Abstract
Ultrasound elastography is gaining attention for its diagnostic potential across various medical fields, and its physical properties make it valuable in modern clinical medicine. However, its specific attributes, especially in the context of recent medical advancements, remain relatively unexplored. This study aimed to identify instrument-specific characteristics and applications of real-time ultrasound elastography, shear wave elastography, and strain elastography, particularly within gastroenterology. Following PRISMA guidelines, the study examined elastography articles on databases like PubMed, resulting in 78 included articles. Data on patient demographics, organ involvement, specificity, sensitivity, accuracy, positive predictive value, and negative predictive value were extracted. Statistical analysis involved SPSS version 21, with significance set at p < 0.05. The majority of patients were male (50.50%), with a mean age of 42.73 ± 4.41 years. Shear wave elastography was the most prevalent technique (48.7%), and liver investigations were predominant in gastroenterology (34.6%). Gastrointestinal applications showed higher sensitivity, positive predictive value, and negative predictive values (p < 0.05) but lower specificity (p < 0.05). Real-time ultrasound elastography exhibited increased specificity, accuracy, and predictive values (p < 0.05). Ultrasound elastography appears more accurate and effective in gastroenterological settings. Nonetheless, its performance depends on instrument-specific and operator-dependent factors. While promising, further studies are necessary to ascertain optimal utilization in both gastrointestinal and non-gastrointestinal conditions.
1. Introduction
Ultrasound elastography, a technique from the 1990s, has gained widespread attention in recent years [1]. Given its properties of elasticity, the modality has been modified to allow for quantification of many characteristics of diseases [2]. With the different methods of wave propagation (longitudinal and perpendicular), the elastic modulus varies, leading to multiple radiological variations that can be applied [2]. Ultrasound elastography is further divided into shear, strain, and acoustic force elastography and real-time tissue elastography. Shear wave elastography uses an ultrasound transducer to generate and propagate shear waves within the tissue being examined. These waves are essentially waves of deformation that travel through the tissue. The equipment measures the speed at which these shear waves travel through the tissue. The velocity of shear wave propagation is directly related to the tissue’s stiffness. Stiffer tissues transmit the shear waves faster, while softer or more elastic tissues transmit them more slowly. This information about differences in velocity is then utilized to create an elastogram [2]. Real-time elastography is often performed using an ultrasound transducer, that emits high-frequency sound waves into the body, and the resulting echoes are used to create images of the underlying tissues. The technique uses specialized software to analyze the deformation of the tissue in response to natural physiological motion (such as cardiac pulsations or respiratory movements). This deformation is linked to tissue elasticity, with stiffer tissues deforming less than softer ones. This information is then displayed simultaneously with the ultrasound images.
Strain elastography is a technique used to evaluate the elasticity or rigidity of bodily tissues. Its primary objective is to offer additional insights into tissue characteristics for the purpose of diagnosing and monitoring various medical conditions. In this method, a specialized probe or transducer is employed to apply force or stress to the target tissue, resulting in a temporary deformation or strain. Typically conducted with an ultrasound machine, images of the tissue are taken both before and after this deformation. The initial image serves as a reference, while the subsequent image illustrates how the tissue responds to the applied stress. The software connected to the imaging equipment calculates the displacement of tissue components between these two image sets. This displacement data is then employed to generate an elastogram, which is an image displayed in color-coded or grayscale format. The elastogram visualizes the relative elasticity or stiffness of the tissue, with stiffer regions typically represented in one color and more elastic areas in another [3]. However, strain elastography might be more suitable for superficial structures and not deeper organs, for example, liver, thyroid, and breast [4]. While most of the studies discuss liver fibrosis being quantified by elastography, the method is also being used to investigate other diseases—for example, pathologies of the rectum, appendix, pancreas, prostate, and other soft tissues [5,6,7].
It is important to note that elastography is still limited by operator dependent characteristics, which can influence many instrumental markers. For example, positive and negative predictive values and specificity and sensitivity [4]. Therefore, the primary objective of this review was to determine demographic characteristics and instrument-specific characteristics of various elastography modalities being used. The secondary objective was to determine if the instrument-specific values were dependent on the nature of the organs and type of modality being used.
2. Methodology
2.1. Protocol Development and Search Strategy
The protocol was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The review methods were established prior to the study. This study was conducted using PICO (patient, intervention, comparison, and outcomes) strategy for our research question. The keywords used in the search strategy are as described in Supplementary Table S1.
2.2. Data Extraction
We screened PubMed and Web of Science for conducting our search using Boolean operators (“OR”, “AND”) for the MESH terms described in Supplementary Table S1. This systematic review included articles published in English during the last fifteen years that fulfilled the following inclusion criteria: (1) articles discussing ultrasound elastography, shear wave elastography or strain elastography; (2) articles specifically documenting optimal sensitivity, specificity, area under the curve, positive and negative likelihood ratio, positive predictive value, and negative predictive values. Using the PICO strategy, the patients who had been evaluated using ultrasound elastography, shear wave elastography or strain elastography were included. The interventions in this regard were the types of elastography used (ultrasound elastography, shear wave elastography or strain elastography). The outcome variables were characteristics such as optimal sensitivity, specificity, area under the curve, positive and negative likelihood ratio, positive predictive value, and negative predictive values. These outcomes were compared to the patients with non-gastrointestinal pathologies. The systematic review included observational studies and randomized-controlled trials to minimize population bias and improve measurement of qualitative variables. During the initial search, 321 articles were found on PubMed and 40 articles on Web of Science. Duplicates were deleted after the first search. Two independent reviewers screened the remaining studies based on inclusion criteria and reviewed the abstracts initially progressing to full-text articles if criteria were met. Zotero and Rayyan applications were utilized. A third reviewer was called in to resolve any potential disagreements.
