Abstract
The aim of the study was to analyse papers describing the use of Electrochemotherapy (ECT) in local treatment of primary and secondary liver tumours located at different sites and with different histologies. Other Local Ablative Therapies (LAT) are also discussed. Analyses of these papers demonstrate that ECT use is safe and effective in lesions of large size, independently of the histology of the treated lesions. ECT performed better than other thermal ablation techniques in lesions > 6 cm in size and can be safely used to treat lesions distant, close, or adjacent to vital structures. ECT spares vessel and bile ducts, is repeatable, and can be performed between chemotherapeutic cycles. ECT can fill the gap in local ablative therapies due to being lesions too large or localized in highly challenging anatomical sites.
1. Introduction
Liver cancer is the sixth leading cause of tumours worldwide, and the fourth for cancer deaths. The reason for the increase in the number of new cases of liver cancer is probably due to changing risk factors, such as obesity, alcohol consumption, and chronic infections with hepatitis B and C, diabetes type II, aflatoxin B1 [1,2]. The liver is also the most frequent site of metastasis from colon and rectum cancer [1] and the management of these malignancies is multidisciplinary. The surgical approach represents the gold standard for patients with primary or secondary liver cancer, but only 25% of these patients are eligible for surgery [3,4] due to factors such as age, pathological condition, severity of disease, location and size of lesions, insufficient proportion of the liver remaining after surgery, or the patient’s own decision [5,6,7,8,9,10,11,12,13,14]. In recent years, minimally invasive treatments for liver metastases have been developed (both thermal and not thermal) for liver metastases, and now, local treatment represents an option for the treatment of these patients.
Local treatment of metastatic colorectal cancer (CRC) has been included in consensus guidelines by the European Society of Medical Oncology (ESMO) [15]. Percutaneous ablation techniques are performed under imaging guidance using specific applicators of different diameter and shape [16,17,18,19]. The shape of the electrodes and the amount of energy delivered affect the ablation volume that depends also on the tumour environment and proximity to a large vessel that leads to the heat sink effect [20].
Thermal ablation techniques, including radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation (CRYO) are used for the treatment of primary and secondary liver tumours. The choice depends on lesion numbers, their localization, size, and local tumour environment. Both RFA and MWA cause a 3-fold elevation of temperature in the target tissue causing the coagulative necrosis of targeted cells directly or indirectly with desiccation of the tissue and destruction of microvasculature [21]. CRYO causes a drop in temperature below −40° within the target tissues and induces a freeze–thaw cycle due to the expansion of argon gas, leading to cell death in a small radius near the probe [21].
Each technique has its advantages and disadvantages and the choice of which to employ depends on the size of the tumour that must be at a maximum of 3.5 cm to be treated with RFA, or 5 cm with MWA and CRYO [21,22,23,24,25].
Non-thermal techniques include trans-arterial therapy (TAE) or trans-arterial che-moembolization (TACE). Both procedures are suitable for patients with unresectable disease involving less than 50% of the liver and without cirrhosis [26]. Tumours within the main portal vein, biliary obstruction and hepatic encephalopathy are contraindications for these treatments. These procedures are generally suggested to patients with palliative intent when curative treatment is not possible.
ECT is a not thermal local ablative procedure that by transient pore formation in the cellular membrane created by electroporation is able to induce enhancement of drug delivery in target cells [27,28]. ECT has been largely used, according to the Standard Operating Procedure developed by the European Standard Operating Procedure for Electrochemotherapy (ESOPE) with the Cliniporator TM Device (IGEA SpA, Carpi, Italy), to treat cutaneous and subcutaneous tumours, and mucosal tumours regardless of histology and body location [28,29,30,31].
The feasibility, safety and effectiveness of ECT has also been shown in deep-seated tumours, such as liver tumours [32,33,34,35] and metastases located near large liver vessels [36,37]. ECT is well-tolerated, with limited side effects [38,39,40]. ECT is specifically suitable for the treatment of liver metastases located centrally, close to the capsule or in proximity of the major vessels, which are not resectable and unsuitable for radiofrequency ablation or microwave ablation due to the heat sink effect [40,41,42,43,44,45,46].
The aim of our study was to analyse papers describing the use of ECT in the local treatment of primary and secondary liver tumours at different sites and with different histologies. In addition, ECT is compared with other thermal ablative percutaneous techniques.
2. Methods
This review is the result of a self-study without protocol and registration number. PRISMA guidelines were used for this systematic review.
In order to ensure an adequate variety of the assessed studies, several electronic databases were considered: PubMed (US National Library of Medicine, http://www.ncbi.nlm.nih.gov/pubmed accessed on 15 October 2022), Scopus (Elsevier, http://www.scopus.com/ accessed on 15 October 2022), Web of Science (Thomson Reuters, http://apps.webofknowledge.com/ accessed on 15 October 2022) and Google Scholar (https://scholar.google.it/ accessed on 15 October 2022).
Only clinical studies published between 2011 and 2022 were analysed, considering this time window consistent with the recent developments concerning the Electrochemotherapy application. Papers not indexed in the electronic databases were evaluated through the references of included studies. Eight [44,47,48,49,50,51,52,53] out of 16 studies were selected. Preclinical studies, review articles and case reports with a single enrolled patient were excluded. However, case reports could be reported in the Section 4.
The selection of papers was made by two reviewers according to a specific procedure (Figure 1). Only papers that met the inclusion criteria and which were written in English were considered. The two investigators extracted data from the included papers and recorded the number of patients treated with ECT, lesion size, percentage of lesions localized in challenging location, and local tumour control. Overall survival was added when available.
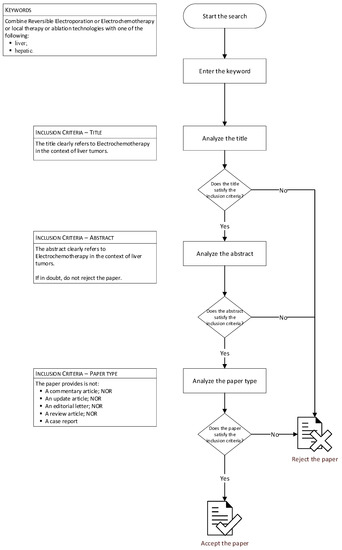
Figure 1.
Flowchart of research methods.
