Medical Imaging Applications of Federated Learning
Abstract
:1. Introduction
2. Review Methodology
2.1. Research Questions
2.2. Search Process
2.3. Inclusion/Exclusion of Literature
3. Results
4. Background
4.1. What Is FL?
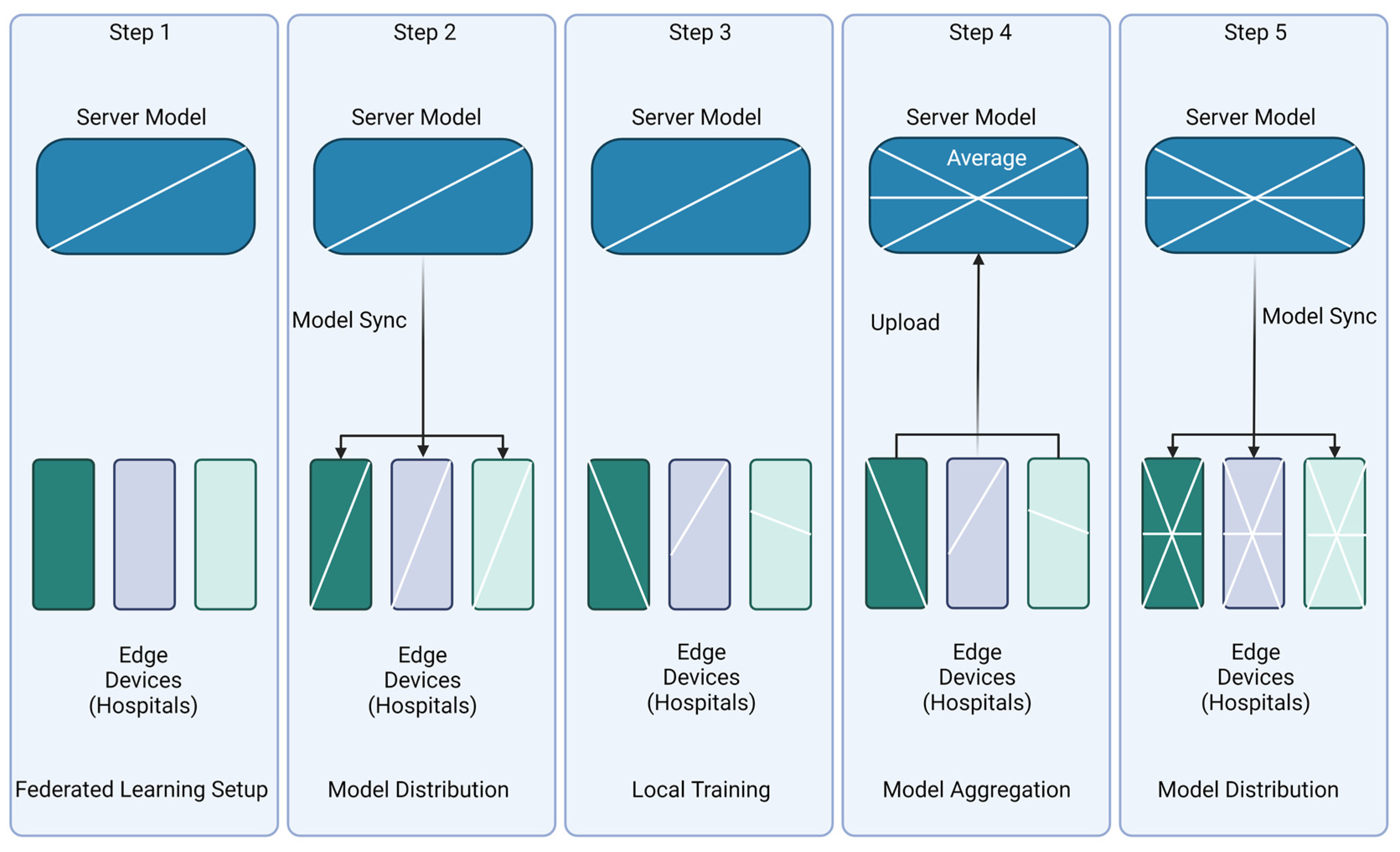
4.2. Setup/Architecture
4.3. Security
4.4. Learning Schemes
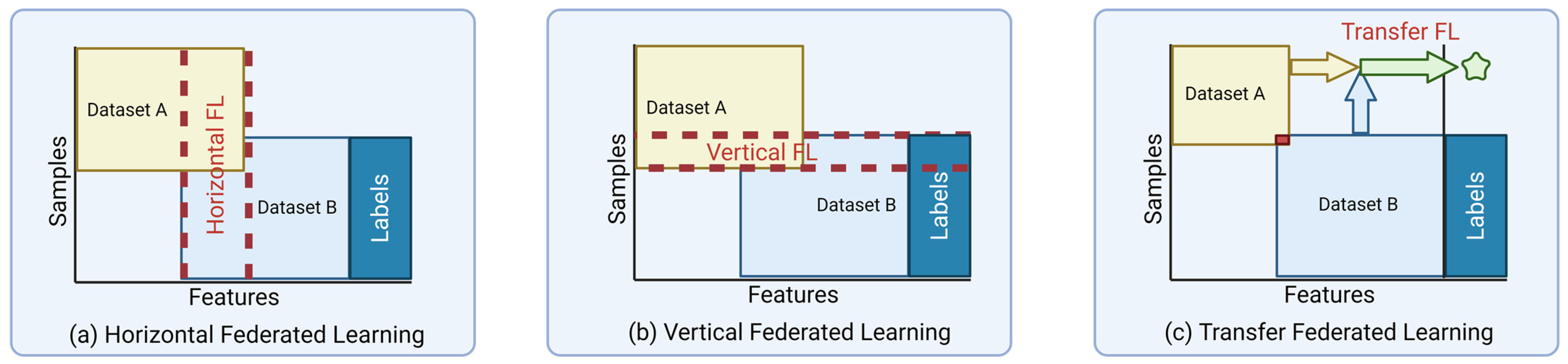
4.5. Data Partitioning
4.6. Aggregation Methods
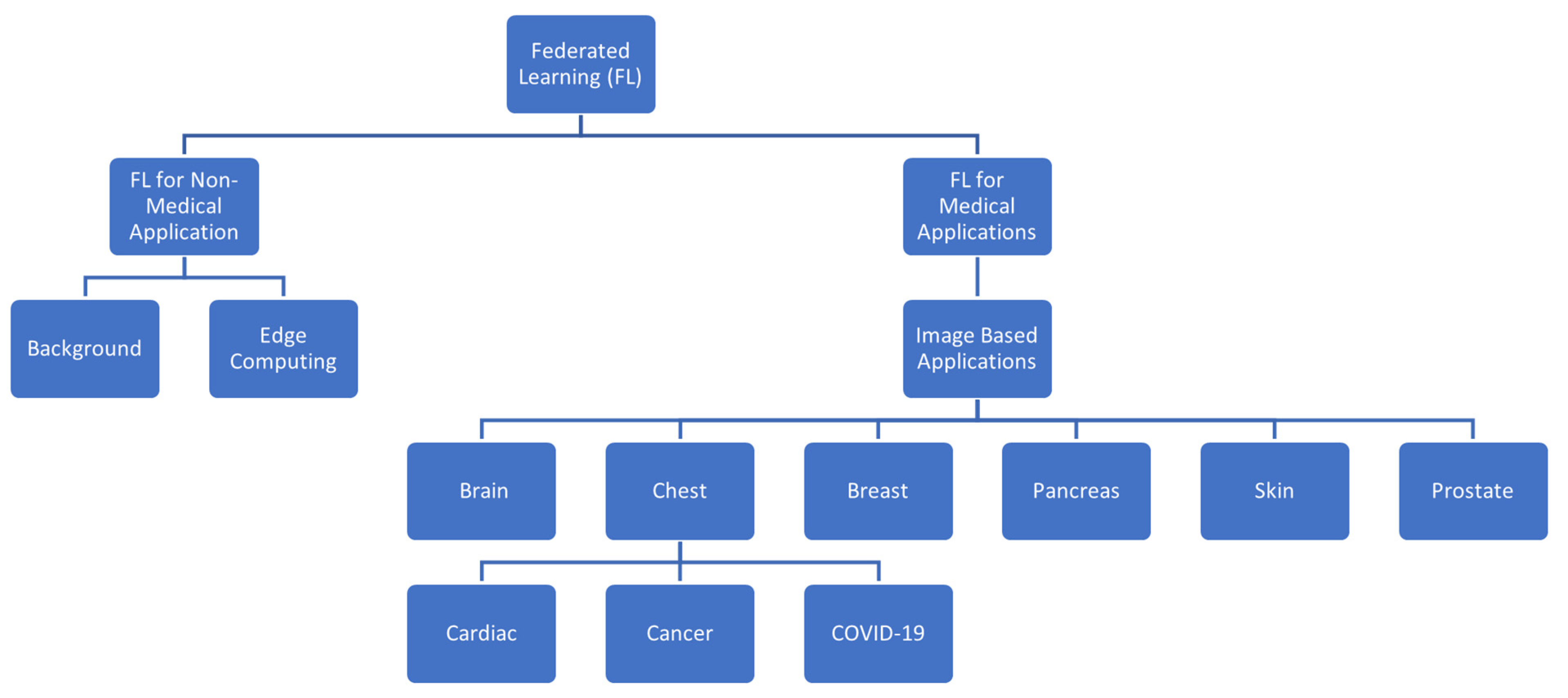
5. Medical Imaging Applications
5.1. Brain
5.1.1. Brain Tumor Detection
5.1.2. Alzheimer’s/Parkinson’s
5.1.3. General Brain Structure Classification
5.1.4. Others
Dementia
Autism
Multiple Sclerosis
Brain Metastasis
Schizophrenia and Depressive Disorders
MRI Reconstruction
5.2. Chest and Abdomen
5.2.1. COVID-19
COVID-19 Chest X-rays
COVID-19 CXR + EMR
COVID-19 CT
COVID-19 CT + Clincal Data
COVID-19 CT/X-rays
COVID-19 X-ray and Ultrasound
5.2.2. General Chest X-rays
5.3. Pancreas
5.4. Breast
| Author | Task | Goal |
|---|---|---|
| Agbley et al. [85] | Tumor Segmentation | Leverage FL to securely train mathematical models over multiple clients with local no special type images from the BIH dataset. |
| Roth et al. [83] | Breast Density classification | Create an FL model that can classify breast densities using BI-RAD data |
| Sanchez et al. [84] | Breast Cancer Classification | Create a novel memory-aware curriculum learning method for FL. |
5.5. Skin
5.6. Prostate
5.7. Others
6. Discussion
6.1. Research Questions
- RQ1: How does federated learning differ from centralized learning when dealing with medical imaging applications?
- ii.
- RQ2: What are the different tasks/scenarios federated learning is used in for medical imaging applications?
6.2. Future Direction
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McMahan, B.; Moore, E.; Ramage, D.; Hampson, S.; Arcas, B.A.y. Communication-Efficient Learning of Deep Networks from Decentralized Data. Proc. Mach. Learn. Res. 2017, 54, 1273–1282. [Google Scholar]
- Chowdhury, A.; Kassem, H.; Padoy, N.; Umeton, R.; Karargyris, A. A Review of Medical Federated Learning: Applications in Oncology and Cancer Research; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 3–24. [Google Scholar]
- Crowson, M.G.; Moukheiber, D.; Arévalo, A.R.; Lam, B.D.; Mantena, S.; Rana, A.; Goss, D.; Bates, D.W.; Celi, L.A. A systematic review of federated learning applications for biomedical data. PLoS Digit. Health 2022, 1, e0000033. [Google Scholar] [CrossRef] [PubMed]
- Rieke, N.; Hancox, J.; Li, W.; Milletarì, F.; Roth, H.R.; Albarqouni, S.; Bakas, S.; Galtier, M.N.; Landman, B.A.; Maier-Hein, K.; et al. The future of digital health with federated learning. NPJ Digit. Med. 2020, 3, 119. [Google Scholar] [CrossRef]
- Rauniyar, A.; Hagos, D.H.; Jha, D.; Håkegård, J.E.; Bagci, U.; Rawat, D.B.; Vlassov, V. Federated Learning for Medical Applications: A Taxonomy, Current Trends, Challenges, and Future Research Directions. arXiv 2022, arXiv:2208.03392. [Google Scholar]
- Shingi, G. A federated learning based approach for loan defaults prediction. In Proceedings of the 2020 International Conference on Data Mining Workshops (ICDMW), Sorrento, Italy, 17–20 November 2020. [Google Scholar]
- Castiglioni, I.; Rundo, L.; Codari, M.; Di Leo, G.; Salvatore, C.; Interlenghi, M.; Gallivanone, F.; Cozzi, A.; D’Amico, N.C.; Sardanelli, F. AI applications to medical images: From machine learning to deep learning. Phys. Medica 2021, 83, 9–24. [Google Scholar] [CrossRef]
- Joshi, M.; Pal, A.; Sankarasubbu, M. Federated Learning for Healthcare Domain—Pipeline, Applications and Challenges. ACM Trans. Comput. Healthc. 2022, 3, 708. [Google Scholar] [CrossRef]
- Pfitzner, B.; Steckhan, N.; Arnrich, B. Federated Learning in a Medical Context: A Systematic Literature Review. ACM Trans. Internet Technol. 2021, 21, 357. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Pham, Q.-V.; Pathirana, P.N.; Ding, M.; Seneviratne, A.; Lin, Z.; Dobre, O.; Hwang, W.-J. Federated Learning for Smart Healthcare: A Survey. ACM Comput. Surv. 2023, 55, 296. [Google Scholar] [CrossRef]
- Kaissis, G.A.; Makowski, M.R.; Rückert, D.; Braren, R.F. Secure, privacy-preserving and federated machine learning in medical imaging. Nat. Mach. Intell. 2020, 2, 305–311. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Ran, A.R.; Hu, X.; Yang, D.; Jiang, M.; Dou, Q.; Cheung, C.Y. Federated Learning in Ocular Imaging: Current Progress and Future Direction. Diagnostics 2022, 12, 2835. [Google Scholar] [CrossRef]
