Quantitative Edge Analysis of Pancreatic Margins in Patients with Chronic Pancreatitis: A Correlation with Exocrine Function
Abstract
:1. Introduction
2. Material and Methods
2.1. Patient Population
- Patients with chronic pancreatitis from all etiologies, except for cancer-related obstructive pancreatitis;
- Patients who had undergone a fecal elastase test;
- Patients who underwent MRI at our Institute within a maximum of 1 year from the fecal elastase test, with images available for review on PACS.
- Pediatric patients;
- No MR imaging available or imaging performed within more than 1 year from the fecal elastase test.
- Group A, with fecal elastase > 200 μg/g (without exocrine pancreatic insufficiency);
- Group B, with fecal elastase between 100 and 200 μg/g (mild to moderate exocrine pancreatic insufficiency);
- Group C, with fecal elastase < 100 μg/g (severe exocrine pancreatic insufficiency).
2.2. Image Acquisition
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
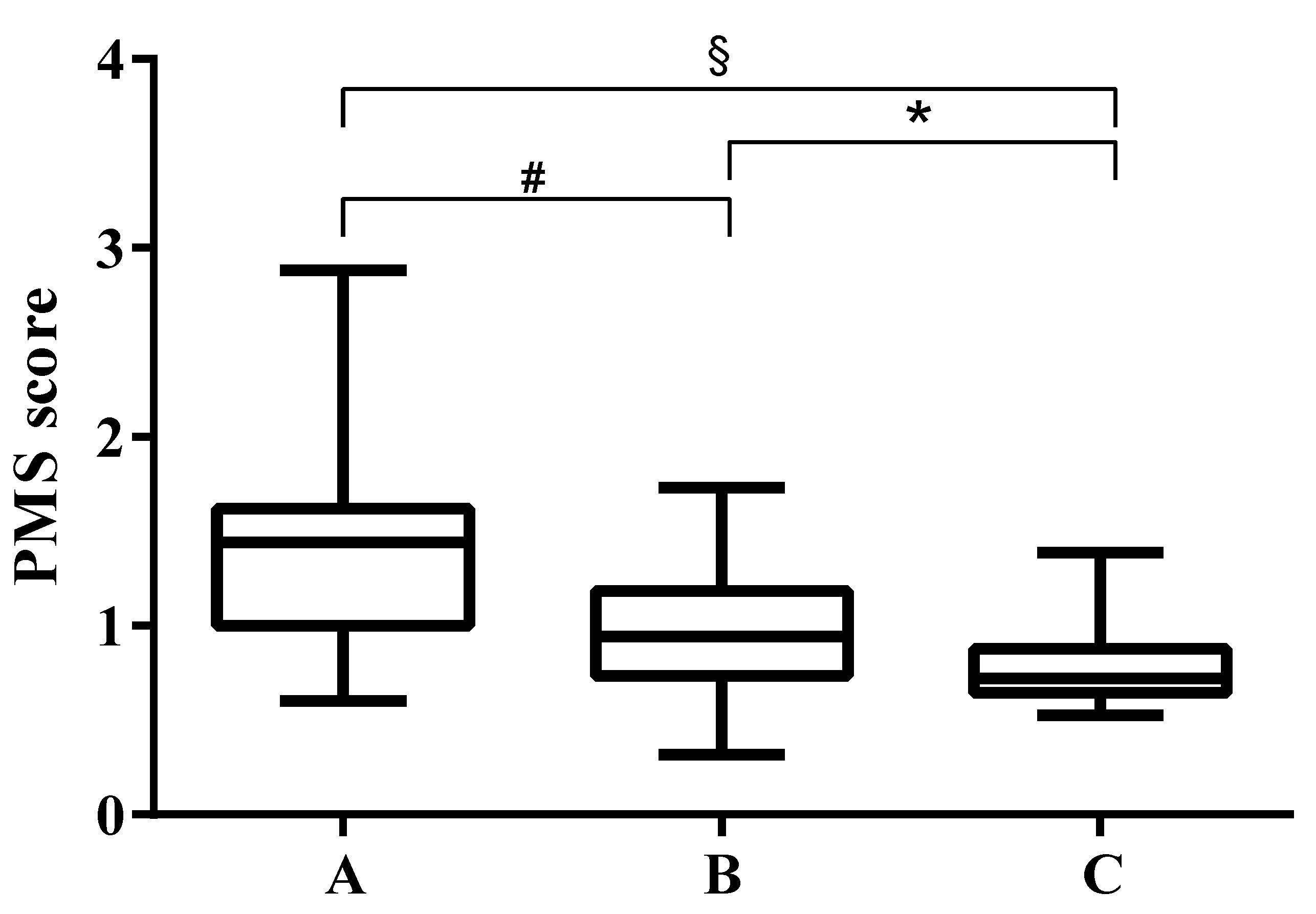
3.1. PMS Values in Patients with Different Fecal Elastase Values
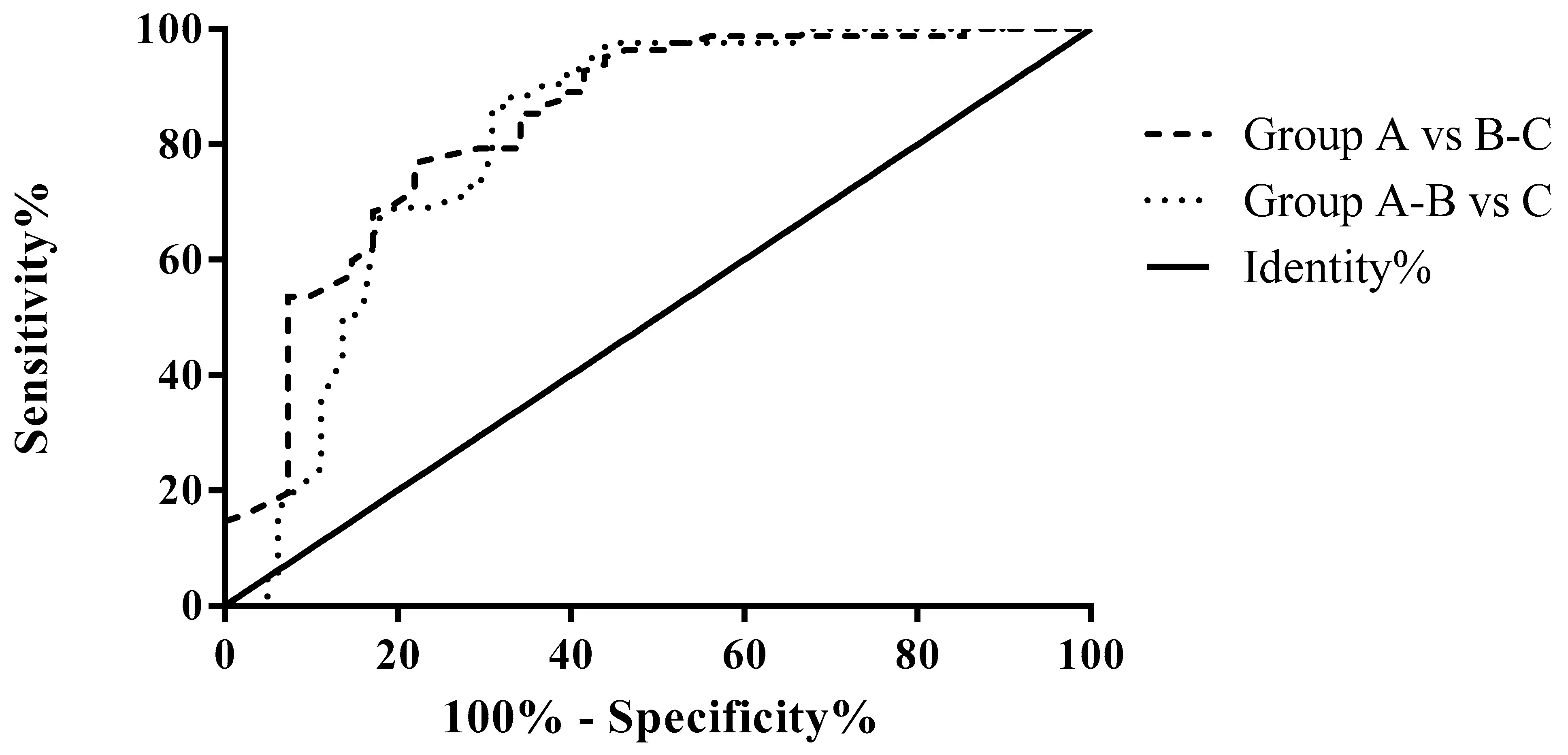
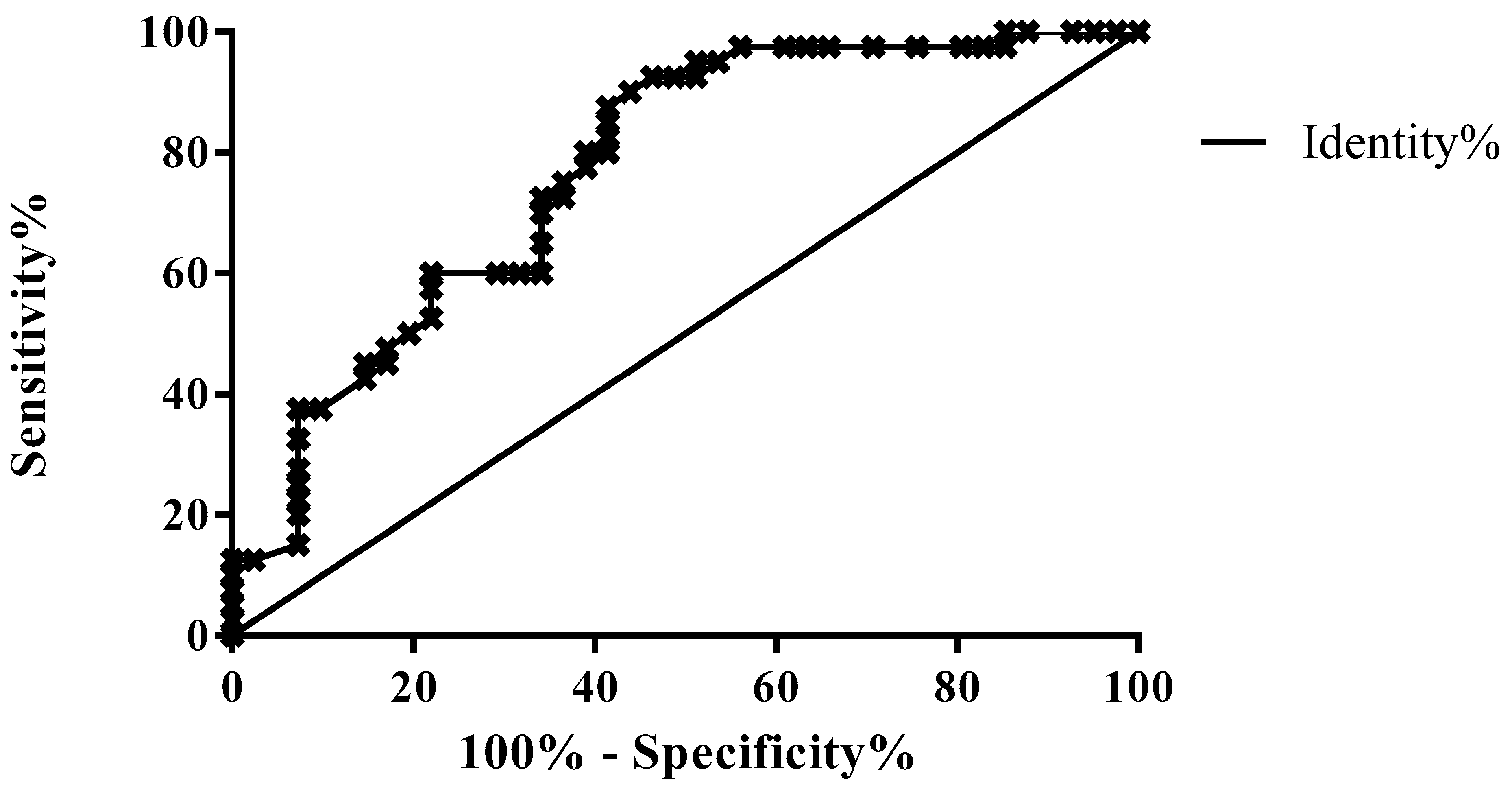
3.2. ROC Analysis for Differential Diagnosis According to the Fecal Elastase Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef]
- Machicado, J.D.; Dudekula, A.; Tang, G.; Xu, H.; Wu, B.U.; Forsmark, C.E.; Yadav, D. Period prevalence of chronic pancreatitis diagnosis from 2001-2013 in the commercially insured population of the United States. