Optimal Bowel Preparation Method to Visualize the Distal Ileum via Small Bowel Capsule Endoscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient and Ethics
2.2. SBCE Examination
2.3. Assessment of the SBCE Images and Scoring System
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Study Overview
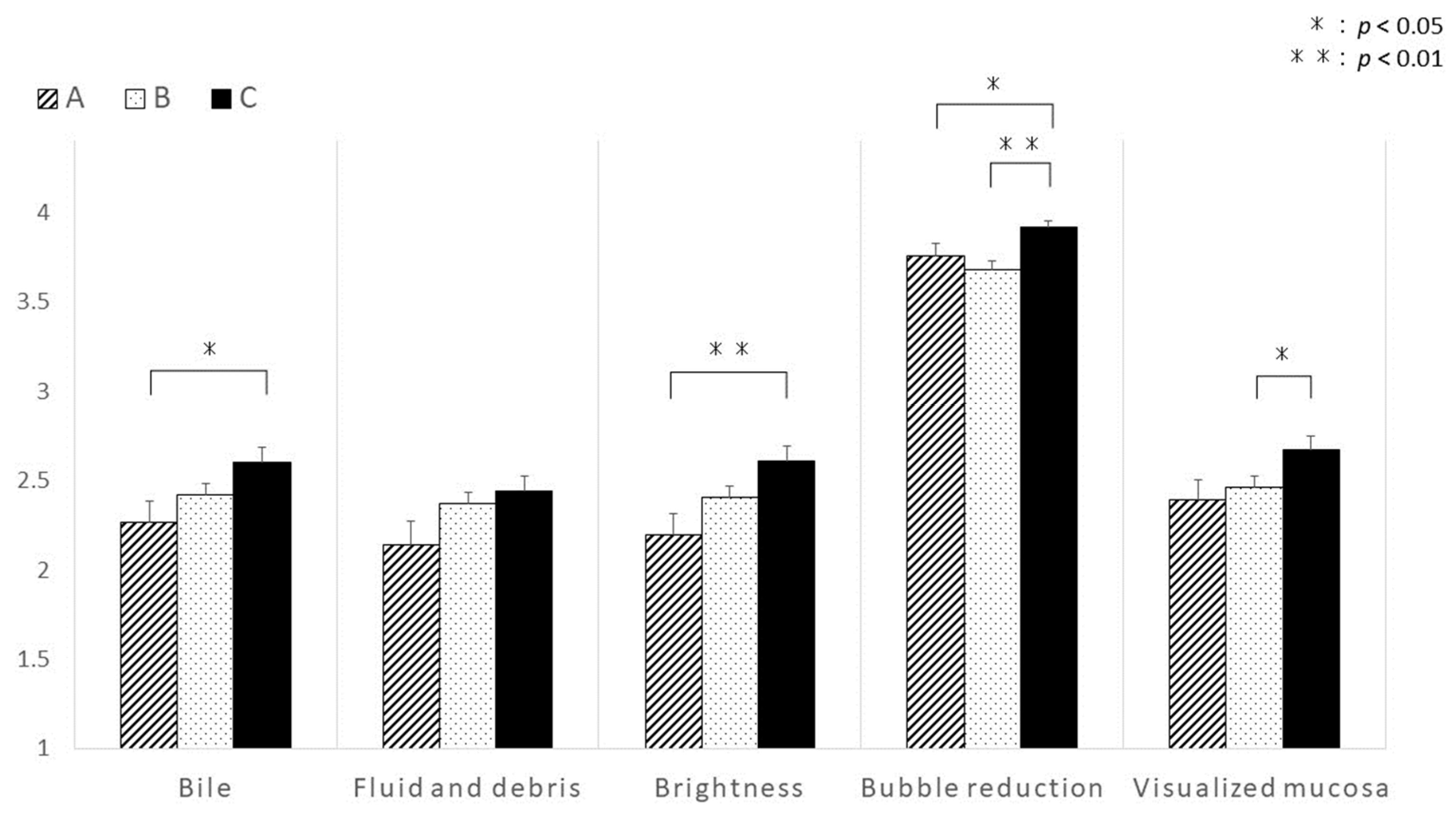
3.2. Differences in Visibility at the Last 10 min during the Period Observing the Distal Ileum
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niv, Y. Efficiency of bowel preparation for capsule endoscopy examination: A meta-analysis. World J. Gastroenterol. 2008, 14, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- van Tuyl, S.A.; den Ouden, H.; Stolk, M.F.; Kuipers, E.J. Optimal preparation for videocapsule endoscopy: A prospective, randomized, single-blind study. Endoscopy 2007, 39, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Wi, J.H.; Moon, J.S.; Choi, M.G.; Kim, J.O.; Do, J.H.; Ryu, J.K.; Shim, K.N.; Lee, K.J.; Jang, B.I.; Korea Gut Image Study Group; et al. Bowel Preparation for Capsule Endoscopy: A Prospective Randomized Multicenter Study. Gut Liver 2009, 3, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Nouda, S.; Morita, E.; Murano, M.; Imoto, A.; Kuramoto, T.; Inoue, T.; Murano, N.; Toshina, K.; Umegaki, E.; Higuchi, K. Usefulness of polyethylene glycol solution with dimethylpolysiloxanes for bowel preparation before capsule endoscopy. J. Gastroenterol. Hepatol. 2010, 25, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Esaki, M.; Matsumoto, T.; Kudo, T.; Yanaru-Fujisawa, R.; Nakamura, S.; Iida, M. Bowel preparations for capsule endoscopy: A comparison between simethicone and magnesium citrate. Gastrointest. Endosc. 2009, 69, 94–101. [Google Scholar] [CrossRef]
- Choi, C.W.; Lee, S.J.; Hong, S.N.; Kim, E.R.; Chang, D.K.; Kim, Y.H.; Lim, Y.J.; Shim, K.N.; Lee, H.S. Small Bowel Capsule Endoscopy within 6 Hours Following Bowel Preparation with Polyethylene Glycol Shows Improved Small Bowel Visibility. Diagnostics 2023, 13, 469. [Google Scholar] [CrossRef]
- Hansel, S.L.; Murray, J.A.; Alexander, J.A.; Bruining, D.H.; Larson, M.V.; Mangan, T.F.; Dierkhising, R.A.; Almazar, E.; Rajan, E. Evaluating a combined bowel preparation for small-bowel capsule endoscopy: A prospective randomized–controlled study. Gastroenterol. Rep. 2020, 8, 31–35. [Google Scholar] [CrossRef]