- Kamble, V.; Phophalia, A. Medical Image Analysis Using Federated Learning Frameworks: Technical Review. In Proceedings of the 2022 IEEE 10th Region 10 Humanitarian Technology Conference (R10-HTC), Hyderabad, India, 16–18 September 2022. [Google Scholar]
- Abreha, H.G.; Hayajneh, M.; Serhani, M.A. Federated Learning in Edge Computing: A Systematic Survey. Sensors 2022, 22, 450. [Google Scholar] [CrossRef]
- Aouedi, O.; Sacco, A.; Piamrat, K.; Marchetto, G. Handling Privacy-Sensitive Medical Data with Federated Learning: Challenges and Future Directions. IEEE J. Biomed. Health Inform. 2022, 27, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, E.T.M.; Pérez, M.Q.; Sánchez, P.M.S.; Bernal, S.L.; Bovet, G.; Pérez, M.G.; Pérez, G.M.; Celdrán, A.H. Decentralized Federated Learning: Fundamentals, State-of-the-art, Frameworks, Trends, and Challenges. IEEE Commun. Surv. Tutor. 2022, 1. [Google Scholar] [CrossRef]
- Adamidi, E.S.; Mitsis, K.; Nikita, K.S. Artificial intelligence in clinical care amidst COVID-19 pandemic: A systematic review. Comput. Struct. Biotechnol. J. 2021, 19, 2833–2850. [Google Scholar] [CrossRef]
- Mahlool, D.H.; Abed, M.H. A Comprehensive Survey on Federated Learning: Concept and Applications. In Mobile Computing and Sustainable Informatics; Springer Nature: Singapore, 2022; pp. 539–553. [Google Scholar]
- Zhu, H.; Zhang, H.; Jin, Y. From federated learning to federated neural architecture search: A survey. Complex Intell. Syst. 2021, 7, 639–657. [Google Scholar] [CrossRef]
- Li, L.; Fan, Y.; Tse, M.; Lin, K.-Y. A review of applications in federated learning. Comput. Ind. Eng. 2020, 149, 106854. [Google Scholar] [CrossRef]
- Narmadha, K.; Varalakshmi, P. Federated Learning in Healthcare: A Privacy Preserving Approach. In Studies in Health Technology and Informatics; IOS Press: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Ng, D.; Lan, X.; Yao, M.M.; Chan, W.P.; Feng, M. Federated learning: A collaborative effort to achieve better medical imaging models for individual sites that have small labelled datasets. Quant. Imaging Med. Surg. 2021, 11, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Rootes-Murdy, K.; Gazula, H.; Verner, E.; Kelly, R.; Deramus, T.; Plis, S.; Sarwate, A.; Turner, J.; Calhoun, V. Federated Analysis of Neuroimaging Data: A Review of the Field. Neuroinformatics 2022, 20, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, Y.; Chen, T.; Tong, Y. Federated Machine Learning: Concepts and Applications. ACM Trans. Intell. Syst. Technol. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Zhou, S.K.; Greenspan, H.; Davatzikos, C.; Duncan, J.S.; Van Ginneken, B.; Madabhushi, A.; Prince, J.L.; Rueckert, D.; Summers, R.M. A Review of Deep Learning in Medical Imaging: Imaging Traits, Technology Trends, Case Studies with Progress Highlights, and Future Promises. Proc. IEEE 2021, 109, 820–838. [Google Scholar] [CrossRef]
- AltexSoft. Federated Learning Explained. Available online: https://www.altexsoft.com/blog/federated-learning/ (accessed on 12 November 2022).
- Long, G.; Shen, T.; Tan, Y.; Gerrard, L.; Clarke, A.; Jiang, J. Federated Learning for Privacy-Preserving Open Innovation Future on Digital Health. In Humanity Driven AI: Productivity, Well-Being, Sustainability and Partnership; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Roy, A.G.; Siddiqui, S.; Pölsterl, S.; Navab, N.; Wachinger, C. BrainTorrent: A Peer-to-Peer Environment for Decentralized Federated Learning. arXiv 2019, arXiv:1905.06731. [Google Scholar]
- Zhang, M.; Qu, L.; Singh, P.; Kalpathy-Cramer, J.; Rubin, D.L. SplitAVG: A Heterogeneity-Aware Federated Deep Learning Method for Medical Imaging. IEEE J. Biomed. Health Inf. 2022, 26, 4635–4644. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, B.; Barnawi, A.; Xi, S.; Kumar, N.; Wu, Y. FedDPGAN: Federated Differentially Private Generative Adversarial Networks Framework for the Detection of COVID-19 Pneumonia. Inf. Syst. Front. 2021, 23, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Stripelis, D.; Saleem, H.; Ghai, T.; Dhinagar, N.; Gupta, U.; Anastasiou, C.; Ver Steeg, G.; Ravi, S.; Naveed, M.; Thompson, P.M.; et al. Secure Neuroimaging Analysis Using Federated Learning with Homomorphic Encryption; SPIE: Cergy, France, 2021; Volume 12088. [Google Scholar]
- Ziegler, J.; Pfitzner, B.; Schulz, H.; Saalbach, A.; Arnrich, B. Defending against Reconstruction Attacks through Differentially Private Federated Learning for Classification of Heterogeneous Chest X-ray Data. Sensors 2022, 22, 5195. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Zoha, A.; Mohjazi, L.; Sajid, H.; Abbasi, Q.; Imran, M.A. When Federated Learning Meets Vision: An Outlook on Opportunities and Challenges. In Body Area Networks. Smart IoT and Big Data for Intelligent Health Management; Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 308–319. [Google Scholar]