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2019, 19, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, D.M.; Greer, P.J.; Ohlsen, C.S.; Mance, S.; Ellison, M.; Breze, C.; Busby, B.; Whitcomb, D.C.; Haupt, M. Acute and Chronic Pancreatitis Disease Prevalence, Classification, and Comorbidities: A Cohort Study of the UK BioBank. Clin. Transl. Gastroenterol. 2022, 13, e00455. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Barthet, M.; Mollard, B.R.; Amouretti, M.; Marion-Audibert, A.-M.; Dyard, F. Estimation of the prevalence and incidence of chronic pancreatitis and its complications: A prospective survey in adults attending gastroenterologists in France. Gastroentérol. Clin. Biol. 2006, 30, 838–844. [Google Scholar] [CrossRef]
- Kleeff, J.; Whitcomb, D.C.; Shimosegawa, T.; Esposito, I.; Lerch, M.M.; Gress, T.; Mayerle, J.; Drewes, A.M.; Rebours, V.; Akisik, F.; et al. Chronic pancreatitis. Nat. Rev. Dis. Prim. 2017, 3, 17060. [Google Scholar] [CrossRef] [PubMed]
- Brock, C.; Nielsen, L.M.; Lelic, D.; Drewes, A.M. Pathophysiology of chronic pancreatitis. World J. Gastroenterol. 2013, 19, 7231–7240. [Google Scholar] [CrossRef]
- Layer, P.; Yamamoto, H.; Kalthoff, L.; Clain, J.E.; Bakken, L.J.; DiMagno, E.P. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology 1994, 107, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Löhr, J.M.; Dominguez-Munoz, E.; Rosendahl, J.; Besselink, M.; Mayerle, J.; Lerch, M.M.; Haas, S.; Akisik, F.; Kartalis, N.; Iglesias-Garcia, J.; et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United Eur. Gastroenterol. J. 2017, 5, 153–199. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Munoz, J.E.; Drewes, A.M.; Lindkvist, B.; Ewald, N.; Czakó, L.; Rosendahl, J.; Löhr, J.M. Recommendations from the United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2018, 18, 847–854. [Google Scholar] [CrossRef]