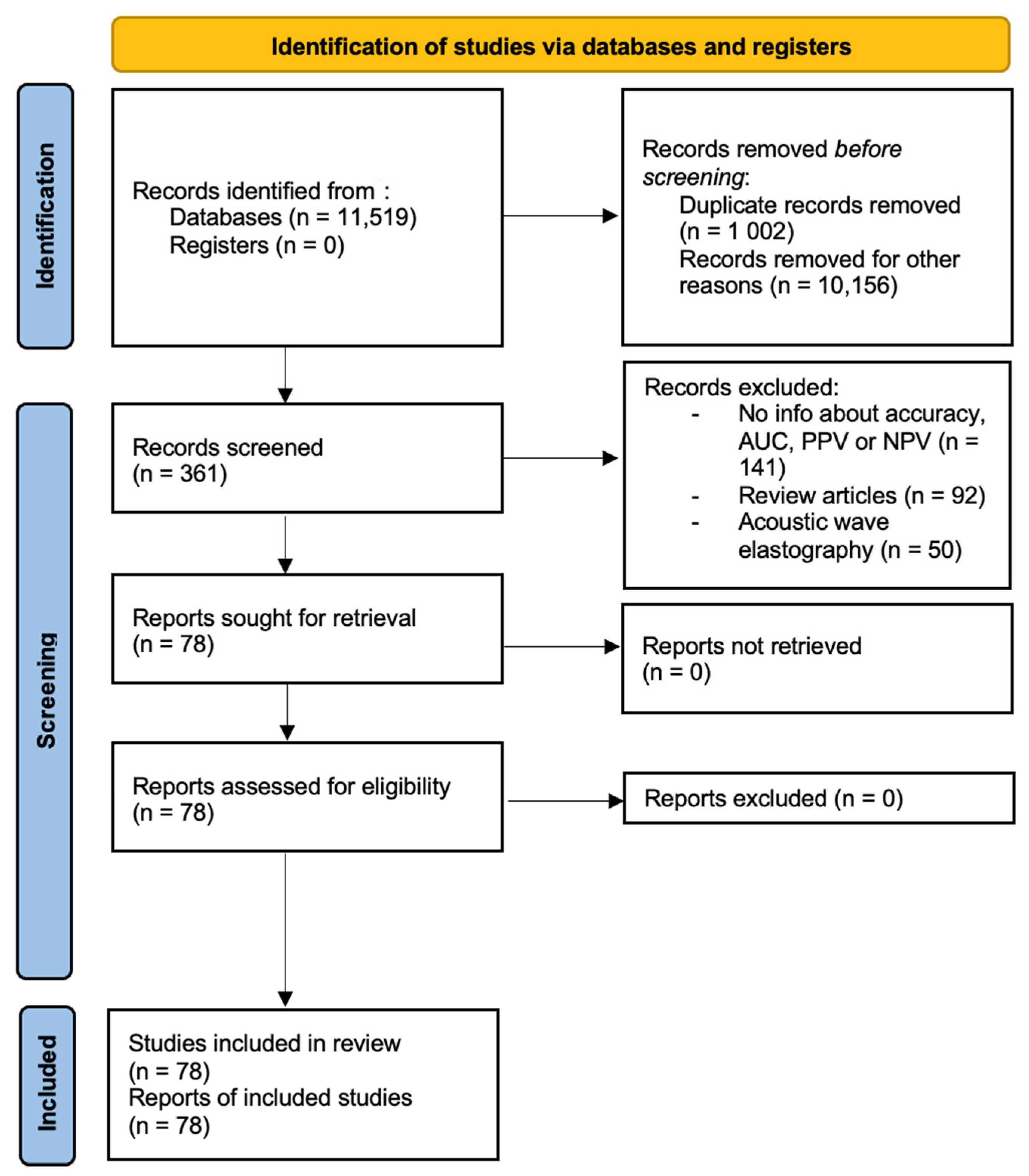
Information about study design, year, location where study was conducted, published journal, demographic data, diagnostic modalities, pathologies, and modality-specific values were extracted from the records by one reviewer. The extracted data was double-checked for accuracy and completeness. Studies were removed from the analysis if translations of the articles were not available, copies of complete articles were not available or if information about device-specific characteristics was complete. Papers that had not been peer-reviewed were also excluded. The preliminary data was recorded in an excel spreadsheet and final analysis contained 78 studies. This has been presented in Figure 1.
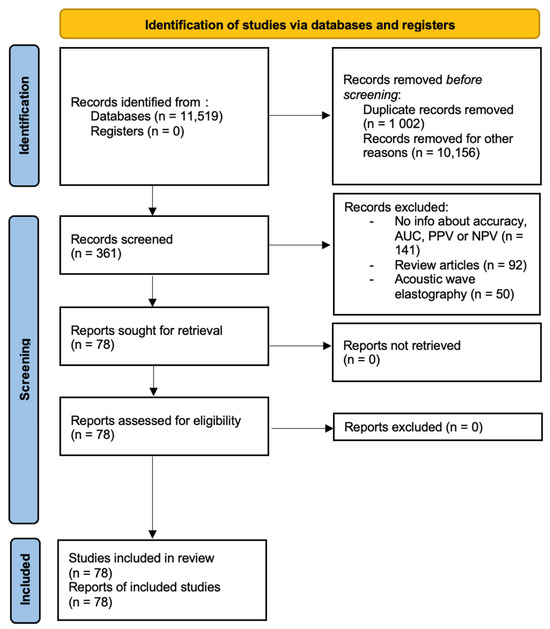
Figure 1.
PRISMA diagram.
2.3. Risk of Bias
The quality of the studies was assessed by tools published by Cochrane Reviews [8] [Table 1, Supplementary Table S2]

Table 1.
Quality Assessment.
Statistical analysis was conducted through Statistical Package for the Social Sciences (SPSS) version 21 (IBM Corp., Armonk, NY, USA). Mean ± standard deviation was used for continuous variables. Frequency or percentages were used for qualitative variables. Statistical tests including chi-square, independent t-test, and analysis of variance were utilized. Logistic regression models were used to determine predictors for multiple outcomes. A p-value < 0.05 was considered significant.
3. Results
3.1. Demographic Characteristics
The review included 78 articles: 69 prospective studies, 7 retrospective studies, and 2 randomized controlled trials. The total number of cases included in the review was 13,689. Data regarding gender was available for 8787 cases. The majority of the cases were male (50.50%). Information about age was available for 12,753 cases. The mean age of the study population was 42.73 ± 4.41 years. The data extraction for the articles has been mentioned in Table 2 [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].

Table 2.
Findings of the studies discussed.
The types of elastography techniques utilized have been discussed in Supplementary Table S3 and the sources of funding have been discussed in Supplementary Table S4.
The lowest positive predictive value of elastography was observed by Casariego et al., estimated to be 8.3% for real-time ultrasound elastography [9]. However, relatively higher positive predictive values were observed by Yoon et al. and Lin et al. [18,71]. The negative predictive values of elastography were relatively similar, ranging from 70% to 100%. However, lower negative predictive values were noted for shear wave elastography by Sporea et al. [28]. The lowest sensitivity was observed by Casariego et al., estimated to be 25% for real-time ultrasound elastography [9]. The lowest specificity was noted by Verhoeven et al. for strain elastography [9].