- Ads, O.S.; Alfares, M.M.; Salem, M.A.-M. Multi-limb Split Learning for Tumor Classification on Vertically Distributed Data. In Proceedings of the 2021 Tenth International Conference on Intelligent Computing and Information Systems (ICICIS), Cairo, Egypt, 5–7 December 2021. [Google Scholar]
- Li, X.; Gu, Y.; Dvornek, N.; Staib, L.H.; Ventola, P.; Duncan, J.S. Multi-site fMRI analysis using privacy-preserving federated learning and domain adaptation: ABIDE results. Med. Image Anal. 2020, 65, 101765. [Google Scholar] [CrossRef] [PubMed]
- Knolle, M.; Kaissis, G.; Jungmann, F.; Ziegelmayer, S.; Sasse, D.; Makowski, M.; Rueckert, D.; Braren, R. Efficient, high-performance semantic segmentation using multi-scale feature extraction. PLoS ONE 2021, 16, e0255397. [Google Scholar] [CrossRef]
- Yang, D. Federated semi-supervised learning for COVID region segmentation in chest CT using multi-national data from China, Italy, Japan. Med. Image Anal. 2021, 70, 101992. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Kalra, S.; Cresswell, J.C.; Taylor, G.W.; Tizhoosh, H.R. Federated learning and differential privacy for medical image analysis. Sci. Rep. 2022, 12, 1953. [Google Scholar] [CrossRef] [PubMed]
- Stripelis, D.; Ambite, J.L.; Lam, P.; Thompson, P. Scaling Neuroscience Research Using Federated Learning. In Proceedings of the 2021 IEEE 18th International Symposium on Biomedical Imaging (ISBI), Nice, France, 13–16 April 2021. [Google Scholar]
- Plis, S.M.; Sarwate, A.D.; Wood, D.; Dieringer, C.; Landis, D.; Reed, C.; Panta, S.R.; Turner, J.A.; Shoemaker, J.M.; Carter, K.W.; et al. COINSTAC: A Privacy Enabled Model and Prototype for Leveraging and Processing Decentralized Brain Imaging Data. Front. Neurosci. 2016, 10, 365. [Google Scholar] [CrossRef]
- Sheller, M.J.; Reina, G.A.; Edwards, B.; Martin, J.; Bakas, S. Multi-institutional Deep Learning Modeling Without Sharing Patient Data: A Feasibility Study on Brain Tumor Segmentation. In Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 92–104. [Google Scholar]
- Sheller, M.J.; Edwards, B.; Reina, G.A.; Martin, J.; Pati, S.; Kotrotsou, A.; Milchenko, M.; Xu, W.; Marcus, D.; Colen, R.R.; et al. Federated learning in medicine: Facilitating multi-institutional collaborations without sharing patient data. Sci. Rep. 2020, 10, 12598. [Google Scholar] [CrossRef]
- Li, W.; Milletarì, F.; Xu, D.; Rieke, N.; Hancox, J.; Zhu, W.; Baust, M.; Cheng, Y.; Ourselin, S.; Cardoso, M.J.; et al. Privacy-Preserving Federated Brain Tumour Segmentation. In Machine Learning in Medical Imaging; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 133–141. [Google Scholar]
- Fay, D.; Sjölund, J.; Oechtering, T.J. Decentralized Differentially Private Segmentation with PATE. arXiv 2020, arXiv:2004.06567. [Google Scholar]
- Mächler, L.; Ezhov, I.; Kofler, F.; Shit, S.; Paetzold, J.C.; Loehr, T.; Zimmer, C.; Wiestler, B.; Menze, B.H. FedCostWAvg: A New Averaging for Better Federated Learning. In Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 383–391. [Google Scholar]
- He, Y.; Zenk, M.; Fritz, M. CosSGD: Nonlinear Quantization for Communication-efficient Federated Learning. arXiv 2020, arXiv:2012.08241. [Google Scholar]
- Rawat, A.; Zizzo, G.; Kadhe, S.; Epperlein, J.P.; Braghin, S. Robust Learning Protocol for Federated Tumor Segmentation Challenge. arXiv 2022, arXiv:2212.08290. [Google Scholar]
- Islam, M.; Reza, M.T.; Kaosar, M.; Parvez, M.Z. Effectiveness of Federated Learning and CNN Ensemble Architectures for Identifying Brain Tumors Using MRI Images. Neural Process. Lett. 2022, 55, 3779–3809. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Baid, U.; Edwards, B.; Sheller, M.; Wang, S.-H.; Reina, G.A.; Foley, P.; Gruzdev, A.; Karkada, D.; Davatzikos, C.; et al. Federated learning enables big data for rare cancer boundary detection. Nat. Commun. 2022, 13, 7346. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Gutman, B.A.; Romero, E.; Thompson, P.M.; Altmann, A.; Lorenzi, M. Federated Learning in Distributed Medical Databases: Meta-Analysis of Large-Scale Subcortical Brain Data. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019. [Google Scholar]
- Silva, S.; Altmann, A.; Gutman, B.; Lorenzi, M. Fed-BioMed: A general open-source frontendframework for federated learning in healthcare. In Proceedings of the MICCAI 2020—23rd International Conference on Medical Image Computing and Computer Assisted Intervention—1st Workshop on Distributed and Collaborative Learning, Lima, Peru, 4 October 2020; pp. 201–210. [Google Scholar]