3. Results
Data collected from all the papers included in the analysis are summarized in Table 1. Table 2 shows the reported side effect for each manuscript. For each study, Electrochemotherapy was performed using the electric protocol defined by ESOPE guidelines (electric pulses of 100 µsec at 1000 V/cm) and Bleomycin was administrated intravenously (15,000 IU/m2). Linear fixed configuration, hexagonal fixed configuration or variable geometry manufactured by IGEA S.p.A. using multiple insertion of a single needle was used in the studies as reported in Table 1. Electric pulses were delivered using a generator (IGEA S.p.A., Italy) with the following parameters: 8–96 pulses at 400–3000 V (910–1000 V/cm), of 100 μs duration, at 1–5000 Hz of repetition frequency or a single pulse for a single relived R-wave (ECG synchronization). No combined treatments with ECT were reported by each included study.

Table 1.
Summary table of the studies analysed in this manuscript.

Table 2.
Reported side effect for each manuscript.
The first evidence of feasibility, safety, and efficacy of intraoperative ECT in the treatment of colorectal liver metastases was published by Edhemovic et al. [44]. Twenty-nine metastases in 16 patients were treated in 16 sessions of ECT. Radiological evaluation of all the treated metastases showed 85% complete responses and 15% partial responses. In a group of seven patients that underwent a second operation at 6–12 weeks after the first one, during which ECT was performed, the histology of resected metastases treated by ECT showed less viable tissue (p = 0.001) compared to non-treated ones. No immediate (intraoperative) and/or postoperative serious adverse related events were observed. No differences were observed in treatment responses in the central versus peripheral location of the lesions.
ECT has been already used to treat hepatocellular carcinoma (HCC) [45,46] and to treat a prospective case series of patients with liver cirrhosis and Vp3-Vp4-portal vein tumour thrombus (PVTT) from hepatocellular carcinoma (HCC). In patients with cirrhosis, ECT seems effective and safe for curative treatment of Vp3-Vp4 PVTT from HCC [47]. All patients underwent three-phase computed tomography (CT), contrast enhanced ultrasound (CEUS) and ultrasound-guided percutaneous biopsy of the thrombus before ECT. CEUS examination after treatment showed a complete absence of enhancement of the treated thrombus in all cases. Post-treatment biopsy showed apoptosis and necrosis of tumour cells in all cases. Follow-up ranged from 9 to 20 months (median, 14 months). In two patients, the follow-up CT and CEUS showed complete patency of the treated portal vein without any intravascular or perivascular recurrence during follow-up. The other three patients had a persistent avascular non-tumoral shrinked thrombus at CEUS and CT during follow-up. No local recurrence was observed at follow-up CT and CEUS in 5/6 patients. In the remaining patient, 24 h post-treatment CEUS showed an absence of enhancement of the treated thrombus, but this patient was lost for follow-up because of death from gastrointestinal haemorrhage 5 weeks after ECT. The high risk of haemorrhage from gastroesophageal varices after ECT treatment of the main, right or left PV must be considered in the pre-treatment evaluation of the patients [47].
ECT was also used to treat perihilar cholangiocarcinoma (PHCCA) [48]. Five patients with PHCCA underwent ECT. Three patients underwent percutaneous ECT of a single PHCCA nodule. One patient underwent resection of a nodule in the IV segment and intraoperative ECT of a large PHCCA in the VIII segment. Another patient underwent percutaneous ECT of a large PHCCA recurrence after left lobectomy and RF ablation of a synchronous metastasis in the VI segment. The CT evaluation at 4 weeks post-treatment showed a complete response in three cases and incomplete response in two cases. The follow-up ranged from 10 to 30 months. Two of these five patients were alive at 30 months, with no local or distant intrahepatic recurrences in other segments. The case series was the first study to investigate the safety and efficacy of ECT in the treatment of patients with inoperable PHCCA. It demonstrates that ECT is feasible, safe, and effective and may be a suitable option for the treatment of PHCCA in selected clinical situations.
Electrochemotherapy with bleomycin was recently performed by Djokic et al. on 17 hepatocellular carcinomas in 10 patients [49]. The median size of the treated lesions was 24 mm (range 8 and 41 mm), located either centrally, that is, near the major hepatic vessels, or peripherally. The complete response rate at 3–6 months was 80% per patient and 88% per treated lesion. At the last observation (medium observation time of 20.5 months), the results showed a complete response in 15 out of 17 lesions. ECT is predominantly applicable in patients with impaired liver function due to liver cirrhosis and/or with lesions where a high-risk operation is needed to achieve curative intent, given the intra/perioperative risk for high morbidity and mortality [49]. ECT of hepatocellular carcinoma proved to be a feasible and safe treatment in all 10 patients included in this study.
A prospective pilot study to evaluate the feasibility, safety, and efficacy of intraoperative ECT for otherwise unresectable colorectal liver metastases was performed by Coletti et al. [50]. In this study, five patients with nine colorectal liver metastasis of dimension < 3 cm were treated with ECT with bleomycin pre-treatment followed by open liver resection [50].
Edhemovich et al. [51] demonstrated ECT’s long-term effectiveness and safety in a prospective study on 39 patients with unresectable metachronous colorectal liver metastases. In this paper, the authors reported an objective response rate equal to 75% (63% of complete response, 12% of partial response) and a median duration of the response equal to 20.8 months for metastases in a complete response and 9.8 months for metastases in a partial response. The therapy was significantly more effective for metastases smaller than 3 cm in diameter than for larger ones. There was no difference in response according to the metastatic location, that is, metastases in central vs. peripheral locations.
However, there was no difference in overall survival for metastases smaller than 3 cm in diameter than for larger ones, with a median overall survival time of 29.0 months.
