Percutanous Electrochemotherapy (ECT) in Primary and Secondary Liver Malignancies: A Systematic Review
Abstract
1. Introduction
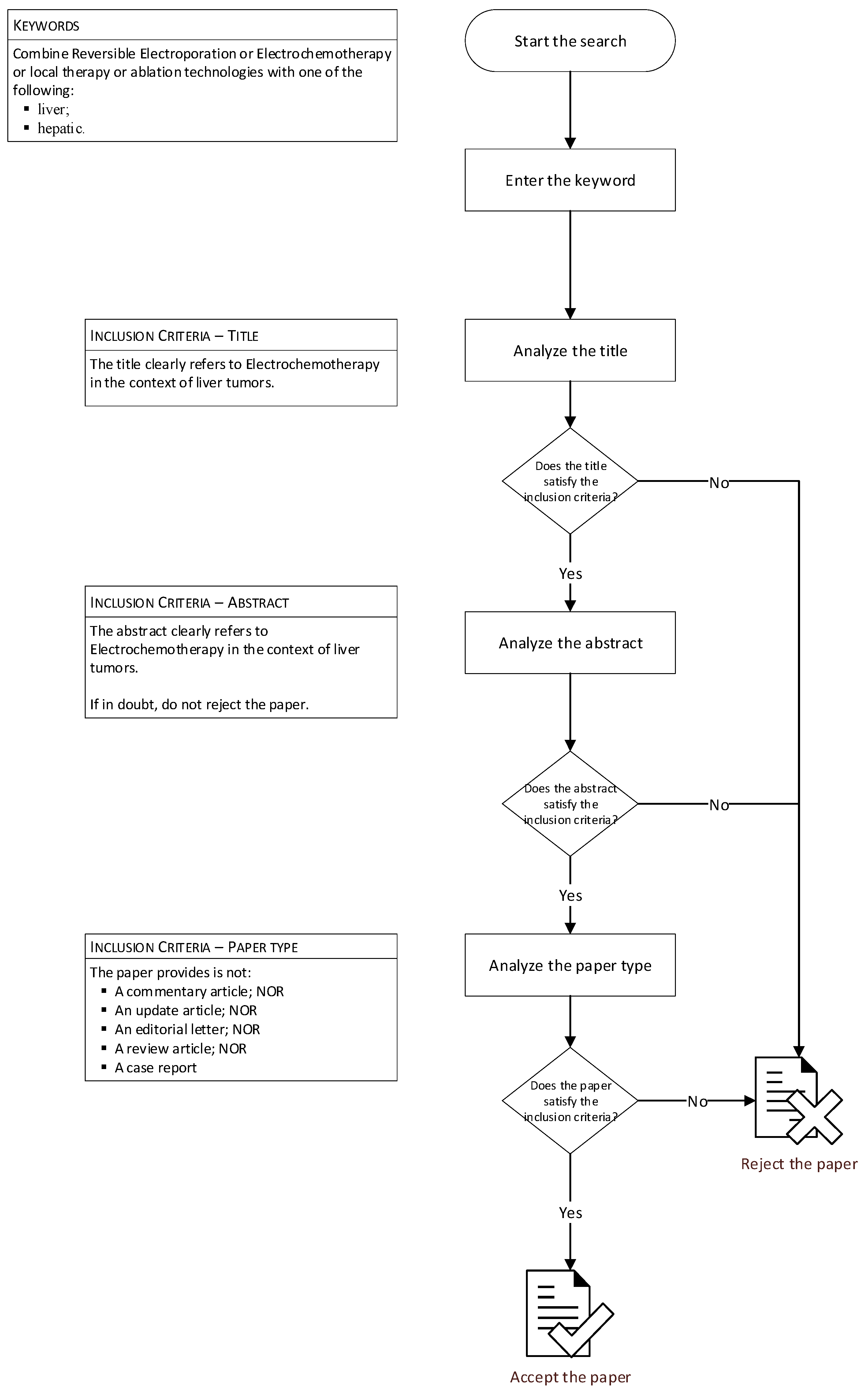
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LAT | Local Ablative Therapies |
| ECT | Electrochemotherapy |
| CRC | Colorectal Cancer |
| ESMO | European Society of Medical Oncology |
| RFA | Radiofrequency ablation |
| MWA | Microwave ablation |
| CRYO | Cryoablation |
| TAE | Transarterial therapies |
| TACE | Transarterial chemoembolization |
| ESOPE | European Standard Operating Procedure for Electrochemotherapy |
| CR | Complete Response |
| PR | Partial Response |
| PD | Progression Disease |
| SD | Stable Disease |
| ORR | Overall response rate |
| HCC | Hepatocellular carcinoma |
| BRCA | Breast Carcinoma |
| OS | Overall survival |
| CT | Computed tomography |
| CEIS | Contrast enhanced ultrasound |
| PHCCA | Perihilar cholangiocarcinoma |
| LTC | Local tumour control |
| NSCLC | Non-Small Cell Lung Carcinoma |
| TARE | Radioembolization |
| PFS | Progression free survival |
| HIPEC | Hyperthermic Intraperitoneal chemotherapy |
| IM | Intrahepatic metastases |
| PM | Intraperitoneal metastases |
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; et al. Global, regional, and national cancer Incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524. [Google Scholar] [CrossRef] [PubMed]
- Valery, P.C.; Laversanne, M.; Clark, P.J.; Petrick, J.L.; McGlynn, K.A.; Bray, F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology 2018, 67, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Hackl, C.; Neumann, P.; Gerken, M.; Loss, M.; Klinkhammer-Schalke, M.; Schlitt, H.J. Treatment of colorectal liver metastases in Germany: A ten-year populationbased analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014, 14, 810. [Google Scholar] [CrossRef]
- Gervais, D.A.; Goldberg, S.N.; Brown, D.B. Society of Interventional Radiology Position Statement on Percutaneous Radiofrequency Ablation for the Treatment of Liver Tumors. J. Vasc. Interv. Radiol. 2009, 20 (Suppl. 7), S342–S347. [Google Scholar] [CrossRef]
- Borie, F.; Bouvier, A.-M.; Herrero, A.; Faivre, J.; Launoy, G.; Delafosse, P.; Velten, M.; Buemi, A.; Peng, J.; Grosclaude, P.; et al. Treatment and Prognosis of Hepatocellular Carcinoma: A Population Based Study in France. J. Surg. Oncol. 2008, 98, 505–509. [Google Scholar] [CrossRef]
- Gillams, A.; Goldberg, N.; Ahmed, M. Thermal Ablation of Colorectal Liver Metastases: A Position Paper by an International Panel of Ablation Experts, The Interventional Oncology Sans Frontieres Meeting 2013. Eur. Radiol. 2015, 25, 3438–3454. [Google Scholar] [CrossRef]
- Van Tilborg, A.A.; Scheffer, H.J.; de Jong, M.C. MWA Versus RFA for Perivascular and Peribiliary CRLM: A Retrospective Patient- and Lesion-Based Analysis of Two Historical Cohorts. Cardiovasc. Interv. Radiol. 2016, 39, 1438–1446. [Google Scholar] [CrossRef]
- Sotirchos, V.S.; Petrovic, L.M.; Gonen, M. Colorectal Cancer Liver Metastases: Biopsy of the Ablation Zone and Margins can be Used to Predict Oncologic Outcome. Radiology 2016, 280, 949–959. [Google Scholar] [CrossRef]
- Argalia, G.; Tarantino, G.; Ventura, C.; Campioni, D.; Tagliati, C.; Guardati, P.; Kostandini, A.; Marzioni, M.; Giuseppetti, G.M.; Giovagnoni, A. Shear wave elastography and transient elastography in HCV patients after direct-acting antivirals. Radiol. Med. 2021, 126, 894–899. [Google Scholar] [CrossRef]
- Giovagnoni, A. A farewell from the “old” Editor-in-Chief. Radiol. Med. 2021, 126, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, M.; Simonetti, G. Interventional Magnetic Resonance Imaging Suite (IMRIS): How to build and how to use. Radiol. Med. 2022, 127, 1063–1067. [Google Scholar] [CrossRef]
- Nakamura, Y.; Higaki, T.; Honda, Y.; Tatsugami, F.; Tani, C.; Fukumoto, W.; Narita, K.; Kondo, S.; Akagi, M.; Awai, K. Advanced CT techniques for assessing hepatocellular carcinoma. Radiol. Med. 2021, 126, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Solomon, S.B.; Cornelis, F. Interventetion molecular imaging. J. Nucl. Med. 2016, 57, 493–496. [Google Scholar] [CrossRef]