- Huang, Y.-L.; Yang, H.-C.; Lee, C.-C. Federated Learning via Conditional Mutual Learning for Alzheimer’s Disease Classification on T1w MRI. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021. [Google Scholar]
- Stripelis, D.; Gupta, U.; Saleem, H.; Dhinagar, N.; Ghai, T.; Sanchez, R.; Anastasiou, C.; Asghar, A.; Steeg, G.V.; Ravi, S.; et al. Secure Federated Learning for Neuroimaging. arXiv 2022, arXiv:2205.05249. [Google Scholar]
- Dipro, S.H.; Islam, M.; Al Nahian, A.; Sharmita Azad, M.; Chakrabarty, A.; Reza, T. A Federated Learning Based Privacy Preserving Approach for Detecting Parkinson’s Disease Using Deep Learning. In Proceedings of the 2022 25th International Conference on Computer and Information Technology (ICCIT), Cox’s Bazar, Bangladesh, 17–19 December 2022. [Google Scholar]
- Bercea, C.I.; Wiestler, B.; Rueckert, D.; Albarqouni, S. FedDis: Disentangled Federated Learning for Unsupervised Brain Pathology Segmentation. arXiv 2021, arXiv:2103.03705. [Google Scholar]
- Parekh, V.S.; Lai, S.; Braverman, V.; Leal, J.; Rowe, S.; Pillai, J.J.; Jacobs, M.A. Cross-Domain Federated Learning in Medical Imaging. arXiv 2021, arXiv:2112.10001. [Google Scholar]
- Gupta, U.; Stripelis, D.; Lam, P.K.; Thompson, P.M.; Ambite, J.E.L.; Steeg, G.V. Membership Inference Attacks on Deep Regression Models for Neuroimaging. Proc. Mach. Learn. Res. 2021, 143, 228–251. [Google Scholar]
- Fan, Z.; Su, J.; Gao, K.; Hu, D.; Zeng, L.-L. A Federated Deep Learning Framework for 3D Brain MRI Images. In Proceedings of the 2021 International Joint Conference on Neural Networks (IJCNN), Shenzhen, China, 18–22 July 2021. [Google Scholar]
- Shamseddine, H.; Otoum, S.; Mourad, A. On the Feasibility of Federated Learning for Neurodevelopmental Disorders: ASD Detection Use-Case. In Proceedings of the GLOBECOM 2022—2022 IEEE Global Communications Conference, Rio de Janeiro, Brazil, 4–8 December 2022. [Google Scholar]
- Liu, D.; Cabezas, M.; Wang, D.; Tang, Z.; Bai, L.; Zhan, G.; Luo, Y.; Kyle, K.; Ly, L.; Yu, J.; et al. MS Lesion Segmentation: Revisiting Weighting Mechanisms for Federated Learning. arXiv 2022, arXiv:2205.01509. [Google Scholar]
- Huang, Y.; Bert, C.; Fischer, S.; Schmidt, M.; Dörfler, A.; Maier, A.; Fietkau, R.; Putz, F. Continual Learning for Peer-to-Peer Federated Learning: A Study on Automated Brain Metastasis Identification. arXiv 2022, arXiv:2204.13591. [Google Scholar]
- Zeng, L.-L.; Fan, Z.; Su, J.; Gan, M.; Peng, L.; Shen, H.; Hu, D. Gradient Matching Federated Domain Adaptation for Brain Image Classification. IEEE Trans. Neural Netw. Learn. Syst. 2022, 144. [Google Scholar] [CrossRef]
- Guo, P.; Wang, P.; Zhou, J.; Jiang, S.; Patel, V.M. Multi-institutional Collaborations for Improving Deep Learning-based Magnetic Resonance Image Reconstruction Using Federated Learning. In Proceedings of the 2021 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Virtual, 19–25 June 2021. [Google Scholar]
- Elmas, G.; Dar, S.U.; Korkmaz, Y.; Ceyani, E.; Susam, B.; Ozbey, M.; Avestimehr, S.; Cukur, T. Federated Learning of Generative Image Priors for MRI Reconstruction. IEEE Trans. Med. Imaging 2022, 42, 1996–2009. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yan, B.; Zhou, Y.; Yang, Y.; Zhang, Y. Experiments of Federated Learning for COVID-19 Chest X-ray Images. arXiv 2020, arXiv:2007.05592. [Google Scholar]
- Xu, Y.; Ma, L.; Yang, F.; Chen, Y.; Ma, K.; Yang, J.; Yang, X.; Chen, Y.; Shu, C.; Fan, Z.; et al. A collaborative online AI engine for CT-based COVID-19 diagnosis. medRxiv 2020. [Google Scholar] [CrossRef]
- Kumar, R.; Khan, A.A.; Kumar, J.; Zakria; Golilarz, N.A.; Zhang, S.; Ting, Y.; Zheng, C.; Wang, W. Blockchain-Federated-Learning and Deep Learning Models for COVID-19 Detection Using CT Imaging. IEEE Sens. J. 2021, 21, 16301–16314. [Google Scholar] [CrossRef] [PubMed]
- Laxmi Lydia, E.; Anupama, C.S.S.; Beno, A.; Elhoseny, M.; Alshehri, M.D.; Selim, M.M. Cognitive computing-based COVID-19 detection on Internet of things-enabled edge computing environment. Soft Comput. 2021, 146. [Google Scholar] [CrossRef] [PubMed]
- Dayan, I.; Roth, H.R.; Zhong, A.; Harouni, A.; Gentili, A.; Abidin, A.Z.; Liu, A.; Costa, A.B.; Wood, B.J.; Tsai, C.-S.; et al. Federated learning for predicting clinical outcomes in patients with COVID-19. Nat. Med. 2021, 27, 1735–1743. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, T.; Lu, Q.; Wang, X.; Zhu, C.; Sun, H.; Wang, Z.; Lo, S.K.; Wang, F.-Y. Dynamic-Fusion-Based Federated Learning for COVID-19 Detection. IEEE Internet Things J. 2021, 8, 15884–15891. [Google Scholar] [CrossRef]