Local tumor control was evaluated in patients with liver malignancies treated by local ablative therapies (LAT). Target lesions were characterised by histology, dimensions in three spatial axes, volume, vascularisation and challenging (CL) location. RFA, MWA, CRYO, ECT and Interstitial Brachytherapy (IBT) were used for local treatment. The study included 155 patients and 211 LAT were performed. Follow-up including MRI for all patients was 11 months. Larger lesions were treated with ECT and IBT and were significantly more often located in challenging location in comparison to those treated with RFA, MWA and CRYO. Best local tumor control (LTC) at 12 months resulted after RFA (93%), followed by ECT (81%), CRYO (70%), IBT (68%) and MWA (61%). Depending on the primary, best results were observed for hepatocellular carcinoma (HCC) (93%), followed by CRC (83%) and breast cancer (BrC) (72%), without statistically significant differences. Local tumor control at 12 months was higher for hypervascular lesions (92% p = 0.07) followed by intermediate (82% p = 0.01) and then hypovascular lesions (64%). Neither diameter nor challenging location had a significant impact on local tumor control even if a tendency to decrease was observed in the larger lesions. In challenging location, the best LTC resulted after RFA (82%) ECT (76%) and IBT (76%). [52]
Spalleck et al. [53] performed a retrospective analysis of patients with liver tumors or metastases treated with percutaneous ECT. Eighteen consecutive patients with measurable liver tumors of different histopathologic origins, mainly colorectal cancer, breast cancer, and hepatocellular cancer were recruited. Only mild or moderate side effects were observed after ECT. The objective response rate was 85.7% (complete response 61.9%, partial response 23.8%), the mean progression-free survival (PFS) was 9.0 ± 8.2 months, and the overall survival (OS) was 11.3 ± 8.6 months. In Table 1, the PFS and OS per different sizes of lesions are shown. ECT performed best (PFS and OS) in lesions within 3 and 6 cm diameters (p = 0.0242, p = 0.0297 respectively). PFS was higher for patients with lesions with lesions < 6 cm (12.0 ± 9.2 months) vs. patients with lesions > 6 cm (4.7 ± 5.4). The lesion localization distant, close or adjacent to vital structures did not influence the effectiveness of ECT. Progression-free survival and overall survival were independent of the primary histology considered. These results seem to demonstrate that ECT is an effective and valuable option for the treatment of unresectable liver metastases that cannot be treated with other ablative techniques.
Analysis of these studies demonstrate that ECT is effective in the treatment of liver lesions (metastases or primitive cancers) of different origin, size and localization, providing a long-term local tumor control method as well as long progression-free survival.
4. Discussion
A wide range of therapeutic options has been developed for treatment of primary and secondary liver tumors to compensate for the limited effectiveness of systemic therapies [54,55,56,57,58,59,60,61,62,63,64,65].
Surgical resection and/or transplantation, as well as local ablative therapies and regional or locoregional therapies, have been validated by prospective studies demonstrating improved patient survival. Surgical resection is the optimal therapeutic option when patients are suitable. However, surgical resection can lead to complications in the presence of cirrhosis or prolonged chemotherapy.
Liver loco-regional treatments, like trans-arterial chemoembolization (TACE) or radio embolization (TARE), have been employed for the treatment of unresectable intrahepatic metastasis (IM) with benefit on overall survival. For large or multinodular tumours locoregional therapies are preferred if the liver function is preserved [65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80]. Thermal ablation techniques including RFA, MWA and CRYO, are used for treatment of primary and secondary liver tumors. Local ablation has minimal morbidity, lower cost and shorter hospital stays [81]. Each local therapies have its advantages and disadvantages and the choice depends by size of the lesion and location with the aim to induce localized cytotoxicity of the tumor preserving near normal tissue [82,83,84,85,86,87,88,89,90,91,92].
Electrochemotherapy of colorectal liver metastases has proven to be a feasible, safe, and efficient treatment method. It allows the treatment of metastases located near the major hepatic vessels that cannot be removed by surgery or radiofrequency ablation.
In addition, ECT treatment of deep-seated tumors of colorectal liver metastases does not affect cardiac function, and no major cardiac rhythm changes or pathological morphological changes were observed. Ten patients treated with ECT and monitored with Holter electrocardiographic (ECG) signals during the periods of 24 h before and after the surgical procedure involving ECT showed only minor significant but clinically irrelevant changes in heart rate and long-term heart rate variability (HRV) parameters during intra-abdominal ECT treatment [92].
ECT has already used to treat hepatocellular carcinoma [45,46] and was also safe and effective for treatment of patients with liver cirrhosis and Vp3-Vp4-portal vein tumor thrombus (PVTT) from hepatocellular carcinoma [47]. ECT represents a suitable option for the management of PHCCA in selected clinical settings, as shown by Tarantino et al. [48]. These results were confirmed in a 71-year-old male affected by a CCA at hepatic hilum and treated with ECT according to ESOPE guidelines. No complications occurred during the ECT procedure and no progression of the disease was found at 18 months with a computed tomography (CT) assessment [93].
In recent years, the combination of ECT for intrahepatic metastases (IM) and cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has been used in a small number of cases with encouraging results. A first synchronous application of ECT and CRS and HIPEC to treat a patient with IM and intraperitoneal metastases (PM) from CCA was described by Stefano et al. [94] A man (47 yrs old) with CCA underwent hepatic resection and systemic therapy. After 14 months, for the occurrence of IM, the patient underwent a second hepatic resection and other chemotherapy cycle. Nonetheless, after 38 months from the first HR a new recurrence occurred and cytoreductive surgery and HIPEC with cisplatin and mitomycin for PM and ECT with BLM on a bulky metastasis of the hepatic hilum were performed. At the computed tomography performed 11 days after treatment complete necrosis of the treated IM was detected. CT scans after 3 and 6 months and magnetic resonance after 9 months showed necrosis of the treated IM and PM, but progression of the residual liver lesions was observed. After 3 months, the patient received SC and underwent TACE after 8 months and TARE after 9 months for the residual liver metastases. At 14 months from CRS and HIPEC, the patient was alive, in good condition, and with stability of the disease [94].
We conducted our study with the intention of determining whether the use of ECT may be beneficial compared with other local thermal techniques in the treatment of particularly large lesions and lesions located in particularly difficult sites.
Combination of local ablative therapy and other options have been explored such as RFA and TACE [95]. Several meta-analyses accumulated data from randomized clinical trials available for RFA plus TACE suggesting that the combination of TACE with RFA improved outcomes compared to RFA alone [96,97,98].
Our attention has mainly focused on liver metastases originating from colorectal cancer, but also metastases of different origin were included in our selection [99,100,101]. We analysed height studies in which ECT or other thermal local procedures were used to treat primary and secondary liver tumors. In these studies, larger lesions were predominantly treated with ECT or IBT while smaller lesions were treated with RFA, CRYO and MWA. Lesions located in challenging positions were treated using ECT rather than with other local ablative therapies. The best local tumor control method depending on the primary tumor was obtained with HCC followed by CRC and BrC, but no statistically significant differences were highlighted.
Local tumor control seems to be higher in hypervascular lesions in comparison to hypovascular ones and not dependent on diameter or volume nor on challenging locations of the target lesion [52], thanks to the careful pre-selection of the most suitable therapy based on current evidence.
Then, large lesions are not an obstacle to local ablative therapies if the right technique and probe are chosen. In the vicinity of large vessels, a method such as ECT, which is not limited by the heat sink effect, can be used safely.