3.2. Imaging Modalities and Their Utility
Shear wave elastography was most involved (38/78; 48.7%), followed by real-time ultrasound elastography (26/78; 33.3%) and strain elastography (14/78; 17.9%). Organs involved in gastroenterology-based diseases were investigated in 34/78 cases (45.3%), of which liver (21/78; 34.6%) was the most common, followed by the pancreas (10/78; 12.8%). Other common organs that were investigated include the thyroid (21.8%) and lymph nodes (29.5%). Elastography was commonly used in diagnosis of malignancy for lymph nodes (26.3%), thyroid (18.8%), pancreas (5.0%), and prostate (2.5%) and for diagnostic accuracy in liver fibrosis (15.0%) and thyroid (11.3%).
3.3. Subgroup Analyses
The subgroup analysis for different organs is shown in Table 3.

Table 3.
Subgroup analysis for different organs.
Real-time ultrasound elastography was commonly used for lymph nodes. Shear wave elastography was commonly used for liver. Strain elastography was also used for both pancreas and lymph nodes. However, the specificity of the elastography was greatest for the appendix. Similar results were noted for sensitivities; higher sensitivities were observed for the rectum, pancreas, and appendix. Higher accuracies were also observed for rectum. Additionally, higher positive predictive values were noted for elastography focused on appendix and rectum.
Table 4 discusses the applications of elastography in organs.

Table 4.
Applications in Various Organs.
Modalities utilized were significantly associated with organ-based pathologies (p < 0.05). Although data for accuracy was limited as seen in Table 4, higher values were observed for the pancreas (p < 0.05). In the cases of the esophagus and rectum, the elastography modalities were associated with endoscopic ultrasound. The study focused on soft tissue and discussed the importance of elastography in patellar tendons. The different measures of all elastography modalities were used to determine indicators of better performance via multivariate regression analysis as shown in Table 5 and Table 6.

Table 5.
Measures in gastrointestinal organs.

Table 6.
Measures in real-time ultrasound elastography.
Elastography for gastrointestinal organs was associated with higher sensitivity, positive predictive value, and negative predictive values (p < 0.05) but lower specificity (p < 0.05). Additionally, real-time ultrasound elastography was associated with increased specificity, accuracy, and positive and negative predictive values (p < 0.05).
4. Discussion
This is the first study to provide an overview of elastography in gastrointestinal organs. About 50.5% of the study population was male (50.5%), and the mean age of the participants was 42.73 ± 4.41 years. Unalp-Arida et al. discussed the application of elastography in liver stiffness and observed a comparatively lower proportion of males in the study population and an older age group [87]. The shear wave technique was commonly involved in elastography, particularly for the liver [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. While shear wave technique might be hampered by inflammation, cholestasis, and congestion, strain technique remains relatively unaffected, increasing the diagnostic yield of elastography [88]. Furthermore, higher estimates of positive predictive value and accuracy were noted for strain elastography for the liver. This is consistent with results from a previous study [89]. The same mechanism can be attributed to the use in lymph nodes.
Higher accuracy was associated with pancreatic imaging (p < 0.05). This finding was different compared to a previous study [90]. Usually, elastography has been considered difficult to perform due to the size of the pancreas [90]. However, strain elastography has an advantage in this regard because an additional static force is present in addition to aortic pulsations allowing for better study of the pancreatic body. However, the use in the pancreatic head and tail might still be limited [90].
Elastography involving gastrointestinal organs were also associated with higher sensitivity, positive predictive value, and negative predictive values (p <0.05) but lower specificity (p < 0.05). Apart from pancreatic diseases, rectal diseases also add to the increased likelihood of these values. Tissue stiffness is usually increased in malignancy and therefore, rectal malignancies of advanced stages would be better diagnosed using elastography as seen in the studies discussed [89]. Additionally, real-time ultrasound elastography was associated with increased specificity, accuracy, and positive and negative predictive values (p < 0.05). These findings were discussed by another study on breast disease, in which increased specificity, accuracy, and positive and negative predictive values were observed for breast lesions in cases of real-time ultrasound elastography [91].
4.1. Use in Non-Gastrointestinal Tissues
Elastography is widely employed in non-gastrointestinal organs due to its distinctive characteristics. It is particularly useful in the evaluation of thyroid nodules. Evaluation of small nodules is limited because of increased distance from the transducer and in patients with larger neck circumference. The index of compression on elastography can help in determining both the size and nature of the nodule [11]. The varying tissue elasticity is also of immense importance in the evaluation of lymph nodes. Altered tissue composition represented by a higher tissue stiffness is more suggestive of malignancy. Malignant lesions are usually suspected in cases of excessive keratin deposition or microcalcifications that hamper stiffness index; these features are more suggestive of cortical damage [47].
Desmoplastic reaction might be limited in cases of non-Hodgkin lymphoma and require the use of shear-wave elastography [47]. In a few cases, the combined use of elastography with other techniques can allow preoperative assessment of axillary lymph nodes in patients with breast cancer [57]. The technique can be optimized via the use of various reference points—for example, the carotid artery and neck muscles in patients with thyroid cancer have good sensitivity and better negative predictive values for determining benign and malignant natures of thyroid nodules [86].
Shear wave elastography is more utilized in cases of prostatic malignancies. Calcifications on shear wave elastography might present false positive results, but extracapsular extension of malignancies can be better visualized [73].
4.2. Use in Gastrointestinal Tissues
Elastography is also being used in many gastrointestinal diseases, specifically the liver and pancreas. Transient elastography is an accurate predictor of liver fibrosis and provides an important correlation with the recurrence of viral illnesses, including hepatitis C [27,36]. Perisinusoidal fibrosis was observed to influence the results of transient elastography in this set of patients leading to a better correlation with recurrence of disease [27]. The varied forms of elastography, including Fibro Scan, can also provide insight to hepatic steatosis, leading to better diagnosis of nonalcoholic fatty liver disease in morbidly obese patients [30]. Other forms of chronic liver disease can also be evaluated, including cirrhotic liver disease and portal hypertensive gastropathy [24,25].
