The Potential of Ultrasound Radiomics in Carpal Tunnel Syndrome Diagnosis: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Protocol Registration
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Study Characteristics
3.3. Quality Assessment
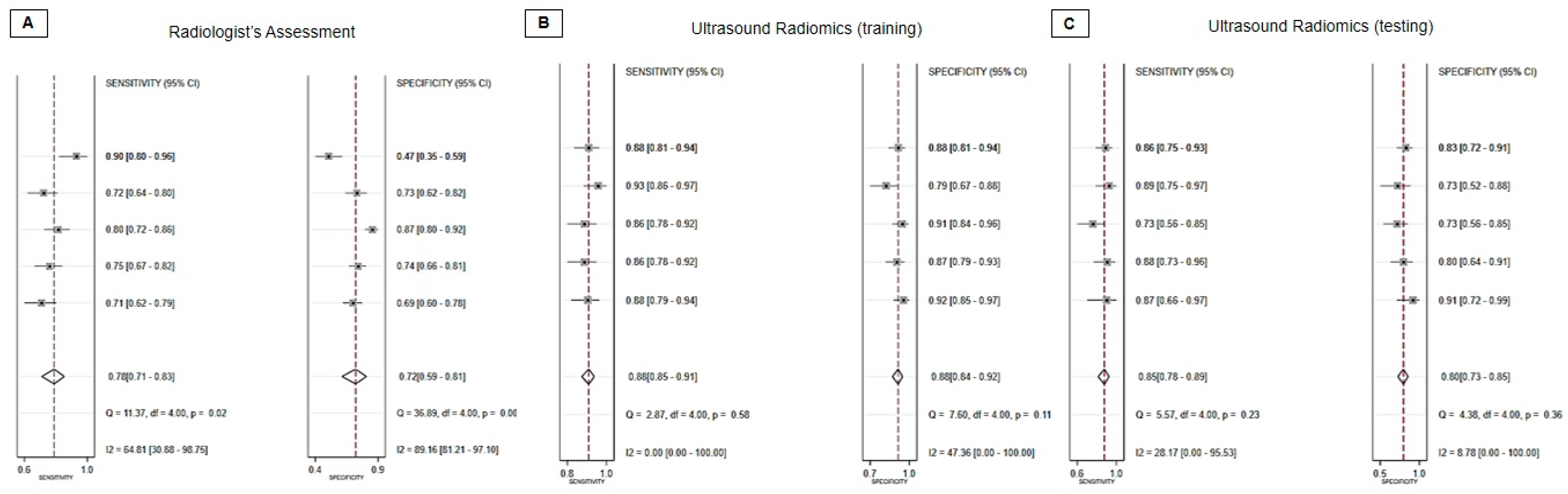
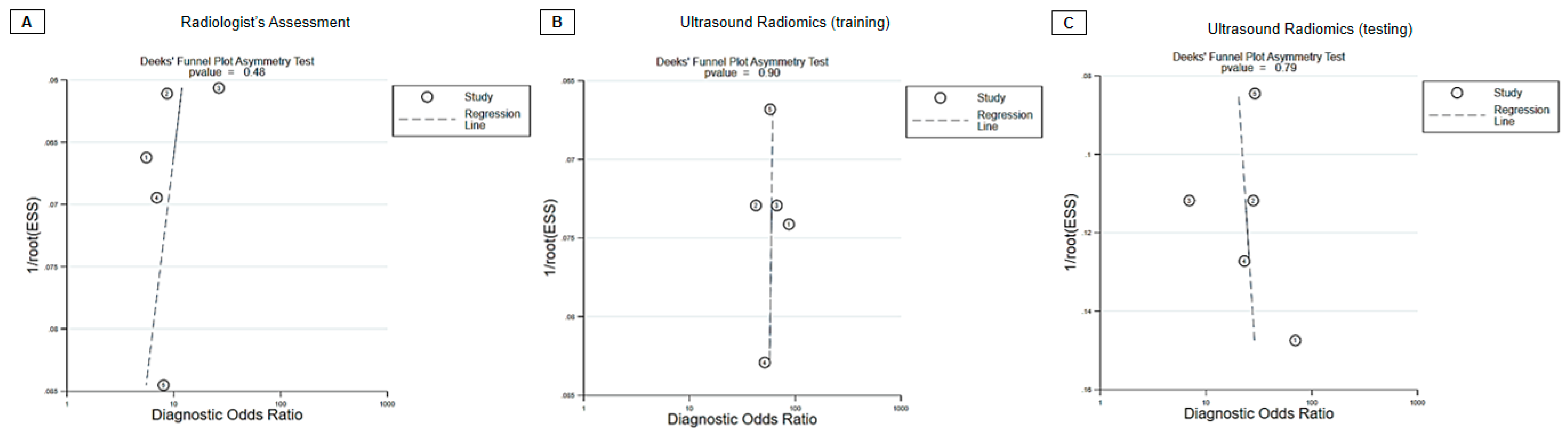
3.4. Diagnostic Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, Y.; Yang, J.; Zhu, Y.; Nie, F.; Wu, H.; Duan, Y.; Chen, K. Ultrasound-based radiomics: Current status, challenges and future opportunities. Med. Ultrason. 2022, 24, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhu, W.; Tang, H.; Zhou, L. Ultrasound radiomics in the prediction of microvascular invasion in hepatocellular carcinoma: A systematic review and meta-analysis. Heliyon 2023, 9, e16997. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Cai, M.; Zha, H.; Chen, B.; Gu, J.; Zhang, M.; Liu, W.; Liu, X.; Liu, X.; Zong, M.; et al. Ultrasound radiomics-based nomogram to predict lymphovascular invasion in invasive breast cancer: A multicenter, retrospective study. Eur. Radiol. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Wei, H.; Yang, L.; Liu, R.; Zhang, B.; Lyu, S. Ultrasound radiomics nomogram for predicting large-number cervical lymph node metastasis in papillary thyroid carcinoma. Front. Oncol. 2023, 13, 1159114. [Google Scholar] [CrossRef] [PubMed]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Into Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Atroshi, I.; Gummesson, C.; Johnsson, R.; Ornstein, E.; Ranstam, J.; Rosén, I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999, 282, 153–158. [Google Scholar] [CrossRef]
- Sperka, P.; Cherry, N.; Burnham, R.; Beach, J. Impact of compensation on work outcome of carpal tunnel syndrome. Occup. Med. 2008, 58, 490–495. [Google Scholar] [CrossRef]
- Chang, K.V.; Wu, W.T.; Ozcakar, L. Ultrasound imaging and guidance in peripheral nerve entrapment: Hydrodissection highlighted. Pain. Manag. 2020, 10, 97–106. [Google Scholar] [CrossRef]
- Chen, I.J.; Chang, K.V.; Lou, Y.M.; Wu, W.T.; Ozcakar, L. Can ultrasound imaging be used for the diagnosis of carpal tunnel syndrome in diabetic patients? A systemic review and network meta-analysis. J. Neurol. 2020, 267, 1887–1895. [Google Scholar] [CrossRef]
- Lin, C.P.; Chen, I.J.; Chang, K.V.; Wu, W.T.; Ozcakar, L. Utility of Ultrasound Elastography in Evaluation of Carpal Tunnel Syndrome: A Systematic Review and Meta-analysis. Ultrasound Med. Biol. 2019, 45, 2855–2865. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Bignotti, B.; Bianchi, L.; Picasso, R.; Martinoli, C.; Tagliafico, A.S. Radiomics of peripheral nerves MRI in mild carpal and cubital tunnel syndrome. La Radiol. Medica 2020, 125, 197–203. [Google Scholar] [CrossRef]