A limitation of this study is the small number of papers. Because of the limited number of studies, it was not possible to perform a subgroup analysis for primitive and secondary liver tumors. However, the eight articles identified are the only ones that met the included criteria. They may be useful and sufficient to provide readers with systematic preliminary results on percutaneous electrochemotherapy for primary and secondary liver malignancies.
5. Conclusions
Analysis of this work shows that ECT is safe and effective in large lesions, regardless of the histology of the lesions treated. ECT performed better than other thermal ablation techniques in lesions > 6 cm and can be safely used to treat lesions that are near or adjacent to vital structures. ECT spares vessels and bile ducts, is repeatable, and can be performed between chemotherapy cycles. ECT can fill the gap in local ablative therapy for lesions that are too large or for lesions in very difficult anatomic locations.
Author Contributions
V.G., V.D. and R.F. wrote the initial manuscript. All authors revised the manuscript for intellectual content and approved the final manuscript as submitted. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in the manuscript and at link https://zenodo.org/record/7503110#.Y7U_y3bMK3A, accessed on 30 December 2022.
Conflicts of Interest
The Authors declare that there are no conflicts of interest.
Abbreviations
| LAT | Local Ablative Therapies |
| ECT | Electrochemotherapy |
| CRC | Colorectal Cancer |
| ESMO | European Society of Medical Oncology |
| RFA | Radiofrequency ablation |
| MWA | Microwave ablation |
| CRYO | Cryoablation |
| TAE | Transarterial therapies |
| TACE | Transarterial chemoembolization |
| ESOPE | European Standard Operating Procedure for Electrochemotherapy |
| CR | Complete Response |
| PR | Partial Response |
| PD | Progression Disease |
| SD | Stable Disease |
| ORR | Overall response rate |
| HCC | Hepatocellular carcinoma |
| BRCA | Breast Carcinoma |
| OS | Overall survival |
| CT | Computed tomography |
| CEIS | Contrast enhanced ultrasound |
| PHCCA | Perihilar cholangiocarcinoma |
| LTC | Local tumour control |
| NSCLC | Non-Small Cell Lung Carcinoma |
| TARE | Radioembolization |
| PFS | Progression free survival |
| HIPEC | Hyperthermic Intraperitoneal chemotherapy |
| IM | Intrahepatic metastases |
| PM | Intraperitoneal metastases |
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; et al. Global, regional, and national cancer Incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524. [Google Scholar] [CrossRef] [PubMed]
- Valery, P.C.; Laversanne, M.; Clark, P.J.; Petrick, J.L.; McGlynn, K.A.; Bray, F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology 2018, 67, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Hackl, C.; Neumann, P.; Gerken, M.; Loss, M.; Klinkhammer-Schalke, M.; Schlitt, H.J. Treatment of colorectal liver metastases in Germany: A ten-year populationbased analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014, 14, 810. [Google Scholar] [CrossRef]
- Gervais, D.A.; Goldberg, S.N.; Brown, D.B. Society of Interventional Radiology Position Statement on Percutaneous Radiofrequency Ablation for the Treatment of Liver Tumors. J. Vasc. Interv. Radiol. 2009, 20 (Suppl. 7), S342–S347. [Google Scholar] [CrossRef]
- Borie, F.; Bouvier, A.-M.; Herrero, A.; Faivre, J.; Launoy, G.; Delafosse, P.; Velten, M.; Buemi, A.; Peng, J.; Grosclaude, P.; et al. Treatment and Prognosis of Hepatocellular Carcinoma: A Population Based Study in France. J. Surg. Oncol. 2008, 98, 505–509. [Google Scholar] [CrossRef]
- Gillams, A.; Goldberg, N.; Ahmed, M. Thermal Ablation of Colorectal Liver Metastases: A Position Paper by an International Panel of Ablation Experts, The Interventional Oncology Sans Frontieres Meeting 2013. Eur. Radiol. 2015, 25, 3438–3454. [Google Scholar] [CrossRef]
- Van Tilborg, A.A.; Scheffer, H.J.; de Jong, M.C. MWA Versus RFA for Perivascular and Peribiliary CRLM: A Retrospective Patient- and Lesion-Based Analysis of Two Historical Cohorts. Cardiovasc. Interv. Radiol. 2016, 39, 1438–1446. [Google Scholar] [CrossRef]
- Sotirchos, V.S.; Petrovic, L.M.; Gonen, M. Colorectal Cancer Liver Metastases: Biopsy of the Ablation Zone and Margins can be Used to Predict Oncologic Outcome. Radiology 2016, 280, 949–959. [Google Scholar] [CrossRef]
- Argalia, G.; Tarantino, G.; Ventura, C.; Campioni, D.; Tagliati, C.; Guardati, P.; Kostandini, A.; Marzioni, M.; Giuseppetti, G.M.; Giovagnoni, A. Shear wave elastography and transient elastography in HCV patients after direct-acting antivirals. Radiol. Med. 2021, 126, 894–899. [Google Scholar] [CrossRef]
- Giovagnoni, A. A farewell from the “old” Editor-in-Chief. Radiol. Med. 2021, 126, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, M.; Simonetti, G. Interventional Magnetic Resonance Imaging Suite (IMRIS): How to build and how to use. Radiol. Med. 2022, 127, 1063–1067. [Google Scholar] [CrossRef]
- Nakamura, Y.; Higaki, T.; Honda, Y.; Tatsugami, F.; Tani, C.; Fukumoto, W.; Narita, K.; Kondo, S.; Akagi, M.; Awai, K. Advanced CT techniques for assessing hepatocellular carcinoma. Radiol. Med. 2021, 126, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Solomon, S.B.; Cornelis, F. Interventetion molecular imaging. J. Nucl. Med. 2016, 57, 493–496. [Google Scholar] [CrossRef]
- Wright, A.S.; Samposo, L.A.; Warner, T.F.; Malvi, D.M.; Lee, F.T. Radiofrequency versus microwawe ablation in hepatic porcine model. Radiology 2005, 236, 132–139. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Stellato, E.; Pellegrino, G.; Bonelli, C.; Cellina, M.; Renzulli, M.; Biondetti, P.; Carrafiello, G. Fluid-dynamic control microcatheter used with glue: Preliminary experience on its feasibility and safety. Radiol. Med. 2022, 127, 272–276. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, Y.S.; Choi, J. Dosimetric analysis of the effects of a temporary tissue expander on the radiotherapy technique. Radiol. Med. 2021, 126, 437–444. [Google Scholar] [CrossRef]
