; Roman, J. Effect of bariatric surgery on fatty liver disease in obese patients: A prospective one year follow-up study. Biomed Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2022, 166, 195–203. [Google Scholar] [CrossRef]
| Author [Ref], Year | AUC (95% CI) FLI | Sensitivity % FLI vs. Other Diagnostic Methods | Specificity % FLI vs. Other Diagnostic Methods | Cut-Off FLI | Reference Methods for NAFLD Diagnosis | * p Value |
|---|---|---|---|---|---|---|
| Bedogni et al. [14] 2006 | 0.84 (0.81–0.87) | 87 61 | 44 86 | <30 ≥60 | AU | missing data |
| Balkau et al. [40] 2010 | OR: 9.33 (5.05–17.25) for males OR: 36.72 (17.12–78.76) for women | missing data | missing data | <20 ≥70 | NAFLD-LFS | p < 0.0001: FLI vs. NAFLD-LFS |
| Zelber-Sagie et al. [41] 2013 | 0.65 FLI vs. AU 0.97 (0.95–0.98): FLI vs. SteatoTest 0.82 (0.77–0.87): FLI vs. HRI: | 80.3 (FLI vs. AU) 85.5 (FLI vs. SteatoTest) 56.3 (FLI vs. HRI) | 87.3 (FLI vs. AU) 92.6 (FLI vs. SteatoTest) 86.5 (FLI vs. HRI) | ≥60 | AU, SteatoTest and HRI | p < 0.001: FLI vs. SteatoTest p < 0.001: FLI vs. HRI |
| Fedchuk et al. [42] 2014 | 0.83 (0.71–0.91) | 76 | 87 | >60 | LBP | p < 0.006: FLI vs. LBP (when is steatosis >33%) |
| Kahl et al. [43] 2014 | 0.72 (0.59–0.85) FLI 0.79 (0.68–0.9) HSI 0.7 (0.53–0.88) NAFLD-FLS | 76 100 35 | 83 91 91 | >60 >36 | 1H-MRS, HSI, NAFLD-FLS | p < 0.01: 1H-MRS p < 0.001: HSI p < 0.05: NAFLD-LFS |
| Goulart et al. [44] 2015 | 0.76 (0.69–0.83) | 89.6 79.1 | 36.7 64.1 | <30 ≥60 | AU, CT | Missing data |
| Xia et al. [45] 2016 | 0.76(0.74–0.77: Chinese pts 0.72(0.66–0.77: Finnish pts | 57 (53–60) in Chinese pts. 85 (79–90) in Finnish pts. | 81 (79–82) Chinese pts. 45 (38–53) Finnish pts. | >27.1 in Chinese pts. >39 in Finnish pts. | AU | p < 0.001: FLI vs. AU Chinese and Finns |
| Motamed et al. [46] 2016 | 0.8656 (0.8548–0.8764) | 82.42 82.33 | 76.87 76.55 | >46.9: males >53.8: women | AU | p < 0.0001: FLI vs. AU |
| Dehnavi et al. [47] 2018 | 0.85 (0.79–0.9) | 83 | 70 | >26.2 | CAP | p < 0.001: FLI vs. CAP |
| Lind et al. [48] 2020 | 0.82 (0.80–0.83) | missing data | missing data | missing data | MRI | p = 0.0019: LFS vs. FLI (in the EFFECT group) p = 0.005: FLI vs. LAP (in the POEM group) |
| Chen et al. [49] 2020 | 0.84 (0.81–0.87): general population 0.793 (0.748–0.839): males 0.765 (0718–0.813): women | 80.3: males 76.1: women | 66.9: males 65.5: women | >20: males >10: women | AU | p < 0.001: FLI vs. AU |
| Jung et al. [50] 2020 | 0.68 (0.64–0.71) | 71.2 34.4 | 57.8 83.4 | <30 ≥60 | MRI | p < 0.01: FLI NAFLD and FLI no NAFLD |
| Zhang et al. [51] 2021 | 0.78 (0.71–0.86) | 87 | 58.5 | >37.64 | MRI-PDFF | missing data |
| Castellana et al. [52] 2021 | 0.14 (0.09–0.19) 0.42 (0.34–0.51) 0.67(0.58–0.75) | 81 (71–88) - 44 (33–55) | 65 (52–76) - 90 (84–94) | <30 30–60 ≥60 | AU vs. CT/MRI | missing data |
| Preciado-Puga et al. [53] 2021 | 0.704 (0.567–0.841) | 71 | 51 | <30 >60 | AU and LBP | p = 0.011: FLI vs. AU p = 0.012: FLI vs. LBP |
| Borges-Canha et al. [54] 2022 | 0.31 (0.28–0.37) 0.22 (−0.81–1.24) | missing data | missing data | >30 | FLI and BAARD score | p < 0.01: FLI vs. WC p < 0.01:FLI vs. WHR |
| Shao et al. [55] 2022 | 0.59 for NAFLD 0.83 for MAFLD 0.17 for non-MAFLD-NAFLD | missing data | missing data | >60 | LBP, AU, MRI-PDFF, and CAP | missing data |
| de Silva et al. [56] 2022 | 0.692 (0.565–0.786) | 58.33 | 69.49 | >30: children (5–15 years) | AU | p = 0.0001: FLI vs. AU |
| Ali et al. [57] 2022 | 0.999 (0.997–1.00): FLI | 98 83 | 100 100 | <30 >60 | AU, CAP | p = 0.0001: FLI vs. HSI p = 0.001: FLI vs. ZJU |
| Kim et al. [58] 2023 | 0.813 for mild steatosis | 85 | 77 | >30 ≥60 | MRI-PDFF | p < 0.001 |
| Mertens et al. [59] 2023 | 0.83 (0.74–0.91) | 62 | 68 | >60 | AU, FLI, CAP and 1H-MRS | AU vs. CAP vs. FLI (p < 0.001) FLI vs. CAP (p = 0.684) |