- Dou, Q.; So, T.Y.; Jiang, M.; Liu, Q.; Vardhanabhuti, V.; Kaissis, G.; Li, Z.; Si, W.; Lee, H.H.C.; Yu, K.; et al. Federated deep learning for detecting COVID-19 lung abnormalities in CT: A privacy-preserving multinational validation study. NPJ Digit. Med. 2021, 4, 60. [Google Scholar] [CrossRef]
- Feki, I.; Ammar, S.; Kessentini, Y.; Khan, M. Federated learning for COVID-19 screening from Chest X-ray images. Appl. Soft Comput. 2021, 106, 107330. [Google Scholar] [CrossRef] [PubMed]
- Abdul Salam, M.; Taha, S.; Ramadan, M. COVID-19 detection using federated machine learning. PLoS ONE 2021, 16, e0252573. [Google Scholar] [CrossRef]
- Alam, M.U.; Rahmani, R. Federated Semi-Supervised Multi-Task Learning to Detect COVID-19 and Lungs Segmentation Marking Using Chest Radiography Images and Raspberry Pi Devices: An Internet of Medical Things Application. Sensors 2021, 21, 5025. [Google Scholar] [CrossRef]
- Liang, H.; Guo, Y.; Chen, X.; Ang, K.-L.; He, Y.; Jiang, N.; Du, Q.; Zeng, Q.; Lu, L.; Gao, Z.; et al. Artificial intelligence for stepwise diagnosis and monitoring of COVID-19. Eur. Radiol. 2022, 32, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-T.; Tran, K.-D.; Huang, Y. FedSGDCOVID: Federated SGD COVID-19 Detection under Local Differential Privacy Using Chest X-ray Images and Symptom Information. Sensors 2022, 22, 3728. [Google Scholar] [CrossRef]
- Qayyum, A.; Ahmad, K.; Ahsan, M.A.; Al-Fuqaha, A.; Qadir, J. Collaborative Federated Learning for Healthcare: Multi-Modal COVID-19 Diagnosis at the Edge. IEEE Open J. Comput. Soc. 2022, 3, 172–184. [Google Scholar] [CrossRef]
- Durga, R.; Poovammal, E. FLED-Block: Federated Learning Ensembled Deep Learning Blockchain Model for COVID-19 Prediction. Front. Public Health 2022, 10, 892499. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.; Cao, X.; Liu, W.; Zhang, Y.; Chen, D.; Dai, H. Integrated CNN and Federated Learning for COVID-19 Detection on Chest X-Ray Images. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022, 319. [Google Scholar] [CrossRef] [PubMed]
- Aldoj, N.; Lukas, S.; Dewey, M.; Penzkofer, T. Semi-automatic classification of prostate cancer on multi-parametric MR imaging using a multi-channel 3D convolutional neural network. Eur. Radiol. 2020, 30, 1243–1253. [Google Scholar] [CrossRef]
- Wang, P.; Shen, C.; Roth, H.R.; Yang, D.; Xu, D.; Oda, M.; Misawa, K.; Chen, P.-T.; Liu, K.-L.; Liao, W.-C.; et al. Automated Pancreas Segmentation Using Multi-institutional Collaborative Deep Learning. In Domain Adaptation and Representation Transfer, and Distributed and Collaborative Learning; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 192–200. [Google Scholar]
- Shen, C.; Wang, P.; Roth, H.R.; Yang, D.; Xu, D.; Oda, M.; Wang, W.; Fuh, C.-S.; Chen, P.-T.; Liu, K.-L.; et al. Multi-task Federated Learning for Heterogeneous Pancreas Segmentation. In Lecture Notes in Computer Science; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 101–110. [Google Scholar]
- Roth, H.R.; Chang, K.; Singh, P.; Neumark, N.; Li, W.; Gupta, V.; Gupta, S.; Qu, L.; Ihsani, A.; Bizzo, B.C.; et al. Federated Learning for Breast Density Classification: A Real-World Implementation. In Domain Adaptation and Representation Transfer, and Distributed and Collaborative Learning; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 181–191. [Google Scholar]
- Jiménez-Sánchez, A.; Tardy, M.; González Ballester, M.A.; Mateus, D.; Piella, G. Memory-aware curriculum federated learning for breast cancer classification. Comput. Methods Programs Biomed. 2023, 229, 107318. [Google Scholar] [CrossRef] [PubMed]
- Agbley, B.L.Y.; Li, J.; Hossin, M.A.; Nneji, G.U.; Jackson, J.; Monday, H.N.; James, E.C. Federated Learning-Based Detection of Invasive Carcinoma of No Special Type with Histopathological Images. Diagnostics 2022, 12, 1669. [Google Scholar] [CrossRef]
- Hashmani, M.A.; Jameel, S.M.; Rizvi, S.S.H.; Shukla, S. An Adaptive Federated Machine Learning-Based Intelligent System for Skin Disease Detection: A Step toward an Intelligent Dermoscopy Device. Appl. Sci. 2021, 11, 2145. [Google Scholar] [CrossRef]
- Mou, Y.; Welten, S.; Jaberansary, M.; Ucer Yediel, Y.; Kirsten, T.; Decker, S.; Beyan, O. Distributed Skin Lesion Analysis Across Decentralised Data Sources. In Studies in Health Technology and Informatics; IOS Press: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Hossen, M.N.; Panneerselvam, V.; Koundal, D.; Ahmed, K.; Bui, F.M.; Ibrahim, S.M. Federated Machine Learning for Detection of Skin Diseases and Enhancement of Internet of Medical Things (IoMT) Security. IEEE J. Biomed. Health Inform. 2022, 27, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Wicaksana, J.; Yan, Z.; Yang, X.; Liu, Y.; Fan, L.; Cheng, K.-T. Customized Federated Learning for Multi-Source Decentralized Medical Image Classification. IEEE J. Biomed. Health Inform. 2022, 26, 5596–5607. [Google Scholar] [CrossRef]