- Frulloni, L.; Falconi, M.; Gabbrielli, A.; Gaia, E.; Graziani, R.; Pezzilli, R.; Uomo, G.; Andriulli, A.; Balzano, G.; Benini, L.; et al. Italian consensus guidelines for chronic pancreatitis. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2010, 42, S381–S406. [Google Scholar] [CrossRef]
- Domínguez-Muñoz, J.E.; Alvarez-Castro, A.; Lariño-Noia, J.; Nieto, L.; Iglesias-García, J. Endoscopic ultrasonography of the pancreas as an indirect method to predict pancreatic exocrine insufficiency in patients with chronic pancreatitis. Pancreas 2012, 41, 724–728. [Google Scholar] [CrossRef]
- Zamboni, G.A.; Ambrosetti, M.C.; Pezzullo, M.; Bali, M.A.; Mansueto, G. Optimum imaging of chronic pancreatitis. Abdom. Radiol. 2020, 45, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Axon, A.T.; Classen, M.; Cotton, P.B.; Cremer, M.; Freeny, P.C.; Lees, W.R. Pancreatography in chronic pancreatitis: International definitions. Gut 1984, 25, 1107–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappeliez, O.; Delhaye, M.; Devière, J.; Le Moine, O.; Metens, T.; Nicaise, N.; Cremer, M.; Stryuven, J.; Matos, C. Chronic pancreatitis: Evaluation of pancreatic exocrine function with MR pancreatography after secretin stimulation. Radiology 2000, 215, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, R.; Perandini, S.; Mantovani, W.; Frulloni, L.; Faccioli, N.; Pozzi Mucelli, R. Quantitative MRCP assessment of pancreatic exocrine reserve and its correlation with faecal elastase-1 in patients with chronic pancreatitis. Radiol. Med. 2012, 117, 282–292. [Google Scholar] [CrossRef]