- Wright, A.S.; Samposo, L.A.; Warner, T.F.; Malvi, D.M.; Lee, F.T. Radiofrequency versus microwawe ablation in hepatic porcine model. Radiology 2005, 236, 132–139. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Stellato, E.; Pellegrino, G.; Bonelli, C.; Cellina, M.; Renzulli, M.; Biondetti, P.; Carrafiello, G. Fluid-dynamic control microcatheter used with glue: Preliminary experience on its feasibility and safety. Radiol. Med. 2022, 127, 272–276. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, Y.S.; Choi, J. Dosimetric analysis of the effects of a temporary tissue expander on the radiotherapy technique. Radiol. Med. 2021, 126, 437–444. [Google Scholar] [CrossRef]
- Bozkurt, M.; Eldem, G.; Bozbulut, U.B.; Bozkurt, M.F.; Kılıçkap, S.; Peynircioğlu, B.; Çil, B.; Lay Ergün, E.; Volkan-Salanci, B. Factors affecting the response to Y-90 microsphere therapy in the cholangiocarcinoma patients. Radiol. Med. 2021, 126, 323–333. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Solomon, S.B. Treatment of primary liver tumours and liver metastases, Part 2: Non-nuclear medicine techniques. J. Nucl. Med. 2018, 59, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Idée, J.-M.; Guiu, B. Use of lipiodol as a drug-delivery system for transcatheter arterial emoembolization of hepatocellular carcinoma: A review. Crit. Rev. Oncol. Hematol. 2013, 88, 530–549. [Google Scholar] [CrossRef] [PubMed]
- Merlotti, A.; Bruni, A.; Borghetti, P.; Ramella, S.; Scotti, V.; Trovò, M.; Chiari, R.; Lohr, F.; Ricardi, U.; Bria, E.; et al. Sequential chemo-hypofractionated RT versus concurrent standard CRT for locally advanced NSCLC: GRADE recommendation by the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Radiol. Med. 2021, 126, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Cionfoli, N.; Paladini, A.; Vallone, M.; Corvino, F.; Teodoli, L.; Moramarco, L.; Quaretti, P.; Catalano, C.; Niola, R.; et al. PHIL® (precipitating hydrophobic injectable liquid): Retrospective multicenter experience on 178 patients in peripheral embolizations. Radiol. Med. 2022, 127, 1303–1312. [Google Scholar] [CrossRef]
- Falcinelli, L.; Mendichi, M.; Chierchini, S.; Tenti, M.V.; Bellavita, R.; Saldi, S.; Ingrosso, G.; Reggioli, V.; Bini, V.; Aristei, C. Pulmonary function in stereotactic body radiotherapy with helical tomotherapy for primary and metastatic lung lesions. Radiol. Med. 2021, 126, 163–169. [Google Scholar] [CrossRef]
- Arslan, A.; Aktas, E.; Sengul, B.; Tekin, B. Dosimetric evaluation of left ventricle and left anterior descending artery in left breast radiotherapy. Radiol. Med. 2021, 126, 14–21. [Google Scholar] [CrossRef]
- Glass, L.F.; Jaroszeski, M.; Gilbert, R.; Reintgen, D.S.; Heller, R. Intralesional bleomycin-mediated electrochemotherapy in 20 patients with basal cell carcinoma. J. Am. Acad. Dermatol. 1997, 37, 596–599. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Clover, A.; de Terlizzi, F.; Bertino, G.; Curatolo, P.; Odili, J.; Campana, L.; Kunte, C.; Muir, T.; Brizio, M.; Sersa, G.; et al. Electrochemotherapy in the treatment of cutaneous malignancy: Outcomes and subgroup analysis from the cumulative results from the pan-European International Network for Sharing Practice in Electrochemotherapy database for 2482 lesions in 987 patients (2008–2019). Eur. J. Cancer 2020, 138, 30–40. [Google Scholar] [CrossRef]
- Plaschke, C.C.; Bertino, G.; McCaul, J.A.; Grau, J.J.; de Bree, R.; Sersa, G.; Occhini, A.; Groselj, A.; Langdon, C.; Heuveling, D.A.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results from the treatment of mucosal cancers. Eur. J. Cancer 2017, 87, 172–181. [Google Scholar] [CrossRef]