- Luining, W.I.; Cysouw, M.C.F.; Meijer, D.; Hendrikse, N.H.; Boellaard, R.; Vis, A.N.; Oprea-Lager, D.E. Targeting PSMA Revolutionizes the Role of Nuclear Medicine in Diagnosis and Treatment of Prostate Cancer. Cancers 2022, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wicaksana, J.; Wang, Z.; Yang, X.; Cheng, K.-T. Variation-Aware Federated Learning with Multi-Source Decentralized Medical Image Data. IEEE J. Biomed. Health Inform. 2021, 25, 2615–2628. [Google Scholar] [CrossRef] [PubMed]
- Sarma, K.V.; Harmon, S.; Sanford, T.; Roth, H.R.; Xu, Z.; Tetreault, J.; Xu, D.; Flores, M.G.; Raman, A.G.; Kulkarni, R.; et al. Federated learning improves site performance in multicenter deep learning without data sharing. J. Am. Med. Inf. Assoc. 2021, 28, 1259–1264. [Google Scholar] [CrossRef]
- Lim, J.S.; Hong, M.; Lam, W.S.; Zhang, Z.; Teo, Z.L.; Liu, Y.; Ng, W.Y.; Foo, L.L.; Ting, D.S. Novel technical and privacy-preserving technology for artificial intelligence in ophthalmology. Curr. Opin. Ophthalmol. 2022, 33, 174–187. [Google Scholar] [CrossRef]
- Lo, J.; Yu, T.T.; Ma, D.; Zang, P.; Owen, J.P.; Zhang, Q.; Wang, R.K.; Beg, M.F.; Lee, A.Y.; Jia, Y.; et al. Federated Learning for Microvasculature Segmentation and Diabetic Retinopathy Classification of OCT Data. Ophthalmol. Sci. 2021, 1, 100069. [Google Scholar] [CrossRef]
- Qu, L.; Balachandar, N.; Zhang, M.; Rubin, D. Handling data heterogeneity with generative replay in collaborative learning for medical imaging. Med. Image Anal. 2022, 78, 102424. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, F.; Gao, W.; Zhuang, X. A New Framework of Swarm Learning Consolidating Knowledge from Multi-Center Non-IID Data for Medical Image Segmentation. IEEE Trans. Med. Imaging 2022, 42, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, D.; Wang, Z.; Shi, Y.; Hu, J. Distributed contrastive learning for medical image segmentation. Med. Image Anal. 2022, 81, 102564. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Qian, K.; Wang, Z.; Chang, Y.; Bao, Z.; Hu, B.; Schuller, B.W.; Yamamoto, Y. A Federated Learning Paradigm for Heart Sound Classification. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022. [Google Scholar]
- Linardos, A.; Kushibar, K.; Walsh, S.; Gkontra, P.; Lekadir, K. Federated learning for multi-center imaging diagnostics: A simulation study in cardiovascular disease. Sci. Rep. 2022, 12, 3551. [Google Scholar] [CrossRef]
- Hansen, C.R.; Price, G.; Field, M.; Sarup, N.; Zukauskaite, R.; Johansen, J.; Eriksen, J.G.; Aly, F.; Mcpartlin, A.; Holloway, L.; et al. Open-source distributed learning validation for a larynx cancer survival model following radiotherapy. Radiother. Oncol. 2022, 173, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Rønn Hansen, C.; Price, G.; Field, M.; Sarup, N.; Zukauskaite, R.; Johansen, J.; Eriksen, J.G.; Aly, F.; Mcpartlin, A.; Holloway, L.; et al. Larynx cancer survival model developed through open-source federated learning. Radiother. Oncol. 2022, 176, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Chai, Y.J.; Joo, H.; Lee, K.; Hwang, J.Y.; Kim, S.-M.; Kim, K.; Nam, I.-C.; Choi, J.Y.; Yu, H.W.; et al. Federated Learning for Thyroid Ultrasound Image Analysis to Protect Personal Information: Validation Study in a Real Health Care Environment. JMIR Med. Inform. 2021, 9, e25869. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.A.; Shaikh, M.J.S.; Nadkarni, P. Tuberculosis conundrum—Current and future scenarios: A proposed comprehensive approach combining laboratory, imaging, and computing advances. World J. Radiol. 2022, 14, 114–136. [Google Scholar] [CrossRef]
- Kassem, H.; Alapatt, D.; Mascagni, P.; Karargyris, A.; Padoy, N. Federated Cycling (FedCy): Semi-Supervised Federated Learning of Surgical Phases. IEEE Trans. Med. Imaging 2023, 42, 1920–1931. [Google Scholar] [CrossRef]
- McMahan, B.; Thakurta, A. Federated Learning with Formal Differential Privacy Guarantees. Available online: https://blog.research.google/2022/02/federated-learning-with-formal.html?m=1 (accessed on 5 May 2023).
- Hablani, N. Federated Learning at the Edge May Out-Compete the Cloud on Privacy, Speed and Cost. Available online: https://venturebeat.com/ai/federated-learning-at-the-edge-may-out-compete-the-cloud-on-privacy-speed-and-cost/ (accessed on 2 October 2023).