- Barral, M.; Taouli, B.; Guiu, B.; Koh, D.-M.; Luciani, A.; Manfredi, R.; Vilgrain, V.; Hoeffel, C.; Kanematsu, M.; Soyer, P. Diffusion-weighted MR imaging of the pancreas: Current status and recommendations. Radiology 2015, 274, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Concia, M.; Sprinkart, A.M.; Penner, A.-H.; Brossart, P.; Gieseke, J.; Schild, H.H.; Willinek, W.A.; Mürtz, P. Diffusion-weighted magnetic resonance imaging of the pancreas: Diagnostic benefit from an intravoxel incoherent motion model-based 3 b-value analysis. Invest. Radiol. 2014, 49, 93–100. [Google Scholar] [CrossRef]
- Akisik, M.F.; Aisen, A.M.; Sandrasegaran, K.; Jennings, S.G.; Lin, C.; Sherman, S.; Lin, J.A.; Rydberg, M. Assessment of chronic pancreatitis: Utility of diffusion-weighted MR imaging with secretin enhancement. Radiology 2009, 250, 103–109. [Google Scholar] [CrossRef]
- Tirkes, T.; Lin, C.; Fogel, E.L.; Sherman, S.S.; Wang, Q.; Sandrasegaran, K. T(1) mapping for diagnosis of mild chronic pancreatitis. J. Magn. Reson. Imaging 2017, 45, 1171–1176. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Gromski, M.A.; Fogel, E.L.; DeWitt, J.M.; Patel, A.A.; Tirkes, T. T1 mapping for the diagnosis of early chronic pancreatitis: Correlation with Cambridge classification system. Br. J. Radiol. 2021, 94, 20200685. [Google Scholar] [CrossRef]
- Steinkohl, E.; Bertoli, D.; Hansen, T.M.; Olesen, S.S.; Drewes, A.M.; Frøkjær, J.B. Practical and clinical applications of pancreatic magnetic resonance elastography: A systematic review. Abdom. Radiol. 2021, 46, 4744–4764. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, F.; Wang, X.; Liu, Y.; Ji, R.; Cang, L.; Shi, Y. Magnetic resonance elastography and T1 mapping for early diagnosis and classification of chronic pancreatitis. J. Magn. Reson. Imaging 2018, 48, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, Y.; Gao, F.; Liu, Y.; Tao, S.; Li, Y.; Glaser, K.J.; Ehman, R.L.; Guo, Q. Pancreatic Stiffness Quantified with MR Elastography: Relationship to Postoperative Pancreatic Fistula after Pancreaticoenteric Anastomosis. Radiology 2018, 288, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Zand, K.A.; Florez, E.; Sirous, R.; Shlapak, D.; Souza, F.; Roda, M.; Bryan, J.; Vasanji, A.; Griswold, M.; et al. Liver surface nodularity score allows Prediction of cirrhosis Decompensation and Death. Radiology 2017, 283, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Branch, C.R.; Zand, K.; Subramony, C.; Zhang, H.; Thaggard, K.; Hosch, R.; Bryan, J.; Vasanji, A.; Griswold, M.; et al. Liver surface nodularity quantification from routine ct images as a biomarker for detection and evaluation of cirrhosis. Radiology 2016, 280, 771–781. [Google Scholar] [CrossRef] [Green Version]
- Gasparini, C.; Ambrosetti, M.C.; Zamboni, G.A.; Ambrosetti, A.; Mansueto, G.; Marchegiani, G.; Perri, G.; Salvia, R.; Montemezzi, S. Quantitative Edge Analysis of Pancreatic Margins in Patients with Pancreatic Head Adenocarcinoma: Correlations between Pancreatic Margins and Onset of Postoperative Pancreatic Fistula. In Proceedings of the ESGAR 2022, SE-090 PE 1, Lisbon, Portugal, 31 May–3 June 2022. [Google Scholar]
- Yagüe-Fabra, J.A.; Ontiveros, S.; Jiménez, R.; Chitchian, S.; Tosello, G.; Carmignato, S. A 3D edge detection technique for surface extraction in computed tomography for dimensional metrology applications. CIRP Ann. Manuf. Technol. 2013, 62, 531–534. [Google Scholar] [CrossRef]
