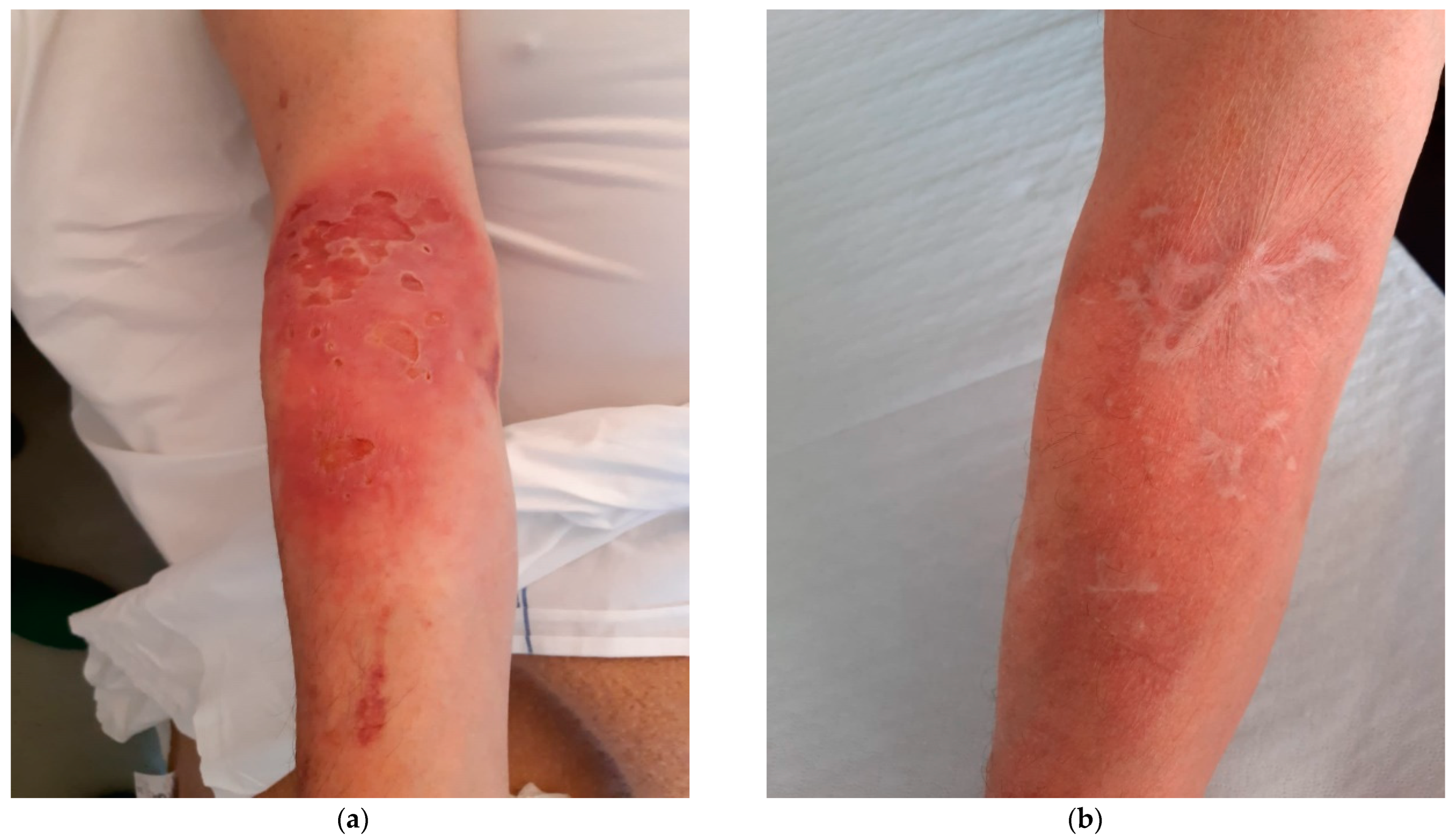
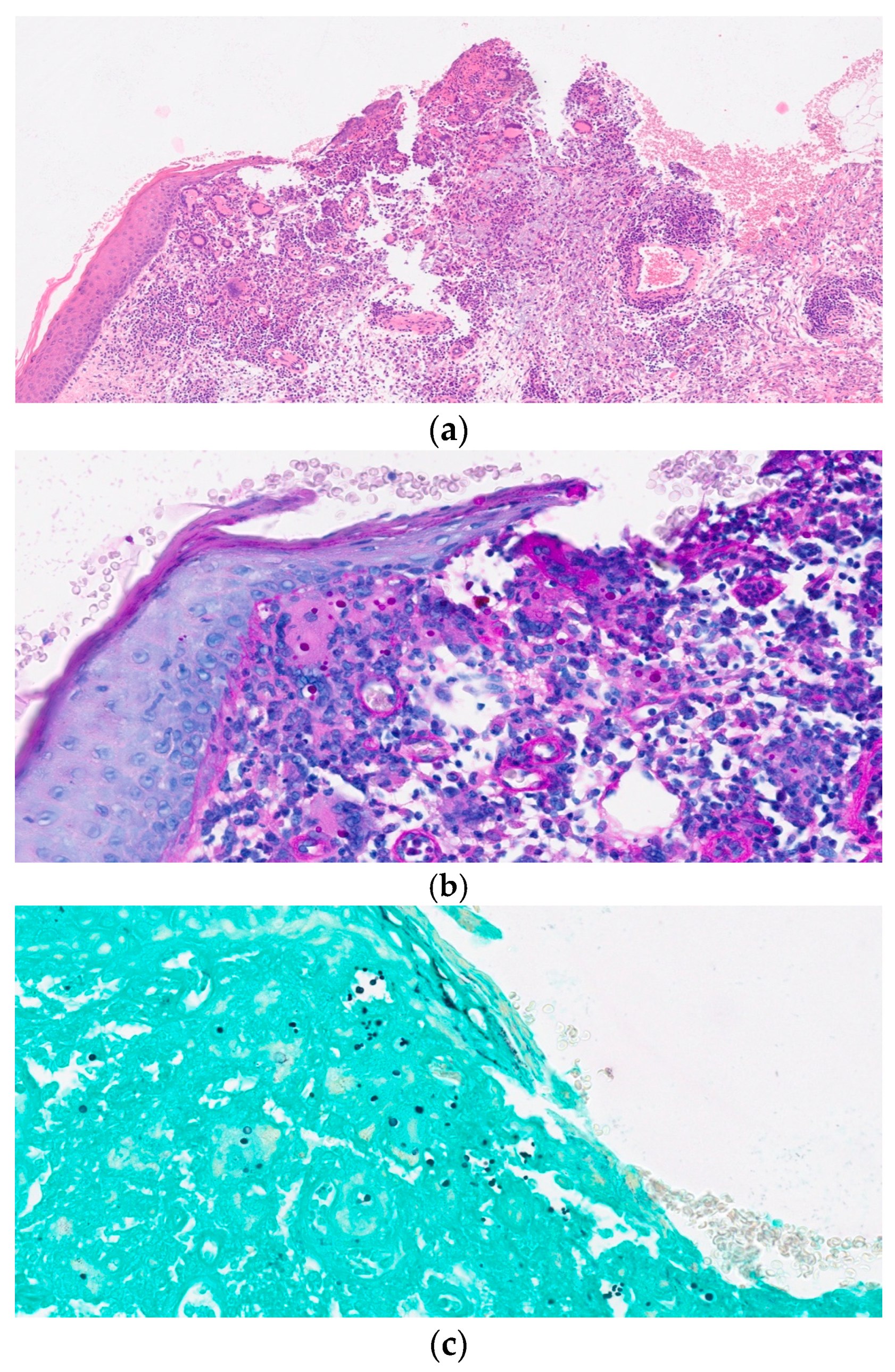
Primary Cutaneous Cryptococcosis in an Immunocompetent Patient: Diagnostic Workflow and Choice of Treatment
Abstract
:Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Severo, L.C.; Zardo, I.B.; Londero, A.T. Cutaneous Cryptococcosis Due to Cryptococcus neoformans var. gattii. Rev. Iberoam. Micol. 2001, 18, 200–201. [Google Scholar] [PubMed]
- Noble, R.C.; Fajardo, L.F. Primary Cutaneous Cryptococcosis: Review and Morphologic Study. Am. J. Clin. Pathol. 1972, 57, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Neuville, S.; Dromer, F.; Morin, O.; Dupont, B.; Ronin, O.; Lortholary, O.; French Cryptococcosis Study Group. Primary Cutaneous Cryptococcosis: A Distinct Clinical Entity. Clin. Infect. Dis. 2003, 36, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Murakawa, G.J.; Kerschmann, R.; Berger, T. Cutaneous Cryptococcus Infection and AIDS: Report of 12 Cases and Review of the Literature. Arch. Dermatol. 1996, 132, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Pema, K.; Diaz, J.; Guerra, L.G.; Nabhan, D.; Verghese, A. Disseminated Cryptococcosis: Comparison of Clinical Manifestations in the Pre-AIDS and AIDS Era. Arch. Intern. Med. 1994, 154, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Dimino-Emme, L.; Gurevitch, A.W. Cutaneous Manifestations of Disseminated Cryptococcosis. J. Am. Acad. Dermatol. 1995, 32, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Yuge, S.; Bastazini, I., Jr.; Coelho, M.C.M.; Soares, C.T. Cutaneous Cryptococcosis in an Immunocompetent Host. Acta. Dermatovenereologica 2006, 86, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Geyer, S.J.; Werber, J.C. Localized Cutaneous Cryptococcosis in an Immunosuppressed Man. Int. J. Dermatol. 