- Bozkurt, M.; Eldem, G.; Bozbulut, U.B.; Bozkurt, M.F.; Kılıçkap, S.; Peynircioğlu, B.; Çil, B.; Lay Ergün, E.; Volkan-Salanci, B. Factors affecting the response to Y-90 microsphere therapy in the cholangiocarcinoma patients. Radiol. Med. 2021, 126, 323–333. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Solomon, S.B. Treatment of primary liver tumours and liver metastases, Part 2: Non-nuclear medicine techniques. J. Nucl. Med. 2018, 59, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Idée, J.-M.; Guiu, B. Use of lipiodol as a drug-delivery system for transcatheter arterial emoembolization of hepatocellular carcinoma: A review. Crit. Rev. Oncol. Hematol. 2013, 88, 530–549. [Google Scholar] [CrossRef] [PubMed]
- Merlotti, A.; Bruni, A.; Borghetti, P.; Ramella, S.; Scotti, V.; Trovò, M.; Chiari, R.; Lohr, F.; Ricardi, U.; Bria, E.; et al. Sequential chemo-hypofractionated RT versus concurrent standard CRT for locally advanced NSCLC: GRADE recommendation by the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Radiol. Med. 2021, 126, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Cionfoli, N.; Paladini, A.; Vallone, M.; Corvino, F.; Teodoli, L.; Moramarco, L.; Quaretti, P.; Catalano, C.; Niola, R.; et al. PHIL® (precipitating hydrophobic injectable liquid): Retrospective multicenter experience on 178 patients in peripheral embolizations. Radiol. Med. 2022, 127, 1303–1312. [Google Scholar] [CrossRef]
- Falcinelli, L.; Mendichi, M.; Chierchini, S.; Tenti, M.V.; Bellavita, R.; Saldi, S.; Ingrosso, G.; Reggioli, V.; Bini, V.; Aristei, C. Pulmonary function in stereotactic body radiotherapy with helical tomotherapy for primary and metastatic lung lesions. Radiol. Med. 2021, 126, 163–169. [Google Scholar] [CrossRef]
- Arslan, A.; Aktas, E.; Sengul, B.; Tekin, B. Dosimetric evaluation of left ventricle and left anterior descending artery in left breast radiotherapy. Radiol. Med. 2021, 126, 14–21. [Google Scholar] [CrossRef]
- Glass, L.F.; Jaroszeski, M.; Gilbert, R.; Reintgen, D.S.; Heller, R. Intralesional bleomycin-mediated electrochemotherapy in 20 patients with basal cell carcinoma. J. Am. Acad. Dermatol. 1997, 37, 596–599. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Clover, A.; de Terlizzi, F.; Bertino, G.; Curatolo, P.; Odili, J.; Campana, L.; Kunte, C.; Muir, T.; Brizio, M.; Sersa, G.; et al. Electrochemotherapy in the treatment of cutaneous malignancy: Outcomes and subgroup analysis from the cumulative results from the pan-European International Network for Sharing Practice in Electrochemotherapy database for 2482 lesions in 987 patients (2008–2019). Eur. J. Cancer 2020, 138, 30–40. [Google Scholar] [CrossRef]
- Plaschke, C.C.; Bertino, G.; McCaul, J.A.; Grau, J.J.; de Bree, R.; Sersa, G.; Occhini, A.; Groselj, A.; Langdon, C.; Heuveling, D.A.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results from the treatment of mucosal cancers. Eur. J. Cancer 2017, 87, 172–181. [Google Scholar] [CrossRef]
- Bertino, G.; Sersa, G.; De Terlizzi, F.; Occhini, A.; Plaschke, C.C.; Groselj, A.; Langdon, C.; Grau, J.J.; McCaul, J.A.; Heuveling, D.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer 2016, 63, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Tafuto, S.; von Arx, C.; De Divitiis, C.; Maura, C.T.; Palaia, R.; Albino, V.; Fusco, R.; Membrini, M.; Petrillo, A.; Granata, V.; et al. Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int. J. Surg. 2015, 21 (Suppl. 1), S78–S82. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Piccirillo, M.; Leongito, M.; Palaia, R.; Granata, F.; Lastoria, S.; Izzo, F.; Petrillo, A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J. Gastroenterol. 2017, 23, 4767–4778. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Catalano, O.; Piccirillo, M.; De Bellis, M.; Izzo, F.; Petrillo, A. Percutaneous ablation therapy of hepatocellular carcinoma with irreversible electroporation: MRI findings. AJR Am. J. Roentgenol. 2015, 204, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Gasljevic, G.; Edhemovic, I.; Cemazar, M.; Brecelj, E.; Gadzijev, E.M.; Music, M.M.; Sersa, G. Histopathological findings in colorectal liver metastases after electrochemotherapy. PLoS ONE 2017, 12, e0180709. [Google Scholar] [CrossRef]
- Brloznik, M.; Boc, N.; Sersa, G.; Zmuc, J.; Gasljevic, G.; Seliskar, A.; Dezman, R.; Edhemovic, I.; Milevoj, N.; Plavec, T.; et al. Radiological findings of porcine liver after electrochemotherapy with bleomycin. Radiol. Oncol. 2019, 53, 415–426. [Google Scholar] [CrossRef]
- Zmuc, J.; Gasljevic, G.; Sersa, G.; Edhemovic, I.; Boc, N.; Seliskar, A.; Plavec, T.; Brloznik, M.; Milevoj, N.; Brecelj, E.; et al. Large Liver Blood Vessels and Bile Ducts Are Not Damaged by Electrochemotherapy with Bleomycin in Pigs. Sci. Rep. 2019, 9, 3649. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Korenbaum, C.; Ben Ammar, M.; Tavolaro, S.; Nouri-Neuville, M.; Lotz, J.P. Multimodal image-guided electrochemotherapy of unresectable liver metastasis from renal cell cancer. Diagn. Interv. Imaging 2019, 100, 309–311. [Google Scholar] [CrossRef]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a New Modality in Interventional Oncology: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818785329. [Google Scholar] [CrossRef]