- Hookey, L.; Louw, J.; Wiepjes, M.; Rubinger, N.; Van Weyenberg, S.; Day, A.G.; Paterson, W. Lack of benefit of active preparation compared with a clear fluid–only diet in small-bowel visualization for video capsule endoscopy: Results of a randomized, blinded, controlled trial. Gastrointest. Endosc. 2017, 85, 187–193. [Google Scholar] [CrossRef]
- Lamba, M.; Ryan, K.; Hwang, J.; Grimpen, F.; Lim, G.; Cornelius, D.; Moss, A.; Lim, E.J.; Brown, G.; Appleyard, M.; et al. Clinical utility of purgative bowel preparation before capsule endoscopy: A multicenter, blinded, randomized controlled trial. Gastrointest. Endosc. 2022, 96, 822–828. [Google Scholar] [CrossRef]
- Pennazio, M.; Rondonotti, E.; Despott, E.J.; Dray, X.; Keuchel, M.; Moreels, T.; Sanders, D.S.; Spada, C.; Carretero, C.; Valdivia, P.C.; et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline-Update 2022. Endoscopy 2023, 55, 58–95. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ogata, H.; Matsumoto, T.; Ohmiya, N.; Ohtsuka, K.; Watanabe, K.; Yano, T.; Matsui, T.; Higuchi, K.; Fujimoto, K. Clinical Practice Guideline for Enteroscopy. Dig. Endosc. 2017, 29, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Brotz, C.; Nandi, N.; Conn, M.; Daskalakis, C.; DiMarino, M.; Infantolino, A.; Katz, L.C.; Schroeder, T.; Kastenberg, D. A validation study of 3 grading systems to evaluate small-bowel cleansing for wireless capsule endoscopy: A quantitative index, a qualitative evaluation, and an overall adequacy assessment. Gastrointest. Endosc. 2009, 69, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef]
- Postgate, A.; Tekkis, P.; Patterson, N.; Fitzpatrick, A.; Bassett, P.; Fraser, C. Are bowel purgatives and prokinetics useful for small-bowel capsule endoscopy? A prospective randomized controlled study. Gastrointest. Endosc. 2009, 69, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Rayner-Hartley, E.; Alsahafi, M.; Cramer, P.; Chatur, N.; Donnellan, F. Low volume polyethylene glycol with ascorbic acid, sodium picosulfate-magnesium citrate, and clear liquid diet alone prior to small bowel capsule endoscopy. World J. Gastrointest. Endosc. 2016, 8, 433–438. [Google Scholar] [CrossRef]
- Pons Beltrán, V.; González Suárez, B.; González Asanza, C.; Pérez-Cuadrado, E.; Fernández Diez, S.; Fernández-Urién, I.; Fernández-Urién, I.; Bilbao, A.M.; Espinós Pérez, J.C.; Menchen Fernández-Pacheco, P.; et al. Evaluation of Different Bowel Preparations for Small Bowel Capsule Endoscopy: A Prospective, Randomized, Controlled Study. Dig. Dis. Sci. 2011, 56, 2900–2905. [Google Scholar] [CrossRef]