| Kamari et al. [60] 2023 | 0.83 (0.825–0.842) | missing data | missing data | >60 | clinical and biological data | p < 0.001: FLI vs. WC p < 0.001: FLI vs. TGs |
| Author [Ref], Year | AUC (95% CI) FLI | Sensitivity % | Specificity % | Cut-Off FLI | Reference Methods for NAFLD Diagnosis | * p Value |
|---|---|---|---|---|---|---|
| Nishi et al. [63] 2015 | 1.18–4.70: for males 1.07–8.19: for women | missing data | missing data | <30 ≥60 | FLI | p = 0.003: for males p < 0.001: for women |
| Li et al. [64] 2018 | 0.74 (0.67–0.81): weak population 0.83 (0.80–0.86): total population 0.71 (0.66–0.77): overweight/obese population | 84.2 | 65.3 | >20 | AU | p < 0.01 |
| Klisic et al. [65] 2018 | 0.909 for model 2 | 89.3: model 2 vs. model 1 | 87.5: model 2 vs. model 1 | <30 ≥60 | FLI | p < 0.001: BMI and WC were statistically higher in the two FLI groups |
| Chen et al. [12] 2019 | 0.802 (0.762–0.839) | 66 | 80 | <30 ≥60 | AU, FLI and HSI | p = 0.0383: FLI vs. HSI |
| Khang et al. [66] 2019 | 0.849 (0.841–0.856) | 0.280 | 0.035 | <20 ≥60 | FLI and AU | p < 0.001 |
| Hsu et al. [67] 2019 | 0.76 (0.73–0.78 | 60.66 | 79.35 | ≥60 | AU | p < 0.001 |
| Motamed et al. [68] 2020 | 0.712 (0.675–0.749): for males 0.721 (0.683–0.759): form women | missing data | missing data | missing data | AU | p < 0.001 |
| Busquets-Cortes et al. [69] 2021 | 6.343 (5.368–7.495) | missing data | missing data | <30 ≥60 | FLI | p < 0.001 |
| Author, Year [Ref] | AUC (95% CI) FLI | Sensitivity % | Specificity % | Cut-Off FLI | Reference Methods for NAFLD Diagnosis | * p Value |
|---|---|---|---|---|---|---|
| Kanerva et al. [81] 2014 | 0.68 (047–0.84) | 61 | 86 | >60 | NAFLD-LFS | p < 0.001 NAFLD-LFS vs. FLI |
| Huh et al. [82] 2015 | 1.75 (1.39–2.20): FLI 1.39 (1.26–1.55): HSI | Missing data | Missing data | ≥60 >36 | HSI, BARD- score | p < 0.001: FLI vs. estimated 24 h sodium excretion |
| Cantero et al. [83] 2017 | 0.84 | Missing data | Missing data | ≥60 | FLI, HSI and NAFLD-LFS | p = 0.793: RESMENA vs. AHA |
| Cantoral et al. [84] 2019 | 0.88 (0.74–1.06) | 77.8 | 61 | ≥30 | MRI | p < 0.01: FLI vs. HSI |
| Gastaldelli et al. [85] 2020 | EXE + DAPA vs. EXE + placebo:−511, −0.73 EXE + DAPA vs. DAPA + placebo:−4.93, −0.62 | Missing data | Missing data | ≥60 | FLI and NAFLD-LFS | p = 0.0092 p = 0.0119 |
| Nucci et al. [86] 2023 | 0.73 (0.33–0.89): before diet 0.85 (0.54–0.95): after diet | Missing data | Missing data | >60 | CAP, FAST score | p < 0.0001 for FLI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biciusca, T.; Stan, S.I.; Balteanu, M.A.; Cioboata, R.; Ghenea, A.E.; Danoiu, S.; Bumbea, A.-M.; Biciusca, V. The Role of the Fatty Liver Index (FLI) in the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review. Diagnostics 2023, 13, 3316. https://doi.org/10.3390/diagnostics13213316
Biciusca T, Stan SI, Balteanu MA, Cioboata R, Ghenea AE, Danoiu S, Bumbea A-M, Biciusca V. The Role of the Fatty Liver Index (FLI) in the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review. Diagnostics. 2023; 13(21):3316. https://doi.org/10.3390/diagnostics13213316
Chicago/Turabian StyleBiciusca, Teodora, Sorina Ionelia Stan, Mara Amalia Balteanu, Ramona Cioboata, Alice Elena Ghenea, Suzana Danoiu, Ana-Maria Bumbea, and Viorel Biciusca. 2023. "The Role of the Fatty Liver Index (FLI) in the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review" Diagnostics 13, no. 21: 3316. https://doi.org/10.3390/diagnostics13213316
APA StyleBiciusca, T., Stan, S. I., Balteanu, M. A., Cioboata, R., Ghenea, A. E., Danoiu, S., Bumbea, A.-M., & Biciusca, V. (2023). The Role of the Fatty Liver Index (FLI) in the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review. Diagnostics, 13(21), 3316. https://doi.org/10.3390/diagnostics13213316