- Bertino, G.; Sersa, G.; De Terlizzi, F.; Occhini, A.; Plaschke, C.C.; Groselj, A.; Langdon, C.; Grau, J.J.; McCaul, J.A.; Heuveling, D.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer 2016, 63, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Tafuto, S.; von Arx, C.; De Divitiis, C.; Maura, C.T.; Palaia, R.; Albino, V.; Fusco, R.; Membrini, M.; Petrillo, A.; Granata, V.; et al. Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int. J. Surg. 2015, 21 (Suppl. 1), S78–S82. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Piccirillo, M.; Leongito, M.; Palaia, R.; Granata, F.; Lastoria, S.; Izzo, F.; Petrillo, A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J. Gastroenterol. 2017, 23, 4767–4778. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Catalano, O.; Piccirillo, M.; De Bellis, M.; Izzo, F.; Petrillo, A. Percutaneous ablation therapy of hepatocellular carcinoma with irreversible electroporation: MRI findings. AJR Am. J. Roentgenol. 2015, 204, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Gasljevic, G.; Edhemovic, I.; Cemazar, M.; Brecelj, E.; Gadzijev, E.M.; Music, M.M.; Sersa, G. Histopathological findings in colorectal liver metastases after electrochemotherapy. PLoS ONE 2017, 12, e0180709. [Google Scholar] [CrossRef]
- Brloznik, M.; Boc, N.; Sersa, G.; Zmuc, J.; Gasljevic, G.; Seliskar, A.; Dezman, R.; Edhemovic, I.; Milevoj, N.; Plavec, T.; et al. Radiological findings of porcine liver after electrochemotherapy with bleomycin. Radiol. Oncol. 2019, 53, 415–426. [Google Scholar] [CrossRef]
- Zmuc, J.; Gasljevic, G.; Sersa, G.; Edhemovic, I.; Boc, N.; Seliskar, A.; Plavec, T.; Brloznik, M.; Milevoj, N.; Brecelj, E.; et al. Large Liver Blood Vessels and Bile Ducts Are Not Damaged by Electrochemotherapy with Bleomycin in Pigs. Sci. Rep. 2019, 9, 3649. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Korenbaum, C.; Ben Ammar, M.; Tavolaro, S.; Nouri-Neuville, M.; Lotz, J.P. Multimodal image-guided electrochemotherapy of unresectable liver metastasis from renal cell cancer. Diagn. Interv. Imaging 2019, 100, 309–311. [Google Scholar] [CrossRef]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a New Modality in Interventional Oncology: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818785329. [Google Scholar] [CrossRef]
- Jarm, T.; Cemazar, M.; Miklavcic, D.; Sersa, G. Antivascular effects of electrochemotherapy: Implications in treatment of bleeding metastases. Expert Rev. Anticancer Ther. 2010, 10, 729–746. [Google Scholar] [CrossRef]
- Barra, S.; Guarnieri, A.; di Monale E Bastia, M.B.; Marcenaro, M.; Tornari, E.; Belgioia, L.; Magrini, S.M.; Ricardi, U.; Corvò, R. Short fractionation radiotherapy for early prostate cancer in the time of COVID-19: Long-term excellent outcomes from a multicenter Italian trial suggest a larger adoption in clinical practice. Radiol. Med. 2021, 126, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Cellini, F.; Di Franco, R.; Manfrida, S.; Borzillo, V.; Maranzano, E.; Pergolizzi, S.; Morganti, A.G.; Fusco, V.; Deodato, F.; Santarelli, M.; et al. Palliative radiotherapy indications during the COVID-19 pandemic and in future complex logistic settings: The NORMALITY model. Radiol. Med. 2021, 126, 1619–1656. [Google Scholar] [CrossRef] [PubMed]
- Lancellotta, V.; Del Regno, L.; Di Stefani, A.; Fionda, B.; Marazzi, F.; Rossi, E.; Balducci, M.; Pampena, R.; Morganti, A.G.; Mangoni, M.; et al. The role of stereotactic radiotherapy in addition to immunotherapy in the management of melanoma brain metastases: Results of a systematic review. Radiol. Med. 2022, 127, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Edhemovic, I.; Brecelj, E.; Gasljevic, G.; Marolt Music, M.; Gorjup, V.; Mali, B.; Jarm, T.; Kos, B.; Pavliha, D.; Kuzmanov Grcar, B.; et al. Intraoperative Electrochemotherapy of Colorectal Liver Metastases. J. Surg. Oncol. 2014, 110, 320–327. [Google Scholar] [CrossRef]
- Trotovsek, B.; Hadzialjevic, B.; Cemazar, M.; Sersa, G.; Djokic, M. Laparoscopic electrochemotherapy for the treatment of hepatocellular carcinoma: Technological advancement. Front Oncol. 2022, 12, 996269. [Google Scholar] [CrossRef]