Rectal lesions have also been assessed using elastography for preoperative assessment [74]. Pancreatic lesions have also been commonly assessed with strain elastography. A few measures have been discussed in this regard. Malignant lesions usually have a significantly higher lesion-to-parenchyma strain ratio and lesion-to-wall ratio [78]. The technique does not increase operation time or risk of adverse effects on evaluation [78]. Endoscopic ultrasound elastography has also been used in the identification of pancreatic duct dilatation that correlated with higher stiffness index and head-based locations in patients with pancreatic cancer [77]. The elastic modulus as shown by elastography is also useful in appendicitis and other infections. However, there are a few limitations in the evaluation of the appendix. The signal can be displaced by an anteriorly located cecum in the retrocecal appendix [72]. Most importantly, because of the location of the appendix, the use of shear wave elastography might be limited because the shear waves decrease in intensity during propagation [72].
The esophagus is also being evaluated using elastography. Reflux esophagitis is caused by acid retention or reflux in the lower esophagus. The esophagogastric junction prevents reflux and has varying pressure depending on the contraction of the lower esophageal sphincter [75]. The change in stiffness with appropriate reference points—for example, the liver—correlated with motility of the esophagogastric junction. Therefore, a greater change in stiffness indirectly implied normal movements of the esophagogastric junction [75]. Similar underlying principles have been used in esophageal cancer for evaluation of lymph nodes [53].
Limitations of the Study
The main strength of the study is the comparison of measures in various organs and pathologies using elastography on a large scale. There are a few limitations to the study. There was limited availability of data regarding accuracy. Additionally, multiple studies used qualitative measures that could not be compared in a pair-wise fashion despite producing significant results in the studies discussed.
5. Conclusions
Ultrasound-based elastography is gradually becoming a widely used source in many diseases. However, the use is still more common and apparently accurate in gastrointestinal diseases. Shear wave elastography was commonly used for the liver, and strain elastography was commonly utilized for the pancreas. However, accuracy and positive predictive value of strain elastography in the liver would help in navigating through differential diagnoses of various pathologies. Elastography techniques might help in minimizing the impact of inflammation in visualizing lesions in both gastrointestinal and non-gastrointestinal tissues, for example, prostate. The addition of different indices—for example, motility index in the esophagus—can help to diagnose risk of reflux early. The utility and outcomes are, however, dependent on a few instrument-specific characteristics and operator-dependent characteristics—for example, feasibility of use and experience of the operator. Furthermore, the addition of real-time imaging and determination of appropriate cutoff values for optimal results are other factors that must be considered. Despite its promising utility as a tool, further studies are needed to determine the associated factors and optimal output for both gastrointestinal and non-gastrointestinal diseases. Many future applications of elastography are yet to be investigated including cancer detection and monitoring and use in minimally invasive procedures. With the promising advent of artificial intelligence, further studies using both artificial intelligence and elastography might present a new era of interventions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics13213302/s1, Supplementary Table S1 details the MESH terms used in the search. Supplementary Table S2 discusses the risk of bias of the studies included. Supplementary Table S3 includes the types of elastography techniques used. Supplementary Table S4 includes the sources of funding of all the included studies. Supplementary Table S5 represents the PRISMA checklist.
Author Contributions
Conceptualization, N.J., and H.G.; methodology, N.J.; software, N.J.; validation, H.G., A.J., and H.P.; formal analysis, N.J.; investigation, N.J., H.G., and A.J.; resources, H.P.; data curation, H.P.; writing—original draft preparation, N.J., H.G., and A.J.; writing—review and editing, H.G., A.J., and H.P.; supervision, H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be made available by special request addressed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Drakonaki, E.E.; Allen, G.M.; Wilson, D.J. Ultrasound elastography for musculoskeletal applications. Br. J. Radiol. 2012, 85, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Paluch, Ł.; Nawrocka-Laskus, E.; Wieczorek, J.; Mruk, B.; Frel, M.; Walecki, J. Use of Ultrasound Elastography in the Assessment of the Musculoskeletal System. Pol. J. Radiol. 2016, 81, 240–246. [Google Scholar] [CrossRef]
- Shen, M.; Lee, A.; Lefkowitch, J.H.; Worman, H.J. Vibration-controlled Transient Elastography for Assessment of Liver Fibrosis at a USA Academic Medical Center. J. Clin. Transl. Hepatol. 2022, 10, 197–206. [Google Scholar] [CrossRef]
- Rosenthal, M.H.; Lee, A.; Jajoo, K. Imaging and Endoscopic Approaches to Pancreatic Cancer. Hematol. Oncol. Clin. North Am. 2015, 29, 675–699. [Google Scholar] [CrossRef]
- Risk of Bias Tools. Risk of Bias Info. 2023. Available online: https://www.riskofbias.info/ (accessed on 13 October 2023).