| Keywords | PubMed | ArXiv | IEEE Xplorer |
|---|---|---|---|
| “Federated Learning” | 354 | 3291 | 4063 |
| “Federated Learning” + “Medical” | 151 | 59 | 354 |
| “Federated Learning” + “Healthcare” | 90 | 25 | 192 |
| “Federated Learning” + “Medical Imaging” | 23 | 41 | 38 |
| “Federated Learning” + “COVID-19” | 43 | 19 | 63 |
| “Federated Learning” + “Brain” | 18 | 26 | 58 |
| “Federated Learning” + “Cancer” | 37 | 5 | 29 |
| “Federated Learning” + “Breast” | 2 | 3 | 11 |
| “Federated Learning” + “Pancreas” | 1 | 2 | 2 |
| Author | Primary Focus | Specific to Medical Field | Summary/Strengths of the Review |
|---|---|---|---|
| Kamble et al. [13] | Frameworks | Yes | Summarizes applications of FL on medical imaging tasks. |
| Abreha et al. [14] | Edge Computing | No | Relates FL to Edge computing. Compares methods of learning such as Centralized learning, Deep learning, and Cloud Computing Services. |
| Aouedi et al. [15] | FL in MedIOT | Yes | Aggregates FL works in MedIoT. Provides information about the variations of FL, such as decentralized vs. centralized as well as the different aggregation techniques. Focuses significantly on COVID-19 applications. Extensive discussion section proposing several future directions. |
| Beltran et al. [16] | CFL vs. DFL | Yes | Compares and explains the differences between Decentralized FL and Centralized FL. Reviews the applications of DFL and analyzes DFL framework. |
| Castiglioni et al. [7] | Background of AI in medical imaging | Yes | Provides context surrounding FL. Explains well what AI is and how it is applied to medical images, as well as the challenges at each step. |
| Chowdhury et al. [2] | Cancer Research FL | Yes | Reviews applications of FL to various forms of cancer. |
| Crowson et al. [3] | FL in healthcare | Yes | Evaluates the current state of FL in healthcare. Only includes up to 2020, so only 13 sources. |
| Adamidi et al. [17] | AI in COVID-19 | Yes | Conducts a systematic review of published and preprint reports of AI models for Coronavirus disease 2019. Some of the reports include FL applications. |
| Joshi et al. [8] | FL background and healthcare | Yes | Explains in detail the fundamentals of FL and the possible variations. Introduces various FL applications categorized into prognosis, diagnosis, and clinical workflow. |
| Mahlool et al. [18] | Applications of FL and DL | Yes | Medical applications of FL and DL. |
| Kaissis et al. [11] | Security in FL Medical Imaging | Yes | Provides some context surrounding the challenges of security in FL medical imaging. Demonstrates by discussing the different kinds of attacks and the solutions provided by various other works. |
| Zhang et al. [19] | Security in FL | Yes | Focuses on the challenges of security and proposes novel applications of privacy-preserving FL in the following scenarios: high communication cost, system heterogeneity and statistical heterogeneity. Nicely generalizes how the issues can be fixed. |
| Li et al. [20] | Applications of FL in Industrial Engineering and healthcare | Some | Discusses the numerous issues that tend to arise when talking about FL. Focuses on the applications related to Industrial Engineering and, secondly, healthcare. |
| Narmadha et al. [21] | Applications of FL in healthcare | Yes | A high-level review of FL in healthcare |
| Ng et al. [22] | FL applications with small datasets | Yes | Provides insight into FL in healthcare applications, focusing specifically on how the problem of small datasets can be alleviated through FL and how different applications were trained and implemented. There were only a handful of direct applications. The group highlights four challenges for FL: weight updating, participation incentives, hardware requirement burdens, data heterogeneity and labeling. |
| Nguyen et al. [10] | Systematic review of FL in healthcare | Yes | Provides insight into some of the other reviews published before this one. Talks about the key principles around FL in healthcare, motivations for using FL in healthcare, requirements for FL and advanced FL designs for healthcare. In Section 5, the paper then goes into the applications of FL in healthcare. |
| Pfitzner et al. [9] | Extensive review of parameters and application of FL in healthcare | Yes | Extensive systematic review that discusses the concepts and research in FL relevant to healthcare. |
| Nguyen, T. et al. [12] | FL in Ophthalmology | Yes | FL applications in ophthalmology, as well as some applications on EMR data, Internet of Things in healthcare, as well as medical imaging. |
| Rauniyar et al. [5] | FL applications in medical field | Yes | Focuses on medical applications rather than technical rigor; provides significant background information as well as information regarding frameworks, challenges, and future directions. |
| Rootes-Murdy et al. [23] | FL in Neuroimaging | Yes | Provides a summary of federated neuroimaging data analysis tools. The paper also talks about the different platforms available for neuroimaging, such as COINSTAC. |
| Yang et al. [24] | Technical FL Summary and applications | No | Focuses heavily on the technical aspects and concepts of FL. Provides general applications not specific to the medical field. |
| Zhou et al. [25] | Review of Deep Learning in medical Imaging | Yes | Focuses on the application of Deep Learning, not specifically FL, in the medical imaging field. Provides insight into the strides that have occurred in various fields, organizing each section by the part of the body. |
| Author | Task | Disease | Goal |
|---|---|---|---|
| Sheller et al. [41] | Tumor segmentation | Tumor | Use FL to achieve generalizability of ML models. |
| Li et al. [43] | Tumor segmentation | Tumor | 1. Implement differential privacy and prove feasibility; 2. Test effects of imbalanced training nodes. |
| Silva et al. [50] | Analysis of subcortical thickness and shape features | Alzheimer’s, Parkinson | Introduce an easy-to-use framework to share any biomedical data with a case study that analyzes subcortical thickness and shape features across diseases such as Alzheimer’s and Parkinson’s, while comparing to healthy individuals. |
| Roy et al. [28] | Whole brain segmentation | General | Create a central server-less FL system. |
| Sheller et al. [42] | Tumor segmentation | Tumor | Use FL to increase 1. Generalizability; 2. Performance. |
| Silva et al. [51] | Analysis of subcortical thickness and shape features | Alzheimer’s, Parkinson | A case study that analyzes subcortical thickness and shape features across diseases such as Alzheimer’s and Parkinson’s while comparing them to healthy individuals. |
| Stripelis et al. [39] | Brain Age prediction | Dementia | Demonstrate an approach to address heterogeneous environments by predicting Dementia using Brain Age. |
| Stripelis et al. [31] | Brain Age prediction | Dementia | 1. Demonstrate a successful implementation of Cheon-Kim-Kim-Song scheme for a more secure Transfer supporting fully homomorphic encryption; 2. Demonstrate performance on skewed data. |
| Li et al. [35] | Autism spectrum disorder biomarker discovery | Autism | 1. Privacy-preserving pipeline for fMRI; 2. Address data heterogeneity due to domain shift. |
| Huang et al. [52] | Detection and stage classification of Alzheimer’s | Alzheimer’s | 1. Set up a way to conduct multisite Alzheimer’s classification by 3D convolutional neural network and t1w MRI; 2. Compare results to other models. |
| Bercea et al. [55] | Brain anomaly segmentation | General | Create a framework that can identify anomalies by only sending shape and intensity parameters. |
| Machler et al. [45] | Tumor segmentation | Tumor | Create a better way to average updated model weights. |
| Fan et al. [58] | Autism spectrum disorder diagnosis | Autism | 1. Create an FL framework for analyzing 3D Brain MRI images; 2. Implement privacy measures to enhance security. |
| Parekh et al. [56] | Organ localizing, lesion segmentation | General | 1. Demonstrate the feasibility of training cross-domain; 2. cross-task FL models. |