- Djokic, M.; Dezman, R.; Cemazar, M.; Stabuc, M.; Petric, M.; Smid, L.M.; Jansa, R.; Plesnik, B.; Bosnjak, M.; Tratar, U.L.; et al. Percutaneous image guided electrochemotherapy of hepatocellular carcinoma: Technological advancement. Radiol. Oncol. 2020, 54, 347–352. [Google Scholar] [CrossRef]
- Tarantino, L.; Busto, G.; Nasto, A.; Fristachi, R.; Cacace, L.; Talamo, M.; Accardo, C.; Bortone, S.; Gallo, P.; Tarantino, P.; et al. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: A feasibility study. World J. Gastroenterol. 2017, 23, 906–918. [Google Scholar] [CrossRef]
- Tarantino, L.; Busto, G.; Nasto, A.; Nasto, R.A.; Tarantino, P.; Fristachi, R.; Cacace, L.; Bortone, S. Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: A feasibility study. EJSO 2018, 44, 1603–1609. [Google Scholar] [CrossRef]
- Djokic, M.; Cemazar, M.; Popovic, P.; Kos, B.; Dezman, R.; Bosnjak, M.; Zakelj, M.N.; Miklavcic, D.; Potrc, S.; Stabuc, B.; et al. Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study. Eur. J. Surg. Oncol. 2018, 44, 651–657. [Google Scholar] [CrossRef]
- Coletti, L.; Battaglia, V.; De Simone, P.; Turturici, L.; Bartolozzi, C.; Filipponi, F. Safety and feasibility of electrochemotherapy in patients with unresectable colorectal liver metastases: A pilot study. Int. J. Surg. 2017, 44, 26–32. [Google Scholar] [CrossRef]
- Edhemovic, I.; Brecelj, E.; Cemazar, M.; Boc, N.; Trotovsek, B.; Djokic, M.; Dezman, R.; Ivanecz, A.; Potrc, S.; Bosnjak, M.; et al. Intraoperative electrochemotherapy of colorectal liver metastases: A prospective phase II study. Eur. J. Surg. Oncol. 2020, 46, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Bischoff, P.; Haddad, H.; Zhou, W.; Temming, S.; Schäfer, A.; Spallek, H.; Kaupe, L.; Kovács, G.; Pinkawa, M. Long-Term Comparative Study on the Local Tumour Control of Different Ablation Technologies in Primary and Secondary Liver Malignancies. J. Pers. Med. 2022, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Spallek, H.; Bischoff, P.; Zhou, W.; de Terlizzi, F.; Jakob, F.; Kovàcs, A. Percutaneous electrochemotherapy in primary and secondary liver malignancies-local tumor control and impact on overall survival. Radiol. Oncol. 2022, 56, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.S.; Balbaa, M.F.; Gallazzi, M.B.; Eid, M.E.; Kotb, H.T.; Shafei, M.E.; Ierardi, A.M.; Daolio, P.A.; Barile, A.; Carrafiello, G. Role of percutaneous CT-guided radiofrequency ablation in treatment of intra-articular, in close contact with cartilage and extra-articular osteoid osteomas: Comparative analysis and new classification system. Radiol. Med. 2022, 127, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Grasso, R.F.; Bernetti, C.; Pacella, G.; Altomare, C.; Castiello, G.; Andresciani, F.; Sarli, M.; Zobel, B.B.; Faiella, E. A comparative analysis of thermal ablation techniques in the treatment of primary and secondary lung tumors: A single-center experience. Radiol. Med. 2022, 127, 714–724. [Google Scholar] [CrossRef]
- Fiore, F.; Somma, F.; D’Angelo, R.; Tarotto, L.; Stoia, V. Cone beam computed tomography (CBCT) guidance is helpful in reducing dose exposure to pediatric patients undergoing radiofrequency ablation of osteoid osteoma. Radiol. Med. 2022, 127, 183–190. [Google Scholar] [CrossRef]
- Song, W.; Chen, Q.; Guo, D.; Jiang, C. Preoperative estimation of the survival of patients with unresectable hepatocellular carcinoma achieving complete response after conventional transcatheter arterial chemoembolization: Assessments of clinical and LI-RADS MR features. Radiol. Med. 2022, 127, 939–949. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bianchi, G.; Formiconi, F.; Palumbo, P.; Zugaro, L.; Gravina, G.L.; Barile, A.; Masciocchi, C. CT-guided cryoablation for management of bone metastases: A single center experience and review of the literature. Radiol. Med. 2022, 127, 199–205. [Google Scholar] [CrossRef]