- Jarm, T.; Cemazar, M.; Miklavcic, D.; Sersa, G. Antivascular effects of electrochemotherapy: Implications in treatment of bleeding metastases. Expert Rev. Anticancer Ther. 2010, 10, 729–746. [Google Scholar] [CrossRef]
- Barra, S.; Guarnieri, A.; di Monale E Bastia, M.B.; Marcenaro, M.; Tornari, E.; Belgioia, L.; Magrini, S.M.; Ricardi, U.; Corvò, R. Short fractionation radiotherapy for early prostate cancer in the time of COVID-19: Long-term excellent outcomes from a multicenter Italian trial suggest a larger adoption in clinical practice. Radiol. Med. 2021, 126, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Cellini, F.; Di Franco, R.; Manfrida, S.; Borzillo, V.; Maranzano, E.; Pergolizzi, S.; Morganti, A.G.; Fusco, V.; Deodato, F.; Santarelli, M.; et al. Palliative radiotherapy indications during the COVID-19 pandemic and in future complex logistic settings: The NORMALITY model. Radiol. Med. 2021, 126, 1619–1656. [Google Scholar] [CrossRef] [PubMed]
- Lancellotta, V.; Del Regno, L.; Di Stefani, A.; Fionda, B.; Marazzi, F.; Rossi, E.; Balducci, M.; Pampena, R.; Morganti, A.G.; Mangoni, M.; et al. The role of stereotactic radiotherapy in addition to immunotherapy in the management of melanoma brain metastases: Results of a systematic review. Radiol. Med. 2022, 127, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Edhemovic, I.; Brecelj, E.; Gasljevic, G.; Marolt Music, M.; Gorjup, V.; Mali, B.; Jarm, T.; Kos, B.; Pavliha, D.; Kuzmanov Grcar, B.; et al. Intraoperative Electrochemotherapy of Colorectal Liver Metastases. J. Surg. Oncol. 2014, 110, 320–327. [Google Scholar] [CrossRef]
- Trotovsek, B.; Hadzialjevic, B.; Cemazar, M.; Sersa, G.; Djokic, M. Laparoscopic electrochemotherapy for the treatment of hepatocellular carcinoma: Technological advancement. Front Oncol. 2022, 12, 996269. [Google Scholar] [CrossRef]
- Djokic, M.; Dezman, R.; Cemazar, M.; Stabuc, M.; Petric, M.; Smid, L.M.; Jansa, R.; Plesnik, B.; Bosnjak, M.; Tratar, U.L.; et al. Percutaneous image guided electrochemotherapy of hepatocellular carcinoma: Technological advancement. Radiol. Oncol. 2020, 54, 347–352. [Google Scholar] [CrossRef]
- Tarantino, L.; Busto, G.; Nasto, A.; Fristachi, R.; Cacace, L.; Talamo, M.; Accardo, C.; Bortone, S.; Gallo, P.; Tarantino, P.; et al. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: A feasibility study. World J. Gastroenterol. 2017, 23, 906–918. [Google Scholar] [CrossRef]
- Tarantino, L.; Busto, G.; Nasto, A.; Nasto, R.A.; Tarantino, P.; Fristachi, R.; Cacace, L.; Bortone, S. Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: A feasibility study. EJSO 2018, 44, 1603–1609. [Google Scholar] [CrossRef]
- Djokic, M.; Cemazar, M.; Popovic, P.; Kos, B.; Dezman, R.; Bosnjak, M.; Zakelj, M.N.; Miklavcic, D.; Potrc, S.; Stabuc, B.; et al. Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study. Eur. J. Surg. Oncol. 2018, 44, 651–657. [Google Scholar] [CrossRef]
- Coletti, L.; Battaglia, V.; De Simone, P.; Turturici, L.; Bartolozzi, C.; Filipponi, F. Safety and feasibility of electrochemotherapy in patients with unresectable colorectal liver metastases: A pilot study. Int. J. Surg. 2017, 44, 26–32. [Google Scholar] [CrossRef]
- Edhemovic, I.; Brecelj, E.; Cemazar, M.; Boc, N.; Trotovsek, B.; Djokic, M.; Dezman, R.; Ivanecz, A.; Potrc, S.; Bosnjak, M.; et al. Intraoperative electrochemotherapy of colorectal liver metastases: A prospective phase II study. Eur. J. Surg. Oncol. 2020, 46, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Bischoff, P.; Haddad, H.; Zhou, W.; Temming, S.; Schäfer, A.; Spallek, H.; Kaupe, L.; Kovács, G.; Pinkawa, M. Long-Term Comparative Study on the Local Tumour Control of Different Ablation Technologies in Primary and Secondary Liver Malignancies. J. Pers. Med. 2022, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Spallek, H.; Bischoff, P.; Zhou, W.; de Terlizzi, F.; Jakob, F.; Kovàcs, A. Percutaneous electrochemotherapy in primary and secondary liver malignancies-local tumor control and impact on overall survival. Radiol. Oncol. 2022, 56, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.S.; Balbaa, M.F.; Gallazzi, M.B.; Eid, M.E.; Kotb, H.T.; Shafei, M.E.; Ierardi, A.M.; Daolio, P.A.; Barile, A.; Carrafiello, G. Role of percutaneous CT-guided radiofrequency ablation in treatment of intra-articular, in close contact with cartilage and extra-articular osteoid osteomas: Comparative analysis and new classification system. Radiol. Med. 2022, 127, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Grasso, R.F.; Bernetti, C.; Pacella, G.; Altomare, C.; Castiello, G.; Andresciani, F.; Sarli, M.; Zobel, B.B.; Faiella, E. A comparative analysis of thermal ablation techniques in the treatment of primary and secondary lung tumors: A single-center experience. Radiol. Med. 2022, 127, 714–724. [Google Scholar] [CrossRef]
- Fiore, F.; Somma, F.; D’Angelo, R.; Tarotto, L.; Stoia, V. Cone beam computed tomography (CBCT) guidance is helpful in reducing dose exposure to pediatric patients undergoing radiofrequency ablation of osteoid osteoma. Radiol. Med. 2022, 127, 183–190. [Google Scholar] [CrossRef]
- Song, W.; Chen, Q.; Guo, D.; Jiang, C. Preoperative estimation of the survival of patients with unresectable hepatocellular carcinoma achieving complete response after conventional transcatheter arterial chemoembolization: Assessments of clinical and LI-RADS MR features. Radiol. Med. 2022, 127, 939–949. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bianchi, G.; Formiconi, F.; Palumbo, P.; Zugaro, L.; Gravina, G.L.; Barile, A.; Masciocchi, C. CT-guided cryoablation for management of bone metastases: A single center experience and review of the literature. Radiol. Med. 2022, 127, 199–205. [Google Scholar] [CrossRef]