- Wu, H.; Luo, B.; Zhao, Y.; Yuan, G.; Wang, Q.; Liu, P.; Zhai, L.; Lv, W.; Zhang, J. Radiomics analysis of the optic nerve for detecting dysthyroid optic neuropathy, based on water-fat imaging. Insights Imaging 2022, 13, 154. [Google Scholar] [CrossRef]
- Zha, J.; Ni, J.; Chen, S.; Feng, H.; Che, T.; Qiao, S. Ultrasound Radiomics-Guided Iliac Fascia Block on Postoperative Cognitive Dysfunction in Elderly Patients Undergoing Hip Surgery. Comput. Math. Methods Med. 2022, 2022, 2492667. [Google Scholar] [CrossRef]
- Hashiba, J.; Yokota, H.; Abe, K.; Sekiguchi, Y.; Ikeda, S.; Sugiyama, A.; Kuwabara, S.; Uno, T. Ultrasound-based radiomic analysis of the peripheral nerves for differentiation between CIDP and POEMS syndrome. Acta Radiol. 2023, 64, 2627–2635. [Google Scholar] [CrossRef]
- Wang, M.Y.; Jia, C.G.; Xu, H.Q.; Xu, C.S.; Li, X.; Wei, W.; Chen, J.C. Development and Validation of a Deep Learning Predictive Model Combining Clinical and Radiomic Features for Short-Term Postoperative Facial Nerve Function in Acoustic Neuroma Patients. Curr. Med. Sci. 2023, 43, 336–343. [Google Scholar] [CrossRef]
- Faeghi, F.; Ardakani, A.A.; Acharya, U.R.; Mirza-Aghazadeh-Attari, M.; Abolghasemi, J.; Ejtehadifar, S.; Mohammadi, A. Accurate automated diagnosis of carpal tunnel syndrome using radiomics features with ultrasound images: A comparison with radiologists’ assessment. Eur. J. Radiol. 2021, 136, 109518. [Google Scholar] [CrossRef]
- Lyu, S.; Zhang, M.; Zhang, B.; Yu, J.; Zhu, J.; Gao, L.; Yang, L.; Zhang, Y. Application of ultrasound images-based radiomics in carpal tunnel syndrome: Without measuring the median nerve cross-sectional area. J. Clin. Ultrasound 2023, 51, 1198–1204. [Google Scholar] [CrossRef]
- Lyu, S.; Zhang, Y.; Zhang, M.; Jiang, M.; Yu, J.; Zhu, J.; Zhang, B. Ultrasound-based radiomics in the diagnosis of carpal tunnel syndrome: The influence of regions of interest delineation method on mode. J. Clin. Ultrasound 2023, 51, 498–506. [Google Scholar] [CrossRef]
- Lyu, S.; Zhang, Y.; Zhang, M.; Zhu, J.; Yu, J.; Zhang, B.; Gao, L.; Wei, H. The Application of Ultrasound Image-Based Radiomics in the Diagnosis of Mild Carpal Tunnel Syndrome. J. Ultrasound Med. 2023, 42, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Torres-Cuenca, T.; Mirza-Aghazadeh-Attari, M.; Faeghi, F.; Acharya, U.R.; Abbasian Ardakani, A. Deep Radiomics Features of Median Nerves for Automated Diagnosis of Carpal Tunnel Syndrome with Ultrasound Images: A Multi-Center Study. J. Ultrasound Med. 2023, 42, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Andary, M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve 2011, 44, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chang, K.V.; Wu, W.T.; Ozcakar, L. Ultrasonography for the diagnosis of carpal tunnel syndrome: An umbrella review. J. Neurol. 2022, 269, 4663–4675. [Google Scholar] [CrossRef]