- Granata, V.; de Lutio di Castelguidone, E.; Fusco, R.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Gallipoli, A.D.; Petrillo, A. Irreversible electroporation of hepatocellular carcinoma: Preliminary report on the diagnostic accuracy of magnetic resonance, computer tomography, and contrast-enhanced ultrasound in evaluation of the ablated area. Radiol. Med. 2016, 121, 122–131. [Google Scholar] [CrossRef]
- Avallone, A.; Pecori, B.; Bianco, F.; Aloj, L.; Tatangelo, F.; Romano, C.; Granata, V.; Marone, P.; Leone, A.; Botti, G.; et al. Critical role of bevacizumab scheduling in combination with pre-surgical chemo-radiotherapy in MRI-defined high-risk locally advanced rectal cancer: Results of the BRANCH trial. Oncotarget 2015, 6, 30394–30407. [Google Scholar] [CrossRef]
- Orlacchio, A.; Guastoni, C.; Beretta, G.D.; Cosmai, L.; Galluzzo, M.; Gori, S.; Grassedonio, E.; Incorvaia, L.; Marcantoni, C.; Netti, G.S.; et al. SIRM-SIN-AIOM: Appropriateness criteria for evaluation and prevention of renal damage in the patient undergoing contrast medium examinations-consensus statements from Italian College of Radiology (SIRM), Italian College of Nephrology (SIN) and Italian Association of Medical Oncology (AIOM). Radiol. Med. 2022, 127, 534–542. [Google Scholar] [CrossRef]
- Detti, B.; Scoccianti, S.; Teriaca, M.A.; Maragna, V.; Lorenzetti, V.; Lucidi, S.; Bellini, C.; Greto, D.; Desideri, I.; Livi, L. Bevacizumab in recurrent high-grade glioma: A single institution retrospective analysis on 92 patients. Radiol. Med. 2021, 126, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Criscuolo, M.; GullÃ, C.; Petrosino, A.; Carlo Bianco, N.; Colosimo, C. Systemic mastocytosis revisited with an emphasis on skeletal manifestations. Radiol. Med. 2021, 126, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Paik, J.; Del Grande, F.; Paris, E.S.; Sujlana, P.; Fayad, L.M. Distinct MR features in scleroderma associated myopathy. Radiol. Med. 2021, 126, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Gregucci, F.; Bonaparte, I.; Vitulano, N.; Surgo, A.; Mazzola, R.; Di Monaco, A.; Carbonara, R.; Alongi, F.; Langialonga, T.; et al. Stereotactic Ablative radiation therapy (SABR) for cardiac arrhythmia: A new therapeutic option? Radiol. Med. 2021, 126, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Campi, C.; Bianca, B.; Bortolotto, C.; Buccicardi, D.; Francesca, C.; Prost, R.; Rengo, M.; Faggioni, L. Blockchain in radiology research and clinical practice: Current trends and future directions. Radiol. Med. 2022, 127, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Chiti, G.; Grazzini, G.; Flammia, F.; Matteuzzi, B.; Tortoli, P.; Bettarini, S.; Pasqualini, E.; Granata, V.; Busoni, S.; Messserini, L.; et al. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): A radiomic model to predict tumor grade. Radiol. Med. 2022, 127, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol. Med. 2022, 127, 461–470. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol. Med. 2021, 126, 1044–1054. [Google Scholar] [CrossRef]
- Renzulli, M.; Brandi, N.; Argalia, G.; Brocchi, S.; Farolfi, A.; Fanti, S.; Golfieri, R. Morphological, dynamic and functional characteristics of liver pseudolesions and benign lesions. Radiol. Med. 2022, 127, 129–144. [Google Scholar] [CrossRef]
- Li, N.; Wakim, J.; Koethe, Y.; Huber, T.; Schenning, R.; Gade, T.P.; Hunt, S.J.; Park, B.J. Multicenter assessment of augmented reality registration methods for image-guided interventions. Radiol. Med. 2022, 127, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Ledda, R.E.; Silva, M.; McMichael, N.; Sartorio, C.; Branchi, C.; Milanese, G.; Nayak, S.M.; Sverzellati, N. The diagnostic value of grey-scale inversion technique in chest radiography. Radiol. Med. 2022, 127, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.M.; Cafarelli, F.P.; Paparella, M.T.; Rennie, W.J.; Guglielmi, G. Phosphaturic mesenchymal tumors: Radiological aspects and suggested imaging pathway. Radiol. Med. 2021, 126, 1609–1618. [Google Scholar] [CrossRef]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef]