- Vidal-Casariego, A.; López-González, L.; Jiménez-Pérez, A.; Ballesteros-Pomar, M.D.; Kyriakos, G.; Urioste-Fondo, A.; Álvarez-San Martín, R.; Cano-Rodríguez, I.; Jiménez-García de la Marina, J.M. Accuracy of ultrasound elastography in the diagnosis of thyroid cancer in a low-risk population. Exp. Clin. Endocrinol. Diabetes 2012, 120, 635–638. [Google Scholar] [CrossRef]
- Azizi, G.; Keller, J.M.; Mayo, M.L.; Piper, K.; Puett, D.; Earp, K.M.; Malchoff, C.D. Thyroid Nodules and Shear Wave Elastography: A New Tool in Thyroid Cancer Detection. Ultrasound Med. Biol. 2015, 41, 2855–2865. [Google Scholar] [CrossRef]
- Dighe, M.; Luo, S.; Cuevas, C.; Kim, Y. Efficacy of thyroid ultrasound elastography in differential diagnosis of small thyroid nodules. Eur. J. Radiol. 2013, 82, e274–e280. [Google Scholar] [CrossRef]
- Unlütürk, U.; Erdoğan, M.F.; Demir, O.; Güllü, S.; Başkal, N. Ultrasound elastography is not superior to grayscale ultrasound in predicting malignancy in thyroid nodules. Thyroid 2012, 22, 1031–1038. [Google Scholar] [CrossRef]
- Çakal, E.; Karaköse, M.; Öztürk Ünsal, İ.; Şahin, M.; Uçan, B.; Özbek, M. The diagnostic value of elastography score and strain index for the evaluation of thyroid micronodules. Turk. J. Med. Sci. 2018, 48, 1048–1052. [Google Scholar] [CrossRef]
- Asteria, C.; Giovanardi, A.; Pizzocaro, A.; Cozzaglio, L.; Morabito, A.; Somalvico, F.; Zoppo, A. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid 2008, 18, 523–531. [Google Scholar] [CrossRef]
- Elsayed, N.M.; Elkhatib, Y.A. Diagnostic Criteria and Accuracy of Categorizing Malignant Thyroid Nodules by Ultrasonography and Ultrasound Elastography with Pathologic Correlation. Ultrason. Imaging 2016, 38, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; Lodise, P.; Di Rocco, G.; Grazhdani, H.; Giannotti, D.; Patrizi, G.; Medvedyeva, E.; Olive, M.; Fioravanti, C.; Giacomelli, L.; et al. Diagnostic accuracy and interobserver agreement of Quasistatic Ultrasound Elastography in the diagnosis of thyroid nodules. Ultraschall. Med. 2015, 36, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.R.; Ji, C.L.; Wu, Y.; Gu, X.G. Combination of ultrasound elastography with TI-RADS in the diagnosis of small thyroid nodules (≤10 mm): A new method to increase the diagnostic performance. Eur. J. Radiol. 2018, 109, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Kim, E.K.; Kwak, J.Y.; Park, V.Y.; Moon, H.J. Application of Various Additional Imaging Techniques for Thyroid Ultrasound: Direct Comparison of Combined Various Elastography and Doppler Parameters to Gray-Scale Ultrasound in Differential Diagnosis of Thyroid Nodules. Ultrasound Med. Biol. 2018, 44, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhai, D.; Zhang, T.; Zhang, S. Use of strain ultrasound elastography versus fine-needle aspiration cytology for the differential diagnosis of thyroid nodules: A retrospective analysis. Clinics 2020, 75, e1594. [Google Scholar] [CrossRef] [PubMed]
- Gay, S.; Schiaffino, S.; Santamorena, G.; Massa, B.; Ansaldo, G.; Turtulici, G.; Giusti, M. Role of Strain Elastography and Shear-Wave Elastography in a Multiparametric Clinical Approach to Indeterminate Cytology Thyroid Nodules. Med. Sci. Monit. 2018, 24, 6273–6279. [Google Scholar] [CrossRef]
- Russ, G.; Royer, B.; Bigorgne, C.; Rouxel, A.; Bienvenu-Perrard, M.; Leenhardt, L. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur. J. Endocrinol. 2013, 168, 649–655. [Google Scholar] [CrossRef]
- Li, H.; Kang, C.; Xue, J.; Jing, L.; Miao, J. Influence of lesion size on shear wave elastography in the diagnosis of benign and malignant thyroid nodules. Sci. Rep. 2021, 11, 21616. [Google Scholar] [CrossRef]
- Wu, H.X.; Zhang, B.J.; Wang, J.; Zhu, B.L.; Zang, Y.P.; Cao, Y.L. Conventional ultrasonography and real time ultrasound elastography in the differential diagnosis of degenerating cystic thyroid nodules mimicking malignancy and papillary thyroid carcinomas. Asian. Pac. J. Cancer. Prev. 2013, 14, 935–940. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seong, M.; Shin, J.H.; Hahn, S.Y. Ultrasound Strain Elastography for Circumscribed Solid Thyroid Nodules without Malignant Features Categorized as Indeterminate by B-Mode Ultrasound. Ultrasound Med. Biol. 2016, 42, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.S.; Tong, C.S.; Cho, C.C.; Yuen, E.H.; Lee, Y.Y.; Ahuja, A.T. Shear wave elastography of thyroid nodules in routine clinical practice: Preliminary observations and utility for detecting malignancy. Eur. Radiol. 2012, 22, 2397–2406. [Google Scholar] [CrossRef]
- Huang, S.T.; Zhang, B.; Yin, H.L.; Li, B.; Liao, J.T.; Wang, Y.B. Incremental diagnostic value of shear wave elastography combined with contrast-enhanced ultrasound in TI-RADS category 4a and 4b nodules. J. Med. Ultrason. 2020, 47, 453–462. [Google Scholar] [CrossRef]
- Rigamonti, C.; Donato, M.F.; Fraquelli, M.; Agnelli, F.; Ronchi, G.; Casazza, G.; Rossi, G.; Colombo, M. Transient elastography predicts fibrosis progression in patients with recurrent hepatitis C after liver transplantation. Gut 2008, 57, 821–827. [Google Scholar] [CrossRef]
- Sporea, I.; Sirli, R.; Deleanu, A.; Tudora, A.; Curescu, M.; Cornianu, M.; Lazar, D. Comparison of the liver stiffness measurement by transient elastography with the liver biopsy. World J. Gastroenterol. 2008, 14, 6513–6517. [Google Scholar] [CrossRef]
- Abrams, G.A.; Jamal, H.; Deeter, W.T., 3rd; Patil, N. LOGIQ E9 Shear Wave Elastrography for Detection of Liver Fibrosis in Patients with Chronic Hepatitis C Virus. South. Med. J. 2016, 109, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Aggarwal, S.; Shalimar; Yadav, R.; Datta Gupta, S.; Agarwal, L.; Agarwal, S. Utility of transient elastography (fibroscan) and impact of bariatric surgery on nonalcoholic fatty liver disease (NAFLD) in morbidly obese patients. Surg. Obes. Relat. Dis. 2018, 14, 81–91. [Google Scholar] [CrossRef]