- Gallardo, E.; Noto, Y.; Simon, N.G. Ultrasound in the diagnosis of peripheral neuropathy: Structure meets function in the neuromuscular clinic. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Zhao, C.; Amadio, P.C. Recent Advances in Ultrasound Diagnosis of Carpal Tunnel Syndrome. Diagnostics 2020, 10, 596. [Google Scholar] [CrossRef]
- Pasini, G.; Bini, F.; Russo, G.; Comelli, A.; Marinozzi, F.; Stefano, A. matRadiomics: A Novel and Complete Radiomics Framework, from Image Visualization to Predictive Model. J. Imaging 2022, 8, 221. [Google Scholar] [CrossRef]
- Aboonq, M.S. Pathophysiology of carpal tunnel syndrome. Neurosciences 2015, 20, 4–9. [Google Scholar]
- Noble, W.S. What is a support vector machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef]
- Radovic, M.; Ghalwash, M.; Filipovic, N.; Obradovic, Z. Minimum redundancy maximum relevance feature selection approach for temporal gene expression data. BMC Bioinform. 2017, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Shu, Y.C.; Lin, C.Y.; Wu, W.T.; Chen, L.R.; Lo, Y.C.; Chiu, H.C.; Ozcakar, L.; Chang, K.V. Application of deep learning algorithms in automatic sonographic localization and segmentation of the median nerve: A systematic review and meta-analysis. Artif. Intell. Med. 2023, 137, 102496. [Google Scholar] [CrossRef] [PubMed]

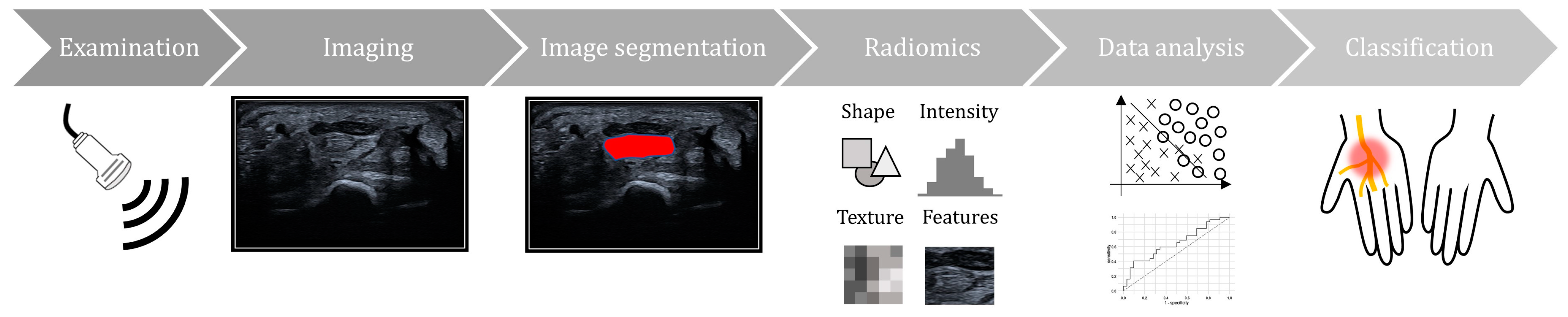

| Author, Year | Country | Study Type | Mean Age of Participants (CTS/non-CTS) | % of Female Participants (CTS/non-CTS) | Ultrasound Machine/Transducer | Software | Hyperparameter | Model for Hyperparameter Selections |
|---|---|---|---|---|---|---|---|---|
| Faeghi et al., 2021 [19] | Iran | Prospective | 51.93/50.28 | 85/84 | AixPlorer Ultimate/5–18 MHz linear-array probe | MATLAB (version R2019a, MathWorks Inc.) | Histogram, AR model gradient, HOG, Gabor, wavelet, GLRLM, GLCM, LBP | SVM model |
| Lyu et al., 2023 a [20] | China | Retrospective | 55.08/54.13 | 80/80 | NA/7–15 MHz linear probe | ITK-SNAP 3.8 | Maximum diameter, sphericity, entropy, median, joint entropy, contrast, correlation, short run low gray level emphasis, gray level non-uniformity, run entropy | Regression model |
| Lyu et al., 2023 b [21] | China | Retrospective | 53.41/54.23 | 84/86 | GE Vivid E9/15 MHz linear probe | Novo Ultrasound Kit (NUK) | Elongation, major axis length, maximum diameter, mesh surface, minor axis length, perimeter, perimeter surface ratio, pixel surface, sphericity, energy, total energy, entropy, 10 percentile, 90 percentile, maximum, minimum, mean, median, robust mean absolute deviation, root mean squared, skewness, kurtosis, uniformity, variance | Forest model |