- De Re, V.; Caggiari, L.; De Zorzi, M.; Repetto, O.; Zignego, A.L.; Izzo, F.; Tornesello, M.L.; Buonaguro, F.M.; Mangia, A.; Sansonno, D.; et al. Genetic diversity of the KIR/HLA system and susceptibility to hepatitis C virus-related diseases. PLoS ONE 2015, 10, e0117420, Erratum in PLoS ONE 2015, 10, e0128849. [Google Scholar] [CrossRef] [PubMed]
- Laurelli, G.; Falcone, F.; Gallo, M.S.; Scala, F.; Losito, S.; Granata, V.; Cascella, M.; Greggi, S. Long-Term Oncologic and Reproductive Outcomes in Young Women With Early Endometrial Cancer Conservatively Treated: A Prospective Study and Literature Update. Int. J. Gynecol. Cancer 2016, 26, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, Y.; Yoshida, K.; Okawa, M.; Maki, T.; Nakajima, S.; Sakata, A.; Okuchi, S.; Hinoda, T.; Kanagaki, M.; Nakamoto, Y. Vessel wall MR imaging in neuroradiology. Radiol. Med. 2022, 30, 1–14. [Google Scholar] [CrossRef]
- Granata, V.; Simonetti, I.; Fusco, R.; Setola, S.V.; Izzo, F.; Scarpato, L.; Vanella, V.; Festino, L.; Simeone, E.; Ascierto, P.A.; et al. Management of cutaneous melanoma: Radiologists challenging and risk assessment. Radiol. Med. 2022, 127, 899–911. [Google Scholar] [CrossRef]
- Cirillo, L.; Rustici, A.; Toni, F.; Zoli, M.; Bartiromo, F.; Gramegna, L.L.; Cicala, D.; Tonon, C.; Caranci, F.; Lodi, R. Vessel Wall MRI: Clinical implementation in cerebrovascular disorders-technical aspects. Radiol. Med. 2022, 127, 645–651. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Grassi, F.; Belli, A.; Silvestro, L.; Ottaiano, A.; et al. Radiomics and machine learning analysis based on magnetic resonance imaging in the assessment of liver mucinous colorectal metastases. Radiol. Med. 2022, 127, 763–772. [Google Scholar] [CrossRef]
- Li, D.; Kang, J.; Golas, B.J.; Yeung, V.W.; Madoff, D.C. Minimally invasive local therapies for liver cancer. Cancer Biol. Med. 2014, 11, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Perillo, T.; Paolella, C.; Perrotta, G.; Serino, A.; Caranci, F.; Manto, A. Reversible cerebral vasoconstriction syndrome: Review of neuroimaging findings. Radiol. Med. 2022, 127, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Polici, M.; Rinzivillo, M.; Zerunian, M.; Nacci, I.; Marasco, M.; Magi, L.; Tarallo, M.; Gargiulo, S.; Iannicelli, E.; et al. CT-based radiomics for prediction of therapeutic response to Everolimus in metastatic neuroendocrine tumors. Radiol. Med. 2022, 127, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yu, N.; Yu, Y.; He, T.; Duan, X. Performance of CT radiomics in predicting the overall survival of patients with stage III clear cell renal carcinoma after radical nephrectomy. Radiol. Med. 2022, 127, 837–847. [Google Scholar] [CrossRef]
- Masci, G.M.; Ciccarelli, F.; Mattei, F.I.; Grasso, D.; Accarpio, F.; Catalano, C.; Laghi, A.; Sammartino, P.; Iafrate, F. Role of CT texture analysis for predicting peritoneal metastases in patients with gastric cancer. Radiol. Med. 2022, 127, 251–258. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Mazzei, M.A.; Meglio, N.D.; Roscio, D.D.; Moroni, C.; Monti, R.; Cappabianca, C.; Picone, C.; Neri, E.; et al. Quantitative imaging decision support (QIDSTM) tool consistency evaluation and radiomic analysis by means of 594 metrics in lung carcinoma on chest CT scan. Cancer Control. 2021, 28, 1073274820985786. [Google Scholar] [CrossRef]
- Zerunian, M.; Pucciarelli, F.; Caruso, D.; Polici, M.; Masci, B.; Guido, G.; De Santis, D.; Polverari, D.; Principessa, D.; Benvenga, A.; et al. Artificial intelligence based image quality enhancement in liver MRI: A quantitative and qualitative evaluation. Radiol. Med. 2022, 10, 1098–1105. [Google Scholar] [CrossRef]
- Kang, Y.J.; Cho, J.H.; Hwang, S.H. Diagnostic value of various criteria for deep lobe involvement in radiologic studies with parotid mass: A systematic review and meta-analysis. Radiol. Med. 2022, 127, 1124–1133. [Google Scholar] [CrossRef]