- Granata, V.; de Lutio di Castelguidone, E.; Fusco, R.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Gallipoli, A.D.; Petrillo, A. Irreversible electroporation of hepatocellular carcinoma: Preliminary report on the diagnostic accuracy of magnetic resonance, computer tomography, and contrast-enhanced ultrasound in evaluation of the ablated area. Radiol. Med. 2016, 121, 122–131. [Google Scholar] [CrossRef]
- Avallone, A.; Pecori, B.; Bianco, F.; Aloj, L.; Tatangelo, F.; Romano, C.; Granata, V.; Marone, P.; Leone, A.; Botti, G.; et al. Critical role of bevacizumab scheduling in combination with pre-surgical chemo-radiotherapy in MRI-defined high-risk locally advanced rectal cancer: Results of the BRANCH trial. Oncotarget 2015, 6, 30394–30407. [Google Scholar] [CrossRef]
- Orlacchio, A.; Guastoni, C.; Beretta, G.D.; Cosmai, L.; Galluzzo, M.; Gori, S.; Grassedonio, E.; Incorvaia, L.; Marcantoni, C.; Netti, G.S.; et al. SIRM-SIN-AIOM: Appropriateness criteria for evaluation and prevention of renal damage in the patient undergoing contrast medium examinations-consensus statements from Italian College of Radiology (SIRM), Italian College of Nephrology (SIN) and Italian Association of Medical Oncology (AIOM). Radiol. Med. 2022, 127, 534–542. [Google Scholar] [CrossRef]
- Detti, B.; Scoccianti, S.; Teriaca, M.A.; Maragna, V.; Lorenzetti, V.; Lucidi, S.; Bellini, C.; Greto, D.; Desideri, I.; Livi, L. Bevacizumab in recurrent high-grade glioma: A single institution retrospective analysis on 92 patients. Radiol. Med. 2021, 126, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Criscuolo, M.; GullÃ, C.; Petrosino, A.; Carlo Bianco, N.; Colosimo, C. Systemic mastocytosis revisited with an emphasis on skeletal manifestations. Radiol. Med. 2021, 126, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Paik, J.; Del Grande, F.; Paris, E.S.; Sujlana, P.; Fayad, L.M. Distinct MR features in scleroderma associated myopathy. Radiol. Med. 2021, 126, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Gregucci, F.; Bonaparte, I.; Vitulano, N.; Surgo, A.; Mazzola, R.; Di Monaco, A.; Carbonara, R.; Alongi, F.; Langialonga, T.; et al. Stereotactic Ablative radiation therapy (SABR) for cardiac arrhythmia: A new therapeutic option? Radiol. Med. 2021, 126, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Campi, C.; Bianca, B.; Bortolotto, C.; Buccicardi, D.; Francesca, C.; Prost, R.; Rengo, M.; Faggioni, L. Blockchain in radiology research and clinical practice: Current trends and future directions. Radiol. Med. 2022, 127, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Chiti, G.; Grazzini, G.; Flammia, F.; Matteuzzi, B.; Tortoli, P.; Bettarini, S.; Pasqualini, E.; Granata, V.; Busoni, S.; Messserini, L.; et al. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): A radiomic model to predict tumor grade. Radiol. Med. 2022, 127, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol. Med. 2022, 127, 461–470. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol. Med. 2021, 126, 1044–1054. [Google Scholar] [CrossRef]
- Renzulli, M.; Brandi, N.; Argalia, G.; Brocchi, S.; Farolfi, A.; Fanti, S.; Golfieri, R. Morphological, dynamic and functional characteristics of liver pseudolesions and benign lesions. Radiol. Med. 2022, 127, 129–144. [Google Scholar] [CrossRef]
- Li, N.; Wakim, J.; Koethe, Y.; Huber, T.; Schenning, R.; Gade, T.P.; Hunt, S.J.; Park, B.J. Multicenter assessment of augmented reality registration methods for image-guided interventions. Radiol. Med. 2022, 127, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Ledda, R.E.; Silva, M.; McMichael, N.; Sartorio, C.; Branchi, C.; Milanese, G.; Nayak, S.M.; Sverzellati, N. The diagnostic value of grey-scale inversion technique in chest radiography. Radiol. Med. 2022, 127, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.M.; Cafarelli, F.P.; Paparella, M.T.; Rennie, W.J.; Guglielmi, G. Phosphaturic mesenchymal tumors: Radiological aspects and suggested imaging pathway. Radiol. Med. 2021, 126, 1609–1618. [Google Scholar] [CrossRef]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef]
- De Re, V.; Caggiari, L.; De Zorzi, M.; Repetto, O.; Zignego, A.L.; Izzo, F.; Tornesello, M.L.; Buonaguro, F.M.; Mangia, A.; Sansonno, D.; et al. Genetic diversity of the KIR/HLA system and susceptibility to hepatitis C virus-related diseases. PLoS ONE 2015, 10, e0117420, Erratum in PLoS ONE 2015, 10, e0128849. [Google Scholar] [CrossRef] [PubMed]
- Laurelli, G.; Falcone, F.; Gallo, M.S.; Scala, F.; Losito, S.; Granata, V.; Cascella, M.; Greggi, S. Long-Term Oncologic and Reproductive Outcomes in Young Women With Early Endometrial Cancer Conservatively Treated: A Prospective Study and Literature Update. Int. J. Gynecol. Cancer 2016, 26, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, Y.; Yoshida, K.; Okawa, M.; Maki, T.; Nakajima, S.; Sakata, A.; Okuchi, S.; Hinoda, T.; Kanagaki, M.; Nakamoto, Y. Vessel wall MR imaging in neuroradiology. Radiol. Med. 2022, 30, 1–14. [Google Scholar] [CrossRef]
- Granata, V.; Simonetti, I.; Fusco, R.; Setola, S.V.; Izzo, F.; Scarpato, L.; Vanella, V.; Festino, L.; Simeone, E.; Ascierto, P.A.; et al. Management of cutaneous melanoma: Radiologists challenging and risk assessment. Radiol. Med. 2022, 127, 899–911. [Google Scholar] [CrossRef]
- Cirillo, L.; Rustici, A.; Toni, F.; Zoli, M.; Bartiromo, F.; Gramegna, L.L.; Cicala, D.; Tonon, C.; Caranci, F.; Lodi, R. Vessel Wall MRI: Clinical implementation in cerebrovascular disorders-technical aspects. Radiol. Med. 2022, 127, 645–651. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Grassi, F.; Belli, A.; Silvestro, L.; Ottaiano, A.; et al. Radiomics and machine learning analysis based on magnetic resonance imaging in the assessment of liver mucinous colorectal metastases. Radiol. Med. 2022, 127, 763–772. [Google Scholar] [CrossRef]