1984, 23, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Saag, M.S.; Graybill, R.J.; Larsen, R.A.; Pappas, P.G.; Perfect, J.R.; Powderly, W.G.; Sobel, J.D.; Dismukes, W.E.; Mycoses Study Group Cryptococcal Subproject. Practice Guidelines for the Management of Cryptococcal Disease. Clin. Infect. Dis. 2000, 30, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, Y.; Gu, J.; Chen, J.; Liao, W.; Liao, W. Systemic Review of Published Reports on Primary Cutaneous Cryptococcosis in Immunocompetent Patients. Mycopathologia 2015, 180, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Werchniak, A.E.; Baughman, R.D. Primary Cutaneous Cryptococcosis in an Elderly Man. Clin. Exp. Dermatol. 2004, 29, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.A.; Bastazini, I., Jr.; Martins, A.L.; Barreto, J.A.; Barbieri D’Elia, M.P.; Lastória, J.C.; Mariangela, E.A.; Marques, M.D. Primary Cutaneous Cryptococcosis in Brazil: Report of 11 Cases in Immunocompetent and Immunosuppressed Patients. Int. J. Dermatol. 2012, 51, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.G.; Guidi, M.; Peterka, T.; Rabufetti, A.; Blum, R.; Mainetti, C. Primary Cutaneous Cryptococcosis Due to Cryptococcus neoformans in an Immunocompetent Host Treated with Itraconazole and Drainage: Case Report and Review of the Literature. Case Rep. Dermatol. 2021, 13, 89–97. [Google Scholar]
- Spiliopoulou, A.; Anastassiou, E.D.; Christofidou, M. Primary Cutaneous Cryptococcosis in Immunocompetent Hosts. Mycoses 2012, 55, E45–E47. [Google Scholar] [CrossRef] [PubMed]