- Scola, E.; Desideri, I.; Bianchi, A.; Gadda, D.; Busto, G.; Fiorenza, A.; Amadori, T.; Mancini, S.; Miele, V.; Fainardi, E. Assessment of brain tumors by magnetic resonance dynamic susceptibility contrast perfusion-weighted imaging and computed tomography perfusion: A comparison study. Radiol. Med. 2022, 127, 664–672. [Google Scholar] [CrossRef]
- Vicini, S.; Bortolotto, C.; Rengo, M.; Ballerini, D.; Bellini, D.; Carbone, I.; Preda, L.; Laghi, A.; Coppola, F.; Faggioni, L. A narrative review on current imaging applications of artificial intelligence and radiomics in oncology: Focus on the three most common cancers. Radiol. Med. 2022, 127, 819–836. [Google Scholar] [CrossRef]
- Mahnken, A.H.; Pereira, P.L.; de Baère, T. Interventional oncologic approaches to liver metastases. Radiology 2013, 266, 407–430. [Google Scholar] [CrossRef] [PubMed]
- Mali, B.; Gorjup, V.; Edhemovic, I.; Brecelj, E.; Cemazar, M.; Sersa, G.; Strazisar, B.; Miklavcic, D.; Jarm, T. Electrochemotherapy of colorectal liver metastases—An observational study of its effects on the electrocardiogram. Biomed. Eng. Online 2015, 14 (Suppl. 3), S5. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Palaia, R.; Albino, V.; Piccirillo, M.; Venanzio Setola, S.; Petrillo, A.; Izzo, F. Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: A case report. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7051–7057. [Google Scholar] [CrossRef] [PubMed]
- Stefano, M.; Prosperi, E.; Fugazzola, P.; Benini, B.; Bisulli, M.; Coccolini, F.; Mastronardi, C.; Palladino, A.; Tomasoni, M.; Agnoletti, V.; et al. Case Report: Cytoreductive Surgery and HIPEC Associated With Liver Electrochemotherapy in a Cholangiocarcinoma Patient With Peritoneal Carcinomatosis and Liver Metastasis Case Report. Front. Surg. 2021, 8, 624817. [Google Scholar] [CrossRef]
- Lencioni, R. Loco-regional treatment of hepatica carcinoma. Hepatology 2010, 52, 762–773. [Google Scholar] [CrossRef]
- Lu, Z.; Wen, F.; Guo, Q.; Liang, H.; Mao, X.; Sun, H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: A meta-analysis of randomized-controlled trials. Eur. J. Gastroenterol. Hepatol. 2013, 25, 187–194. [Google Scholar] [CrossRef]
- Ni, J.Y.; Liu, S.S.; Xu, L.F.; Sun, H.L.; Chen, Y.T. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 3872–3882. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, X.; Ren, S.; Wang, L. Combining transarterial chemoembolization with radiofrequency ablation for hepatocellular carcinoma. Tumour Biol. 2014, 35, 3405–3408. [Google Scholar] [CrossRef]
- De Robertis, R.; Geraci, L.; Tomaiuolo, L.; Bortoli, L.; Beleù, A.; Malleo, G.; D’Onofrio, M. Liver metastases in pancreatic ductal adenocarcinoma: A predictive model based on CT texture analysis. Radiol. Med. 2022, 10, 1079–1084. [Google Scholar] [CrossRef]
- Bracco, S.; Zanoni, M.; Casseri, T.; Castellano, D.; Cioni, S.; Vallone, I.M.; Gennari, P.; Mazzei, M.A.; Romano, D.G.; Piano, M.; et al. Endovascular treatment of acute ischemic stroke due to tandem lesions of the anterior cerebral circulation: A multicentric Italian observational study. Radiol. Med. 2021, 126, 804–817. [Google Scholar] [CrossRef]
- Gurgitano, M.; Angileri, S.A.; Rodà, G.M.; Liguori, A.; Pandolfi, M.; Ierardi, A.M.; Wood, B.J.; Carrafiello, G. Interventional Radiology ex-machina: Impact of Artificial Intelligence on practice. Radiol. Med. 2021, 126, 998–1006. [Google Scholar] [CrossRef] [PubMed]
| Paper | N. of Patients (Lesions) | Number of Treatment with Fixed or Variable Geometry | Dimension of Lesions | Type of Cancer (% of Cases) | Challenging Location | Local Tumour Response | Overall Survival (Months) Mean ± Standard Deviation | |
|---|---|---|---|---|---|---|---|---|
| Edhemovich et al. [44] | 16 (29) secondary liver tumors | Fixed (n. 6) and variable geometry (n. 10) | <3.0 cm | CRC liver metastases | 48% of lesions | CR 85% PR 15% | Not available | |