- Montes Ramirez, M.L.; Pascual-Pareja, J.F.; Sánchez-Conde, M.; Bernardino De la Serna, J.I.; Zamora Vargas, F.X.; Miralles, P.; Castro, J.M.; Ramírez, M.; Gutierrez, I.; Gonzalez-García, J.; et al. Transient elastography to rule out esophageal varices and portal hypertensive gastropathy in HIV-infected individuals with liver cirrhosis. AIDS 2012, 26, 1807–1812. [Google Scholar] [CrossRef]
- Malik, R.; Lai, M.; Sadiq, A.; Farnan, R.; Mehta, S.; Nasser, I.; Challies, T.; Schuppan, D.; Afdhal, N. Comparison of transient elastography, serum markers and clinical signs for the diagnosis of compensated cirrhosis. J. Gastroenterol. Hepatol. 2010, 25, 1562–1568. [Google Scholar] [CrossRef]
- Corpechot, C.; Gaouar, F.; El Naggar, A.; Kemgang, A.; Wendum, D.; Poupon, R.; Carrat, F.; Chazouillères, O. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology 2014, 146, 970–979. [Google Scholar] [CrossRef]
- Miailhes, P.; Pradat, P.; Chevallier, M.; Lacombe, K.; Bailly, F.; Cotte, L.; Trabaud, M.A.; Boibieux, A.; Bottero, J.; Trepo, C.; et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J. Viral. Hepat. 2011, 18, 61–69. [Google Scholar] [CrossRef]
- Lee, M.H.; Cheong, J.Y.; Um, S.H.; Seo, Y.S.; Kim, D.J.; Hwang, S.G.; Yang, J.M.; Han, K.H.; Cho, S.W. Comparison of surrogate serum markers and transient elastography (Fibroscan) for assessing cirrhosis in patients with chronic viral hepatitis. Dig. Dis. Sci. 2010, 55, 3552–3560. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Soejima, Y.; Taketomi, A.; Yoshizumi, T.; Ikegami, T.; Yamashita, Y.; Itoh, S.; Kuroda, Y.; Maehara, Y. Assessment of graft fibrosis by transient elastography in patients with recurrent hepatitis C after living donor liver transplantation. Transplantation 2008, 85, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Gara, N.; Zhao, X.; Kleiner, D.E.; Liang, T.J.; Hoofnagle, J.H.; Ghany, M.G. Discordance among transient elastography, aspartate aminotransferase to platelet ratio index, and histologic assessments of liver fibrosis in patients with chronic hepatitis C. Clin. Gastroenterol. Hepatol. 2013, 11, 303–308.e1. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.X.; Zimmer, S.; Niu, S.; Crotty, P.; Tracey, J.; Pradhan, F.; Shaheen, A.A.; Coffin, C.S.; Heitman, S.J.; Kaplan, G.G.; et al. Liver stiffness by transient elastography predicts liver-related complications and mortality in patients with chronic liver disease. PLoS ONE 2014, 9, e95776. [Google Scholar] [CrossRef]
- Gómez-Dominguez, E.; Mendoza, J.; García-Buey, L.; Trapero, M.; Gisbert, J.P.; Jones, E.A.; Moreno-Otero, R. Transient elastography to assess hepatic fibrosis in primary biliary cirrhosis. Aliment. Pharmacol. Ther. 2008, 27, 441–447. [Google Scholar] [CrossRef]
- Seo, Y.S.; Kim, M.N.; Kim, S.U.; Kim, S.G.; Um, S.H.; Han, K.H.; Kim, Y.S. Risk Assessment of Hepatocellular Carcinoma Using Transient Elastography Vs. Liver Biopsy in Chronic Hepatitis B Patients Receiving Antiviral Therapy. Medicine 2016, 95, e2985. [Google Scholar] [CrossRef]
- Xie, X.; Feng, Y.; Lyu, Z.; Wang, L.; Yang, Y.; Bai, Y.; Liu, C.; Wu, H.; Ren, W.; Zhu, Q. The Combination of Shear Wave Elastography and Platelet Counts Can Effectively Predict High-Risk Varices in Patients with Hepatitis B-Related Cirrhosis. Biomed. Res. Int. 2021, 2021, 6635963. [Google Scholar] [CrossRef]
- Beckebaum, S.; Iacob, S.; Klein, C.G.; Dechêne, A.; Varghese, J.; Baba, H.A.; Sotiropoulos, G.C.; Paul, A.; Gerken, G.; Cicinnati, V.R. Assessment of allograft fibrosis by transient elastography and noninvasive biomarker scoring systems in liver transplant patients. Transplantation 2010, 89, 983–993. [Google Scholar] [CrossRef]
- Obara, N.; Ueno, Y.; Fukushima, K.; Nakagome, Y.; Kakazu, E.; Kimura, O.; Wakui, Y.; Kido, O.; Ninomiya, M.; Kogure, T.; et al. Transient elastography for measurement of liver stiffness measurement can detect early significant hepatic fibrosis in Japanese patients with viral and nonviral liver diseases. J. Gastroenterol. 2008, 43, 720–728. [Google Scholar] [CrossRef]
- Endo, M.; Soroida, Y.; Sato, M.; Kobayashi, T.; Hikita, H.; Sato, M.; Gotoh, H.; Iwai, T.; Sone, S.; Sasano, T.; et al. Ultrasound evaluation of liver stiffness: Accuracy of ultrasound imaging for the prediction of liver cirrhosis as evaluated using a liver stiffness measurement. J. Med. Dent. Sci. 2017, 64, 27–34. [Google Scholar] [CrossRef]
- Ooi, C.C.; Richards, P.J.; Maffulli, N.; Ede, D.; Schneider, M.E.; Connell, D.; Morrissey, D.; Malliaras, P. A soft patellar tendon on ultrasound elastography is associated with pain and functional deficit in volleyball players. J. Sci. Med. Sport. 2016, 19, 373–378. [Google Scholar] [CrossRef]
- Jin, Z.Q.; Lin, M.Y.; Hu, W.H.; Li, W.Y.; Bai, S.J. Gray-scale ultrasonography combined with elastography imaging for the evaluation of papillary thyroid microcarcinoma: As a prognostic clinicopathology factor. Ultrasound Med. Biol. 2014, 40, 1769–1777. [Google Scholar] [CrossRef]
- Chae, S.Y.; Jung, H.N.; Ryoo, I.; Suh, S. Differentiating cervical metastatic lymphadenopathy and lymphoma by shear wave elastography. Sci. Rep. 2019, 9, 12396. [Google Scholar] [CrossRef] [PubMed]
- Desmots, F.; Fakhry, N.; Mancini, J.; Reyre, A.; Vidal, V.; Jacquier, A.; Santini, L.; Moulin, G.; Varoquaux, A. Shear Wave Elastography in Head and Neck Lymph Node Assessment: Image Quality and Diagnostic Impact Compared with B-Mode and Doppler Ultrasonography. Ultrasound Med. Biol. 2016, 42, 387–398. [Google Scholar] [CrossRef]