- Klein, A.; Dashkovsky, M.; Gralnek, I.; Peled, R.; Chowers, Y.; Khamaysi, I.; Har-Noy, O.; Levi, I.; Nadler, M.; Kopylov, U.; et al. Bowel preparation in “real-life” small bowel capsule endoscopy: A two-center experience. Ann. Gastroenterol. 2016, 29, 196–200. [Google Scholar] [CrossRef]
- Inoue, K.; Wiener, I.; Fagan, C.J.; Watson, L.C.; Thompson, J.C. Correlation Between Gallbladder Size and Release of Cholecystokinin After Oral Magnesium Sulfate in Man. Ann. Surg. 1983, 197, 412–415. [Google Scholar] [CrossRef]
- Ninomiya, K.; Yao, K.; Matsui, T.; Sato, Y.; Kishi, M.; Karashima, Y.; Ishihara, H.; Hirai, F. Effectiveness of Magnesium Citrate as Preparation for Capsule Endoscopy: A Randomized, Prospective, Open-Label, Inter-Group Trial. Digestion 2012, 86, 27–33. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, L.; Zheng, P.; Wang, Y.G.; Ding, W.Q.; Yu, Q.; Hui, P.P.; Chen, H.M.; Gao, Y.J.; Ge, Z.Z. Low-dose and same day use of polyethylene glycol improves image of video capsule endoscopy: A multi-center randomized clinical trial. J. Gastroenterol. Hepatol. 2020, 35, 634–640. [Google Scholar] [CrossRef]
- Xavier, S.; Rosa, B.; Monteiro, S.; Arieira, C.; Magalhães, R.; Gonçalves, T.C.; Carvalho, P.B.; Magalhães, J.; Moreira, M.J.; Cotter, J. Bowel preparation for small bowel capsule endoscopy—The later, the better! Dig. Liver Dis. 2019, 51, 1388–1391. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.N.; Farkash, S.; Sompolinsky, Y.; Gafanovich, I.; Goldin, E.; Bar-Gil Shitrit, A. A novel purgative protocol for capsule endoscopy of the small bowel produces better quality of visibility than 2 l of PEG: Timing is of the essence. United Eur. Gastroenterol. J. 2017, 5, 485–490. [Google Scholar] [CrossRef] [PubMed]




| Bile/chyme staining |
| 4 points: No bile |
| 3 points: Some bile/chyme staining present, not interfering with observations |
| 2 points: Quite a lot of bile/chyme staining, slightly hindering observations |
| 1 point: Large amount of bile/chyme staining, hindering observations |
| Fluid and debris |
| 4 points: No fluid and debris |
| 3 points: Some fluid and debris present, not interfering with observations |
| 2 points: Quite a lot of fluid and debris, slightly hindering observations |
| 1 point: Large amount of fluid and debris, hindering observations |
| Brightness |
| 4 points: Bright |
| 3 points: Slightly dark, not interfering with observations |
| 2 points: Quite dark, slightly hindering observations |
| 1 point: Quite dark, hindering observations |
| Bubble reduction |
| 4 points: Bubbles are observed of <20% on the image |
| 3 points: Bubbles are observed of 20–49% on the image |