| He et al. [46] | Image classification | Tumor | Implement a simple cosine-based nonlinear quantization to achieve results in compressing round-trip communication costs. |
| Dipro et al. [54] | Image classification | Parkinson’s | A novel approach to detecting Parkinson’s disease with FL. |
| Zhang et al. [29] | Tumor segmentation | Tumor | Create a new FL method to overcome the performance drops from data heterogeneity. |
| Liu et al. [60] | Lesion segmentation | Multiple Sclerosis | Create a framework that addresses domain shifts that are specific to Multiple Sclerosis lesion segmentation tasks. |
| Stripelis et al. [53] | Brain classification | Alzheimer’s and Brain Age | Build an architecture 1. That encrypts parameters before transmission, computes models via homomorphic encryption and uses methods to limit leakage; 2. Performs well across heterogeneous environments. |
| Islam et al. [48] | Image classification | Tumor | First study to use Complex CNN model for FL MRI-based tumor classification. |
| Huang et al. [61] | Metastasis Segmentation | Brain Metastasis | Overcome catastrophic forgetting by implementing Continual Learning on Brain Metastasis Identification. |
| Zeng et al. [62] | Image classification | Schizophrenia, Major Depressive Disorder | Propose a 2-stage method of gradient matching that aims to reduce domain discrepancy. The group demonstrated the ability of this method on resting-state functional MRIs for diagnostic classification. |
| Ads et al. [34] | Image classification | Tumor | Implement both split learning and Vertical distribution for brain tumor classification. |
| Elmas et al. [64] | MRI reconstruction (Not Diagnostic) | General | Introduce FedGIMP for MRI reconstruction, which leverages a 2-stage approach: cross-site learning of generative MRI prior and prior adaption following injection of the imaging operator. |
| Fay et al. [44] | Tumor segmentation | Tumor | Implement a Private Aggregation of Teacher Ensembles based on the FL model on the BraTS dataset. |
| Guo et al. [63] | MRI reconstruction (Not Diagnostic) | General | 1. Introduce a method called FL-M that enables multi-institutional collaborations for MRI reconstruction; 2. Address domain shift issues by aligning the latent space distribution between the source and target domain; 3. Conduct experiments that provide insights about FL in MRI reconstruction. |
| Gupta et al. [57] | Brain Age prediction | General | Demonstrate the ability to conduct membership interference attacks on deep learning models. |
| Pati et al. [49] | Tumor segmentation | Tumor | Conduct experiments on the largest dataset to date regarding the feasibility and effects of FL on glioblastoma sub-compartment boundary detection. |
| Shamseddine et al. [59] | Autism spectrum disorder diagnosis | Autism | Use FL models to determine if a patient has Autism or not based on: 1. behavioral screening data; 2. A clear facial picture. |
| Rawat et al. [47] | Tumor segmentation | Tumor | Introduce robust learning protocol, which is a combination of server-side adaptive optimization and parameter aggregation schemes to tackle data heterogeneity issues and communication cost of training. |
| Knolle et al. [36] | Pancreas segmentation and tumor segmentation | General pancreas and tumor | Create an FL architecture that can operate in resource-constrained environments by decreasing the amount of image features being used and transferred. |
| Author | Task | Modality | Goal |
|---|---|---|---|
| Liu et al. [65] | Classification | CXR | Compare distributed learning/FL to four other classic models. |
| Xu et al. [66] | Classification | CT |
|
| Kumar et al. [67] | Segmentation/Classification | CT |
|
| Lydia et al. [68] | Classification | CXR | Create an FL-based COVID-19 detection model on an Internet of Things, enabling edge computing environment. |
| Dayan et al. [69] | Classification | CXR + EMR | Provide proof of concept that will demonstrate the ability to create an FL model that can be used across heterogeneous, unharmonized datasets for the prediction of clinical outcomes in patients with COVID-19. |
| Zhang et al. [70] | Classification | CT, CXR |
|
| Dou et al. [71] | Segmentation/Classification | CT |
|
| Feki et al. [72] | Classification | CXR |
|
| Yang et al. [37] | Segmentation/Classification | CT |
|
| Salam et al. [73] | Classification | CXR + EMR |
|
| Alam et al. [74] | Segmentation/Classification | CXR |
|
| Liang et al. [75] | Segmentation/Classification | CT+ EMR |
|
| Zhang et al. [30] | Segmentation | CXR | Create a privacy-preserving data augmentation method enhancing security; |
| Ho et al. [76] | Classification | CXR + EMR., |
|
| Qayyum et al. [77] | Classification | CXR + ultrasound | 1. Create a clustered FL method to develop a multimodal COVID-19 FL detection system using X-ray and ultrasound; |
| Durga et al. [78] | Classification | CT | 1. Propose a novel framework based on blockchain and FL model; |
| Zheng Li et al. [79] | Classification | CXR | 1. Create a FL framework with a dynamic focus on COVID-19 detection on CXR; |
| Author | Task | Goal |
|---|---|---|
| Wang et al. [81] | Pancreas segmentation | Generate and evaluate an FL model for pancreas segmentation. |
| Shen et al. [82] | Pancreas segmentation | Investigate heterogeneous optimization methods that show improvements for the automated segmentation of pancreas and pancreatic tumors in abdominal CT images. |
| Knolle et al. [36] | Pancreas segmentation | Create an FL architecture that can operate in resource-constrained environments by decreasing the amount of image features being used and transferred. |
| Author | Task | Disease | Goal |
|---|---|---|---|
| Hashmani et al. [86] | Segmentation and classification | Skin tumor | Propose an adaptive FL-based skin disease model to create an intelligent dermoscopy device. |
| Mou et al. [87] | Segmentation and classification | Melanoma detection | Present a feasibility study that demonstrated the capabilities of FL on medical records. |
| Hossen et al. [88] | Classification | Skin diseases | 1. Create a custom image dataset prepared with 4 distinct classes of skin disease; 2. Create a novel CNN model to classify the four disease types; 3. Use FL to enhance the security of medical imaging using the custom dataset. |
| Wicaksana et al. [89] | Classification | Skin lesions | Introduce and implement CusFL, a method in which each client trains a private model based on the global model aggregated from all private models trained in the immediate previous iterations. |
| Author | Task | Goal |
|---|---|---|
| Yan et al. [91] | Prostate classification |
|
| Wicaksana et al. [89] | Prostate classification | Introduce and Implement CusFL, a method in which each client trains a private model based on the global model aggregated from all private models trained in the immediate previous iterations. |
| Sarma et al. [92] | Prostate segmentation | Demonstrate the ability to train a FL model across 3 academic institutions while preserving patient privacy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandhu, S.S.; Gorji, H.T.; Tavakolian, P.; Tavakolian, K.; Akhbardeh, A. Medical Imaging Applications of Federated Learning. Diagnostics 2023, 13, 3140. https://doi.org/10.3390/diagnostics13193140
Sandhu SS, Gorji HT, Tavakolian P, Tavakolian K, Akhbardeh A. Medical Imaging Applications of Federated Learning. Diagnostics. 2023; 13(19):3140. https://doi.org/10.3390/diagnostics13193140
Chicago/Turabian StyleSandhu, Sukhveer Singh, Hamed Taheri Gorji, Pantea Tavakolian, Kouhyar Tavakolian, and Alireza Akhbardeh. 2023. "Medical Imaging Applications of Federated Learning" Diagnostics 13, no. 19: 3140. https://doi.org/10.3390/diagnostics13193140
APA StyleSandhu, S. S., Gorji, H. T., Tavakolian, P., Tavakolian, K., & Akhbardeh, A. (2023). Medical Imaging Applications of Federated Learning. Diagnostics, 13(19), 3140. https://doi.org/10.3390/diagnostics13193140