| Tarantino et al. [47] | 6 primitive liver tumors | Variable geometry (n. 6) | 2.5–4.5 cm | HCC | 100% of lesions | CR 100% | 16.6% at 20 months | |
| Tarantino et al. [48] | 5 primitive liver tumors | Variable geometry (n. 5) | 3.0–6.0 cm, mean = 4.2 cm | Cholangio-carcinoma | 100% of lesions | CR 60% | 40% at 30 months | |
| Diokic et al. [49] | 10 primitive liver tumors | Fixed (n. 5) and variable (n. 3) and both (n. 2) geometry | 0.8–4.1 cm | HCC | 100% of lesions | CR 88% | Not available | |
| Coletti et al. [50] | 5 (9) secondary liver tumors | Fixed geometry (n. 9) | mean 2.6 cm (range 0.6–3.0 cm) | CRC liver metastases | Not available | CR 55% SD 45.5% | 100% at 6 months | |
| Edhemovich et al. [51] | 39 secondary liver tumors | Fixed (28) and Variable (11) geometry | mean 2.0 cm (range 0.3–6.0 cm) | CRC liver metastases | Not available | CR 63% PR 12% SD 2% PD 23% | 29 months (median) | |
| Kovacs et al. [52] | 8 primitive liver tumors and 13 secondary liver tumors | Variable geometry (n. 21) | <3 cm 5% 3.0–6.0 cm 38% >6.0 cm 57% <10 cm 5% | HCC (14%), CRC liver metastases (38%), BrC (24%): other primary tumour (24%); | 91% of lesions | ORR: HCC 93% CRC 83% BrCa 72% | OS at 12 months: HCC 83% CRC 62% BrCa 64% | |
| Spalleck et al. [53] | 2 primitive liver tumors and 16 secondary liver tumors | Variable geometry (n. 18) | 3.0–6.0 cm | CRC (39%), BrCa (22%), HCC (11%), Ovarian (11%), Anal (0.5%), NSCLC(0.5%), unknown origin (0.5%) | 90.5% of lesions | All lesions | For lesion < 6 cm CR 90% PR 0% SD 0% PD 0% For lesion > 6 cm CR 36.4% PR 45.4% SD 9.1% PD 0% | For lesion < 6 cm Overall survival 15.1 ± 8.0 months For lesion < 6 cm Overall survival 7.9 ± 7.9 months |
| CRC lesions | CR 50%, PR 25%, SD 0% PD 0% | |||||||
| BrCa lesions | CR 80%, PR 20%, SD 0%, PD 0% | |||||||
| HCC lesions | CR 33.3%, PR 66.7%, SD % 0, PD 0% | |||||||
| Paper | Reported Side Effect |
|---|---|
| Edhemovich et al. [44] | Fever: Two patients |
| Tarantino et al. [47] | No intraoperative or post-operative major complication |
| Tarantino et al. [48] | None |
| Diokic et al. [49] | No intraoperative or postoperative complications during the first 24 h occurred. Two patients presented transient ascites |
| Coletti et al. [50] | Wound dehiscence: One patient Bowel occlusion: One patient eight days after surgery |
| Edhemovich et al. [51] | None |
| Kovacs et al. [52] | None |
| Spalleck et al. [53] | Mild pain: 16 patients Protein C elevation and leucocytosis: One patient Liver capsular hematoma: One patient |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granata, V.; Fusco, R.; D’Alessio, V.; Simonetti, I.; Grassi, F.; Silvestro, L.; Palaia, R.; Belli, A.; Patrone, R.; Piccirillo, M.; et al. Percutanous Electrochemotherapy (ECT) in Primary and Secondary Liver Malignancies: A Systematic Review. Diagnostics 2023, 13, 209. https://doi.org/10.3390/diagnostics13020209
Granata V, Fusco R, D’Alessio V, Simonetti I, Grassi F, Silvestro L, Palaia R, Belli A, Patrone R, Piccirillo M, et al. Percutanous Electrochemotherapy (ECT) in Primary and Secondary Liver Malignancies: A Systematic Review. Diagnostics. 2023; 13(2):209. https://doi.org/10.3390/diagnostics13020209
Chicago/Turabian StyleGranata, Vincenza, Roberta Fusco, Valeria D’Alessio, Igino Simonetti, Francesca Grassi, Lucrezia Silvestro, Raffaele Palaia, Andrea Belli, Renato Patrone, Mauro Piccirillo, and et al. 2023. "Percutanous Electrochemotherapy (ECT) in Primary and Secondary Liver Malignancies: A Systematic Review" Diagnostics 13, no. 2: 209. https://doi.org/10.3390/diagnostics13020209
APA StyleGranata, V., Fusco, R., D’Alessio, V., Simonetti, I., Grassi, F., Silvestro, L., Palaia, R., Belli, A., Patrone, R., Piccirillo, M., & Izzo, F. (2023). Percutanous Electrochemotherapy (ECT) in Primary and Secondary Liver Malignancies: A Systematic Review. Diagnostics, 13(2), 209. https://doi.org/10.3390/diagnostics13020209