- Alam, F.; Naito, K.; Horiguchi, J.; Fukuda, H.; Tachikake, T.; Ito, K. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: Comparison with conventional B-mode sonography. AJR. Am. J. Roentgenol. 2008, 191, 604–610. [Google Scholar] [CrossRef]
- Lo, W.C.; Hsu, W.L.; Wang, C.T.; Cheng, P.W.; Liao, L.J. Incorporation of shear wave elastography into a prediction model in the assessment of cervical lymph nodes. PLoS ONE 2019, 14, e0221062. [Google Scholar] [CrossRef] [PubMed]
- Lenghel, L.M.; Botar Jid, C.; Bolboaca, S.D.; Ciortea, C.; Vasilescu, D.; Baciut, G.; Dudea, S.M. Comparative study of three sonoelastographic scores for differentiation between benign and malignant cervical lymph nodes. Eur. J. Radiol. 2015, 84, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Jia, W.; Shi, J.; Yuan, C.; Zhang, Y.; Chen, M. Role of Elastography in Axillary Examination of Patients with Breast Cancer. J. Ultrasound Med. 2018, 37, 699–707. [Google Scholar] [CrossRef]
- Paterson, S.; Duthie, F.; Stanley, A.J. Endoscopic ultrasound-guided elastography in the nodal staging of oesophageal cancer. World J. Gastroenterol. 2012, 18, 889–895. [Google Scholar] [CrossRef]
- Choi, J.J.; Kang, B.J.; Kim, S.H.; Lee, J.H.; Jeong, S.H.; Yim, H.W.; Song, B.J.; Jung, S.S. Role of sonographic elastography in the differential diagnosis of axillary lymph nodes in breast cancer. J. Ultrasound Med. 2011, 30, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, J.H.; Lim, H.K.; Kim, S.Y.; Han, M.W.; Cho, K.J.; Baek, J.H. Quantitative shear wave elastography in the evaluation of metastatic cervical lymph nodes. Ultrasound Med. Biol. 2013, 39, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Nakajima, T.; Inage, T.; Sata, Y.; Sakairi, Y.; Tamura, H.; Wada, H.; Suzuki, H.; Chiyo, M.; Yoshino, I. The combination of endobronchial elastography and sonographic findings during endobronchial ultrasound-guided transbronchial needle aspiration for predicting nodal metastasis. Thorac. Cancer 2019, 10, 2000–2005. [Google Scholar] [CrossRef]
- Verhoeven, R.L.J.; Trisolini, R.; Leoncini, F.; Candoli, P.; Bezzi, M.; Messi, A.; Krasnik, M.; de Korte, C.L.; Annema, J.T.; van der Heijden, E.H.F.M. Predictive Value of Endobronchial Ultrasound Strain Elastography in Mediastinal Lymph Node Staging: The E-Predict Multicenter Study Results. Respiration 2020, 99, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Omar, N.; Ab Mumin, N.; Ramli Hamid, M.T.; Vijayananthan, A.; Rahmat, K. Diagnostic Accuracy of Shear Wave Elastography as an Adjunct Tool in Detecting Axillary Lymph Nodes Metastasis. Acad. Radiol. 2022, 29 (Suppl. S1), S69–S78. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Sohn, Y.M. Differentiation of benign and metastatic axillary lymph nodes in breast cancer: Additive value of shear wave elastography to B-mode ultrasound. Clin. Imaging 2018, 50, 258–263. [Google Scholar] [CrossRef]
- Ogata, D.; Uematsu, T.; Yoshikawa, S.; Kiyohara, Y. Accuracy of real-time ultrasound elastography in the differential diagnosis of lymph nodes in cutaneous malignant melanoma (CMM): A pilot study. Int. J. Clin. Oncol. 2014, 19, 716–721. [Google Scholar] [CrossRef]
- Taylor, K.; O’Keeffe, S.; Britton, P.D.; Wallis, M.G.; Treece, G.M.; Housden, J.; Parashar, D.; Bond, S.; Sinnatamby, R. Ultrasound elastography as an adjuvant to conventional ultrasound in the preoperative assessment of axillary lymph nodes in suspected breast cancer: A pilot study. Clin. Radiol. 2011, 66, 1064–1071. [Google Scholar] [CrossRef]
- Acu, L.; Oktar, S.Ö.; Acu, R.; Yücel, C.; Cebeci, S. Value of Ultrasound Elastography in the Differential Diagnosis of Cervical Lymph Nodes: A Comparative Study With B-mode and Color Doppler Sonography. J. Ultrasound Med. 2016, 35, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.; Dhalluin, X.; Wallyn, F.; Machuron, F.; Bouchindhomme, B.; Copin, M.C.; Valentin, V. Performance of Endobronchial Ultrasound Elastography in the Differentiation of Malignant and Benign Mediastinal Lymph Nodes: Results in Real-life Practice. J. Bronchology. Interv. Pulmonol. 2019, 26, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Korrungruang, P.; Boonsarngsuk, V. Diagnostic value of endobronchial ultrasound elastography for the differentiation of benign and malignant intrathoracic lymph nodes. Respirology 2017, 22, 972–977. [Google Scholar] [CrossRef]
- Larsen, M.H.; Fristrup, C.; Hansen, T.P.; Hovendal, C.P.; Mortensen, M.B. Endoscopic ultrasound, endoscopic sonoelastography, and strain ratio evaluation of lymph nodes with histology as gold standard. Endoscopy 2012, 44, 759–766. [Google Scholar] [CrossRef]
- Havre, R.F.; Leh, S.M.; Gilja, O.H.; Ødegaard, S.; Waage, J.E.; Baatrup, G.; Nesje, L.B. Differentiation of Metastatic and Non-Metastatic Mesenteric Lymph Nodes by Strain Elastography in Surgical Specimens. Ultraschall. Med. 2016, 37, 366–372. [Google Scholar] [CrossRef]
- Che, D.; Zhou, X.; Sun, M.L.; Wang, X.; Jiang, Z.; Changjun, W. Differentiation of metastatic cervical lymph nodes with ultrasound elastography by virtual touch tissue imaging: Preliminary study. J. Ultrasound Med. 2015, 34, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, M.; Gurbuz, M.K.; Cingi, C.; Adapinar, B.; Değirmenci, A.N.; Acikalin, F.M.; Pinarbaşli, M.Ö.; Colak, E. Diagnostic role of ultrasound elastography on lymph node metastases in patients with head and neck cancer. Braz. J. Otorhinolaryngol. 2019, 85, 297–302. [Google Scholar] [CrossRef]
- Fang, S.; Chang, L.; Chen, F.; Mao, X.; Gu, W. Endobronchial Ultrasound Elastography Combined with Computed Tomography in Differentiating Benign from Malignant Intrathoracic Lymph Nodes. Surg. Innov. 2021, 28, 590–599. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, X.; Mao, X.; Wang, L.; Xiong, H.; Herth, F.J.F.; Han, B. Endobronchial Ultrasound Elastography for Evaluation of Intrathoracic Lymph Nodes: A Pilot Study. Respiration 2017, 93, 327–338. [Google Scholar] [CrossRef]