- Tirkes, T.; Lin, C.; Cui, E.; Deng, Y.; Territo, P.R.; Sandrasegaran, K.; Akisik, F. Quantitative MR Evaluation of Chronic Pancreatitis: Extracellular Volume Fraction and MR Relaxometry. AJR. Am. J. Roentgenol. 2018, 210, 533–542. [Google Scholar] [CrossRef]
- Matos, C.; Nicaise, N.; Metens, T.; Cremer, M.; Devière, J. Secretin-enhanced MR pancreatography. Semin. Ultrasound. CT. MR 1999, 20, 340–351. [Google Scholar] [CrossRef]
- Giuliani, T.; Andrianello, S.; Bortolato, C.; Marchegiani, G.; De Marchi, G.; Malleo, G.; Frulloni, L.; Bassi, C.; Salvia, R. Preoperative fecal elastase-1 (FE-1) adds value in predicting post-operative pancreatic fistula: Not all soft pancreas share the same risk —A prospective analysis on 105 patients. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2020, 22, 415–421. [Google Scholar] [CrossRef]
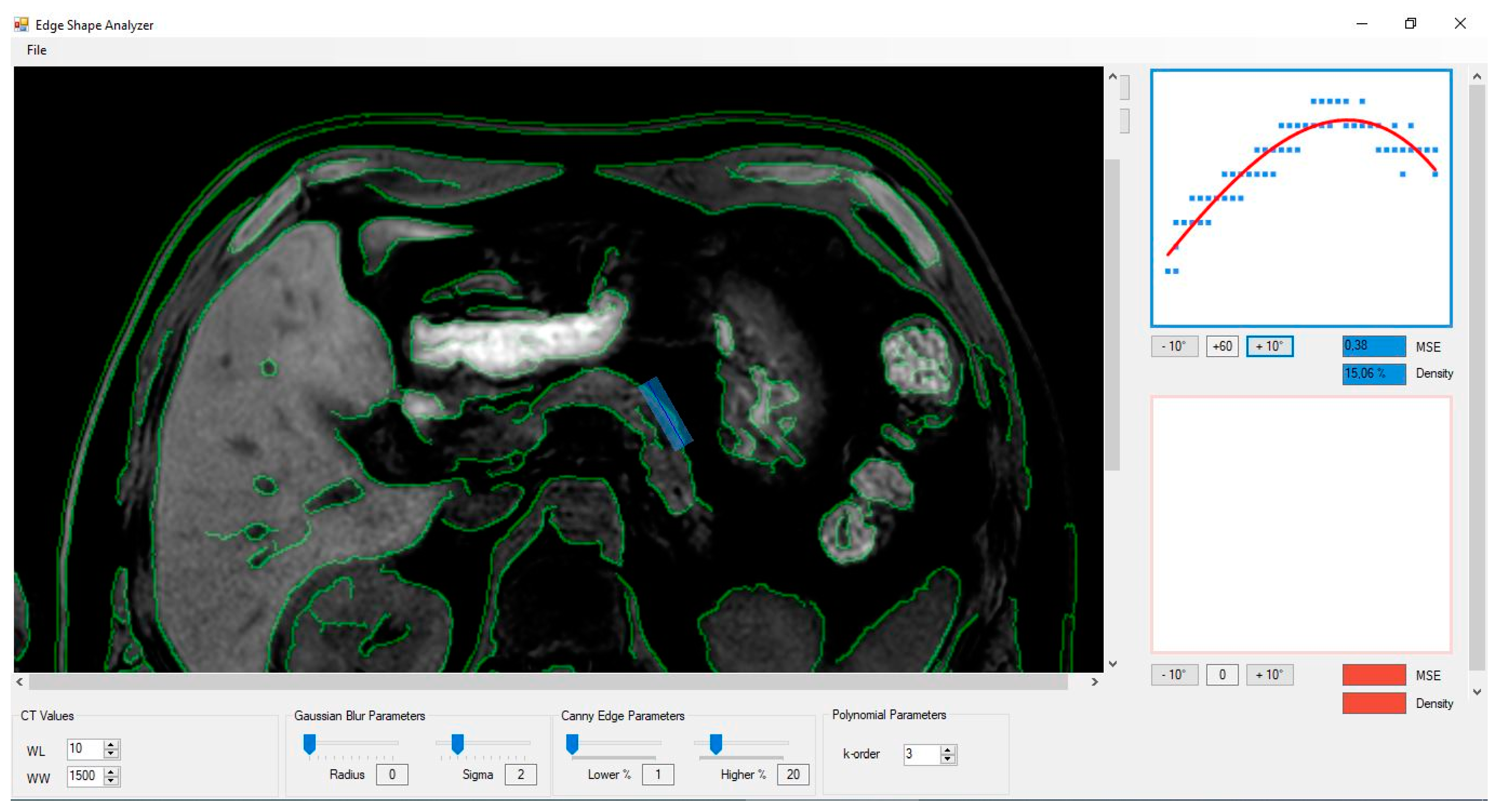


| Group A (Without PEI) | Group B (With Mild to Moderate PEI) | Group C (With Severe PEI) | |
|---|---|---|---|
| TOT | 41 | 40 | 42 |
| M | 26 | 31 | 35 |
| F | 15 | 9 | 7 |
| Mean age (years) | 50 | 55 | 59 |
| SD | 16 | 16 | 13 |
| Group A (Without PEI) | Group B (With Mild to Moderate PEI) | Group C (With Severe PEI) | |
|---|---|---|---|
| CFTR mutation | 5 | 8 | - |
| SPINK-1 mutation | 1 | 1 | - |
| Obstructive Pancreatitis | 10 | 14 | 7 |
| Autoimmune Pancreatitis | 8 | 11 | 28 |
| Paraduodenal Pancreatitis | 2 | - | 3 |
| Pancreas Divisum | 4 | - | 2 |
| Idiopathic | 11 | 6 | 2 |
| Hypertriglyceridaemia | 1 | - | - |