- Rook, A.; Woods, B. Cutaneous Cryptococcosis. Br. J. Dermatol. 1962, 74, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Allegue, F.; de Lis, M.P.; Pérez-Alvarez, R. Primary Cutaneous Cryptococcosis Presenting as a Whitlow. Acta Derm. Venereol. 2007, 87, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Naka, W.; Masuda, M.; Konohama, A.; Konohana, A.; Shinoda, T.; Nishikawa, T. Primary Cutaneous Cryptococcosis and Cryptococcus neoformans Serotype, D. Clin. Exp. Dermatol. 1995, 20, 221–225. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panza, F.; Montagnani, F.; Baldino, G.; Custoza, C.; Tumbarello, M.; Fabbiani, M. Primary Cutaneous Cryptococcosis in an Immunocompetent Patient: Diagnostic Workflow and Choice of Treatment. Diagnostics 2023, 13, 3149. https://doi.org/10.3390/diagnostics13193149
Panza F, Montagnani F, Baldino G, Custoza C, Tumbarello M, Fabbiani M. Primary Cutaneous Cryptococcosis in an Immunocompetent Patient: Diagnostic Workflow and Choice of Treatment. Diagnostics. 2023; 13(19):3149. https://doi.org/10.3390/diagnostics13193149
Chicago/Turabian StylePanza, Francesca, Francesca Montagnani, Gennaro Baldino, Cosimo Custoza, Mario Tumbarello, and Massimiliano Fabbiani. 2023. "Primary Cutaneous Cryptococcosis in an Immunocompetent Patient: Diagnostic Workflow and Choice of Treatment" Diagnostics 13, no. 19: 3149. https://doi.org/10.3390/diagnostics13193149
APA StylePanza, F., Montagnani, F., Baldino, G., Custoza, C., Tumbarello, M., & Fabbiani, M. (2023). Primary Cutaneous Cryptococcosis in an Immunocompetent Patient: Diagnostic Workflow and Choice of Treatment. Diagnostics, 13(19), 3149. https://doi.org/10.3390/diagnostics13193149