- Lin, C.K.; Yu, K.L.; Chang, L.Y.; Fan, H.J.; Wen, Y.F.; Ho, C.C. Differentiating malignant and benign lymph nodes using endobronchial ultrasound elastography. J. Formos. Med. Assoc. 2019, 118, 436–443. [Google Scholar] [CrossRef]
- Cha, S.W.; Kim, I.Y.; Kim, Y.W. Quantitative measurement of elasticity of the appendix using shear wave elastography in patients with suspected acute appendicitis. PLoS ONE 2014, 9, e101292. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Memo, R.; Schaub, C.R. Shear wave ultrasound elastography of the prostate: Initial results. Ultrasound Q. 2012, 28, 13–20. [Google Scholar] [CrossRef]
- Li, T.; Lu, M.; Li, Y.; Li, J.; Hu, Z.; Li, X.; Cheng, X.; Jiang, J.; Tan, B. Quantitative Elastography of Rectal Lesions: The Value of Shear Wave Elastography in Identifying Benign and Malignant Rectal Lesions. Ultrasound Med. Biol. 2019, 45, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Suhara, H.; Hirooka, Y.; Kawashima, H.; Ohno, E.; Ishikawa, T.; Nakamura, M.; Miyahara, R.; Ishigami, M.; Hashimoto, S.; Goto, H. Transabdominal ultrasound elastography of the esophagogastric junction predicts reflux esophagitis. J. Med. Ultrason. 2019, 46, 99–104. [Google Scholar] [CrossRef]
- Rustemović, N.; Kalauz, M.; Grubelić Ravić, K.; Iveković, H.; Bilić, B.; Ostojić, Z.; Opačić, D.; Ledinsky, I.; Majerović, M.; Višnjić, A. Differentiation of Pancreatic Masses via Endoscopic Ultrasound Strain Ratio Elastography Using Adjacent Pancreatic Tissue as the Reference. Pancreas 2017, 46, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Ishikawa, T.; Ohno, E.; Iida, T.; Suzuki, H.; Uetsuki, K.; Furukawa, K.; Nakamura, M.; Honda, T.; Ishigami, M.; et al. Endoscopic ultrasound elastography for small solid pancreatic lesions with or without main pancreatic duct dilatation. Pancreatology 2021, 21, 451–458. [Google Scholar] [CrossRef]
- Carrara, S.; Di Leo, M.; Grizzi, F.; Correale, L.; Rahal, D.; Anderloni, A.; Auriemma, F.; Fugazza, A.; Preatoni, P.; Maselli, R.; et al. EUS elastography (strain ratio) and fractal-based quantitative analysis for the diagnosis of solid pancreatic lesions. Gastrointest. Endosc. 2018, 87, 1464–1473. [Google Scholar] [CrossRef]
- Ignee, A.; Jenssen, C.; Arcidiacono, P.G.; Hocke, M.; Möller, K.; Saftoiu, A.; Will, U.; Fusaroli, P.; Iglesias-Garcia, J.; Ponnudurai, R.; et al. Endoscopic ultrasound elastography of small solid pancreatic lesions: A multicenter study. Endoscopy 2018, 50, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Okasha, H.; Elkholy, S.; El-Sayed, R.; Wifi, M.N.; El-Nady, M.; El-Nabawi, W.; El-Dayem, W.A.; Radwan, M.I.; Farag, A.; El-Sherif, Y.; et al. Real time endoscopic ultrasound elastography and strain ratio in the diagnosis of solid pancreatic lesions. World J. Gastroenterol. 2017, 23, 5962–5968. [Google Scholar] [CrossRef]
- Ahmad, S.; Cao, R.; Varghese, T.; Bidaut, L.; Nabi, G. Transrectal quantitative shear wave elastography in the detection and characterisation of prostate cancer. Surg. Endosc. 2013, 27, 3280–3287. [Google Scholar] [CrossRef]
- Aghaghazvini, L.; Maheronnaghsh, R.; Soltani, A.; Rouzrokh, P.; Chavoshi, M. Diagnostic value of shear wave sonoelastography in differentiation of benign from malignant thyroid nodules. Eur. J. Radiol. 2020, 126, 108926. [Google Scholar] [CrossRef]
- Wang, F.; Chang, C.; Gao, Y.; Chen, Y.L.; Chen, M.; Feng, L.Q. Does Shear Wave Elastography Provide Additional Value in the Evaluation of Thyroid Nodules That Are Suspicious for Malignancy? J. Ultrasound Med. 2016, 35, 2397–2404. [Google Scholar] [CrossRef]
- Azizi, G.; Keller, J.; Lewis, M.; Puett, D.; Rivenbark, K.; Malchoff, C. Performance of elastography for the evaluation of thyroid nodules: A prospective study. Thyroid 2013, 23, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chang, C.; Chen, M.; Gao, Y.; Chen, Y.L.; Zhou, S.C.; Li, J.W.; Zhi, W.X. Does Lesion Size Affect the Value of Shear Wave Elastography for Differentiating Between Benign and Malignant Thyroid Nodules? J. Ultrasound Med. 2018, 37, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Kratky, J.; Vitkova, H.; Bartakova, J.; Lukas, J.; Jiskra, J. Neck Muscles and Content of Carotid Artery as Reference Tissue for Strain Ratio—A Novel Approach to Improve the Diagnostic Performance of Thyroid Elastography? Exp. Clin. Endocrinol. Diabetes 2016, 124, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Unalp-Arida, A.; Ruhl, C.E. Transient Elastography-Assessed Hepatic Steatosis and Fibrosis Are Associated with Body Composition in the United States. Clin. Gastroenterol. Hepatol. 2022, 20, e808–e830. [Google Scholar] [CrossRef]
- Yada, N.; Tamaki, N.; Koizumi, Y.; Hirooka, M.; Nakashima, O.; Hiasa, Y.; Izumi, N.; Kudo, M. Diagnosis of Fibrosis and Activity by a Combined Use of Strain and Shear Wave Imaging in Patients with Liver Disease. Dig. Dis. 2017, 35, 515–520. [Google Scholar] [CrossRef]
- Loft, M.K.; Pedersen, M.R.V.; Rahr, H.B.; Rafaelsen, S.R. Can Ultrasound Elastography Discriminate between Rectal Adenoma and Cancer? A Systematic Review. Cancers 2021, 13, 4158. [Google Scholar] [CrossRef]
- Kawada, N.; Tanaka, S. Elastography for the pancreas: Current status and future perspective. World J. Gastroenterol. 2016, 22, 3712–3724. [Google Scholar] [CrossRef]
- Navarro, B.; Ubeda, B.; Vallespí, M.; Wolf, C.; Casas, L.; Browne, J.L. Role of elastography in the assessment of breast lesions: Preliminary results. J. Ultrasound Med. 2011, 30, 313–321. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).