| Comparison | AUROC | p-Value | Threshold (PMS) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Group A vs. B-C (pts without vs. pts with mild or severe PEI) | 0.8373 | <0.0001 | 1.4 | 95.12 | 56.10 |
| Group A-B vs. C (pts without or with mild vs. pts with severe PEI) | 0.8094 | <0.0001 | 1.005 | 97.62 | 55.56 |
| Groups A vs. B (pts without vs. pts with mild PEI) | 0.7701 | <0.0001 | 1.205 | 80 | 64.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosetti, M.C.; Grecchi, A.; Ambrosetti, A.; Amodio, A.; Mansueto, G.; Montemezzi, S.; Zamboni, G.A. Quantitative Edge Analysis of Pancreatic Margins in Patients with Chronic Pancreatitis: A Correlation with Exocrine Function. Diagnostics 2023, 13, 2272. https://doi.org/10.3390/diagnostics13132272
Ambrosetti MC, Grecchi A, Ambrosetti A, Amodio A, Mansueto G, Montemezzi S, Zamboni GA. Quantitative Edge Analysis of Pancreatic Margins in Patients with Chronic Pancreatitis: A Correlation with Exocrine Function. Diagnostics. 2023; 13(13):2272. https://doi.org/10.3390/diagnostics13132272
Chicago/Turabian StyleAmbrosetti, Maria Chiara, Annamaria Grecchi, Alberto Ambrosetti, Antonio Amodio, Giancarlo Mansueto, Stefania Montemezzi, and Giulia A. Zamboni. 2023. "Quantitative Edge Analysis of Pancreatic Margins in Patients with Chronic Pancreatitis: A Correlation with Exocrine Function" Diagnostics 13, no. 13: 2272. https://doi.org/10.3390/diagnostics13132272
APA StyleAmbrosetti, M. C., Grecchi, A., Ambrosetti, A., Amodio, A., Mansueto, G., Montemezzi, S., & Zamboni, G. A. (2023). Quantitative Edge Analysis of Pancreatic Margins in Patients with Chronic Pancreatitis: A Correlation with Exocrine Function. Diagnostics, 13(13), 2272. https://doi.org/10.3390/diagnostics13132272