| Lyu et al., 2023 c [22] | China | Retrospective | 58.70/55.78 | 77/73 | NA/15 MHz linear probe | ITK-SNAP 3.8 | Sphericity, cluster prominence, short run low gray level emphasis, run entropy, long run high gray level emphasis, large area high gray level emphasis, gray level non uniformity, median, small area low gray level emphasis, size zone non uniformity, low gray level zone emphasis, size zone non uniformity normalized, kurtosis, major axis length | Regression model |
| Mohammadi et al., 2023 [23] | Iran and Colombia | Prospective | 56.35/53.62 | 84/84 | First center: AixPlorer Ultimate/12–18 MHz linear probe Second center: Min- dray MX7/15 MHz linear probe | Not mentioned | 1000 deep radiomic features extracted by deep learning (SqueezeNet) | SVM model; SGD model; KNN model; GradBoost model; RForest model NBayes model; LogReg model; AdaBoost model DTree model |
| Risk of Bias | Applicability Concerns | ||||||
|---|---|---|---|---|---|---|---|
| Author, Year | Patient Selection | Index Text | Reference Standard | Flow and Timing | Patient Selection | Index Text | Reference Standard |
| Faeghi et al., 2021 [19] | Low | Low | Low | Low | Low | Low | Low |
| Lyu et al., 2023 a [20] | Low | Low | Low | High | High | Low | Low |
| Lyu et al., 2023 b [21] | Low | Low | Low | Low | High | Low | Low |
| Lyu et al., 2023 c [22] | Low | Low | Low | High | High | Low | Low |
| Mohammadi et al., 2023 [23] | Low | Low | Low | Low | Low | Low | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.-T.; Lin, C.-Y.; Shu, Y.-C.; Shen, P.-C.; Lin, T.-Y.; Chang, K.-V.; Özçakar, L. The Potential of Ultrasound Radiomics in Carpal Tunnel Syndrome Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 3280. https://doi.org/10.3390/diagnostics13203280
Wu W-T, Lin C-Y, Shu Y-C, Shen P-C, Lin T-Y, Chang K-V, Özçakar L. The Potential of Ultrasound Radiomics in Carpal Tunnel Syndrome Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics. 2023; 13(20):3280. https://doi.org/10.3390/diagnostics13203280
Chicago/Turabian StyleWu, Wei-Ting, Che-Yu Lin, Yi-Chung Shu, Peng-Chieh Shen, Ting-Yu Lin, Ke-Vin Chang, and Levent Özçakar. 2023. "The Potential of Ultrasound Radiomics in Carpal Tunnel Syndrome Diagnosis: A Systematic Review and Meta-Analysis" Diagnostics 13, no. 20: 3280. https://doi.org/10.3390/diagnostics13203280
APA StyleWu, W.-T., Lin, C.-Y., Shu, Y.-C., Shen, P.-C., Lin, T.-Y., Chang, K.-V., & Özçakar, L. (2023). The Potential of Ultrasound Radiomics in Carpal Tunnel Syndrome Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics, 13(20), 3280. https://doi.org/10.3390/diagnostics13203280