- Li, D.; Kang, J.; Golas, B.J.; Yeung, V.W.; Madoff, D.C. Minimally invasive local therapies for liver cancer. Cancer Biol. Med. 2014, 11, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Perillo, T.; Paolella, C.; Perrotta, G.; Serino, A.; Caranci, F.; Manto, A. Reversible cerebral vasoconstriction syndrome: Review of neuroimaging findings. Radiol. Med. 2022, 127, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Polici, M.; Rinzivillo, M.; Zerunian, M.; Nacci, I.; Marasco, M.; Magi, L.; Tarallo, M.; Gargiulo, S.; Iannicelli, E.; et al. CT-based radiomics for prediction of therapeutic response to Everolimus in metastatic neuroendocrine tumors. Radiol. Med. 2022, 127, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yu, N.; Yu, Y.; He, T.; Duan, X. Performance of CT radiomics in predicting the overall survival of patients with stage III clear cell renal carcinoma after radical nephrectomy. Radiol. Med. 2022, 127, 837–847. [Google Scholar] [CrossRef]
- Masci, G.M.; Ciccarelli, F.; Mattei, F.I.; Grasso, D.; Accarpio, F.; Catalano, C.; Laghi, A.; Sammartino, P.; Iafrate, F. Role of CT texture analysis for predicting peritoneal metastases in patients with gastric cancer. Radiol. Med. 2022, 127, 251–258. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Mazzei, M.A.; Meglio, N.D.; Roscio, D.D.; Moroni, C.; Monti, R.; Cappabianca, C.; Picone, C.; Neri, E.; et al. Quantitative imaging decision support (QIDSTM) tool consistency evaluation and radiomic analysis by means of 594 metrics in lung carcinoma on chest CT scan. Cancer Control. 2021, 28, 1073274820985786. [Google Scholar] [CrossRef]
- Zerunian, M.; Pucciarelli, F.; Caruso, D.; Polici, M.; Masci, B.; Guido, G.; De Santis, D.; Polverari, D.; Principessa, D.; Benvenga, A.; et al. Artificial intelligence based image quality enhancement in liver MRI: A quantitative and qualitative evaluation. Radiol. Med. 2022, 10, 1098–1105. [Google Scholar] [CrossRef]
- Kang, Y.J.; Cho, J.H.; Hwang, S.H. Diagnostic value of various criteria for deep lobe involvement in radiologic studies with parotid mass: A systematic review and meta-analysis. Radiol. Med. 2022, 127, 1124–1133. [Google Scholar] [CrossRef]
- Scola, E.; Desideri, I.; Bianchi, A.; Gadda, D.; Busto, G.; Fiorenza, A.; Amadori, T.; Mancini, S.; Miele, V.; Fainardi, E. Assessment of brain tumors by magnetic resonance dynamic susceptibility contrast perfusion-weighted imaging and computed tomography perfusion: A comparison study. Radiol. Med. 2022, 127, 664–672. [Google Scholar] [CrossRef]
- Vicini, S.; Bortolotto, C.; Rengo, M.; Ballerini, D.; Bellini, D.; Carbone, I.; Preda, L.; Laghi, A.; Coppola, F.; Faggioni, L. A narrative review on current imaging applications of artificial intelligence and radiomics in oncology: Focus on the three most common cancers. Radiol. Med. 2022, 127, 819–836. [Google Scholar] [CrossRef]
- Mahnken, A.H.; Pereira, P.L.; de Baère, T. Interventional oncologic approaches to liver metastases. Radiology 2013, 266, 407–430. [Google Scholar] [CrossRef] [PubMed]
- Mali, B.; Gorjup, V.; Edhemovic, I.; Brecelj, E.; Cemazar, M.; Sersa, G.; Strazisar, B.; Miklavcic, D.; Jarm, T. Electrochemotherapy of colorectal liver metastases—An observational study of its effects on the electrocardiogram. Biomed. Eng. Online 2015, 14 (Suppl. 3), S5. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Palaia, R.; Albino, V.; Piccirillo, M.; Venanzio Setola, S.; Petrillo, A.; Izzo, F. Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: A case report. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7051–7057. [Google Scholar] [CrossRef] [PubMed]
- Stefano, M.; Prosperi, E.; Fugazzola, P.; Benini, B.; Bisulli, M.; Coccolini, F.; Mastronardi, C.; Palladino, A.; Tomasoni, M.; Agnoletti, V.; et al. Case Report: Cytoreductive Surgery and HIPEC Associated With Liver Electrochemotherapy in a Cholangiocarcinoma Patient With Peritoneal Carcinomatosis and Liver Metastasis Case Report. Front. Surg. 2021, 8, 624817. [Google Scholar] [CrossRef]
- Lencioni, R. Loco-regional treatment of hepatica carcinoma. Hepatology 2010, 52, 762–773. [Google Scholar] [CrossRef]
- Lu, Z.; Wen, F.; Guo, Q.; Liang, H.; Mao, X.; Sun, H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: A meta-analysis of randomized-controlled trials. Eur. J. Gastroenterol. Hepatol. 2013, 25, 187–194. [Google Scholar] [CrossRef]
- Ni, J.Y.; Liu, S.S.; Xu, L.F.; Sun, H.L.; Chen, Y.T. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 3872–3882. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, X.; Ren, S.; Wang, L. Combining transarterial chemoembolization with radiofrequency ablation for hepatocellular carcinoma. Tumour Biol. 2014, 35, 3405–3408. [Google Scholar] [CrossRef]
- De Robertis, R.; Geraci, L.; Tomaiuolo, L.; Bortoli, L.; Beleù, A.; Malleo, G.; D’Onofrio, M. Liver metastases in pancreatic ductal adenocarcinoma: A predictive model based on CT texture analysis. Radiol. Med. 2022, 10, 1079–1084. [Google Scholar] [CrossRef]
- Bracco, S.; Zanoni, M.; Casseri, T.; Castellano, D.; Cioni, S.; Vallone, I.M.; Gennari, P.; Mazzei, M.A.; Romano, D.G.; Piano, M.; et al. Endovascular treatment of acute ischemic stroke due to tandem lesions of the anterior cerebral circulation: A multicentric Italian observational study. Radiol. Med. 2021, 126, 804–817. [Google Scholar] [CrossRef]
- Gurgitano, M.; Angileri, S.A.; Rodà, G.M.; Liguori, A.; Pandolfi, M.; Ierardi, A.M.; Wood, B.J.; Carrafiello, G. Interventional Radiology ex-machina: Impact of Artificial Intelligence on practice. Radiol. Med. 2021, 126, 998–1006. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).