| 2 points: Bubbles are observed of 50–79% on the image |
| 1 point: Bubbles are observed of ≥80% on the image |
| Visualized mucosa |
| 4 points: Visualization of ≥80% of mucosa |
| 3 points: Visualization of 50–79% of mucosa |
| 2 points: Visualization of 20–49% of mucosa |
| 1 point: Visualization of <20% of mucosa |
| Intestinal collapse |
| Observed (collapsed) or not observed (not collapsed) |
| Group A (No Preparation) | Group B (PEG) | Group C (MC) | p Value | |
|---|---|---|---|---|
| Subjects number | 51 | 123 | 85 | |
| Age (years, mean ± SD) | 63.7 ± 17.5 | 67.1 ± 18.0 | 63.2 ± 17.9 | NS |
| Sex (male/female) | 36/15 | 70/53 | 51/34 | NS |
| Gastric transit time (min, mean ± SD) | 54.2 ± 73.0 | 66.1 ± 62.9 | 55.4 ± 58.1 | NS |
| Small bowel transit time (min, mean ± SD) | 355.7 ± 182.9 | 296.6 ± 149.9 | 311.2 ± 138.9 | NS |
| Indication of VCE n (%) | NS | |||
| OGIB | 36 (70.6) | 84 (68.3) | 45 (52.9) | |
| Abdominal pain | 4 (7.8) | 5 (4.0) | 6 (7.1) | |
| Abnormal bowel movement | 1 (2.0) | 6 (4.9) | 9 (10.6) | |
| IBD (suspected) | 5 (9.8) | 8 (6.5) | 16 (18.8) | |
| Tumor | 3 (5.9) | 14 (11.4) | 7 (8.2) | |
| Others | 2 (3.9) | 6 (4.9) | 2 (2.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kametaka, D.; Ito, M.; Kawano, S.; Ishiyama, S.; Fujiwara, A.; Nasu, J.; Yoshioka, M.; Shiode, J.; Yamamoto, K.; Iwamuro, M.; et al. Optimal Bowel Preparation Method to Visualize the Distal Ileum via Small Bowel Capsule Endoscopy. Diagnostics 2023, 13, 3269. https://doi.org/10.3390/diagnostics13203269
Kametaka D, Ito M, Kawano S, Ishiyama S, Fujiwara A, Nasu J, Yoshioka M, Shiode J, Yamamoto K, Iwamuro M, et al. Optimal Bowel Preparation Method to Visualize the Distal Ileum via Small Bowel Capsule Endoscopy. Diagnostics. 2023; 13(20):3269. https://doi.org/10.3390/diagnostics13203269
Chicago/Turabian StyleKametaka, Daisuke, Mamoru Ito, Seiji Kawano, Shuhei Ishiyama, Akiko Fujiwara, Junichirou Nasu, Masao Yoshioka, Junji Shiode, Kazuhide Yamamoto, Masaya Iwamuro, and et al. 2023. "Optimal Bowel Preparation Method to Visualize the Distal Ileum via Small Bowel Capsule Endoscopy" Diagnostics 13, no. 20: 3269. https://doi.org/10.3390/diagnostics13203269
APA StyleKametaka, D., Ito, M., Kawano, S., Ishiyama, S., Fujiwara, A., Nasu, J., Yoshioka, M., Shiode, J., Yamamoto, K., Iwamuro, M., Kawahara, Y., Okada, H., & Otsuka, M. (2023). Optimal Bowel Preparation Method to Visualize the Distal Ileum via Small Bowel Capsule Endoscopy. Diagnostics, 13(20), 3269. https://doi.org/10.3390/diagnostics13203269






