Establishing and Validating a Morphological Prediction Model Based on CTA to Evaluate the Incidence of Type-B Dissection
Abstract
:1. Introduction
2. Methods
2.1. Patient Sampling
2.2. Outcomes
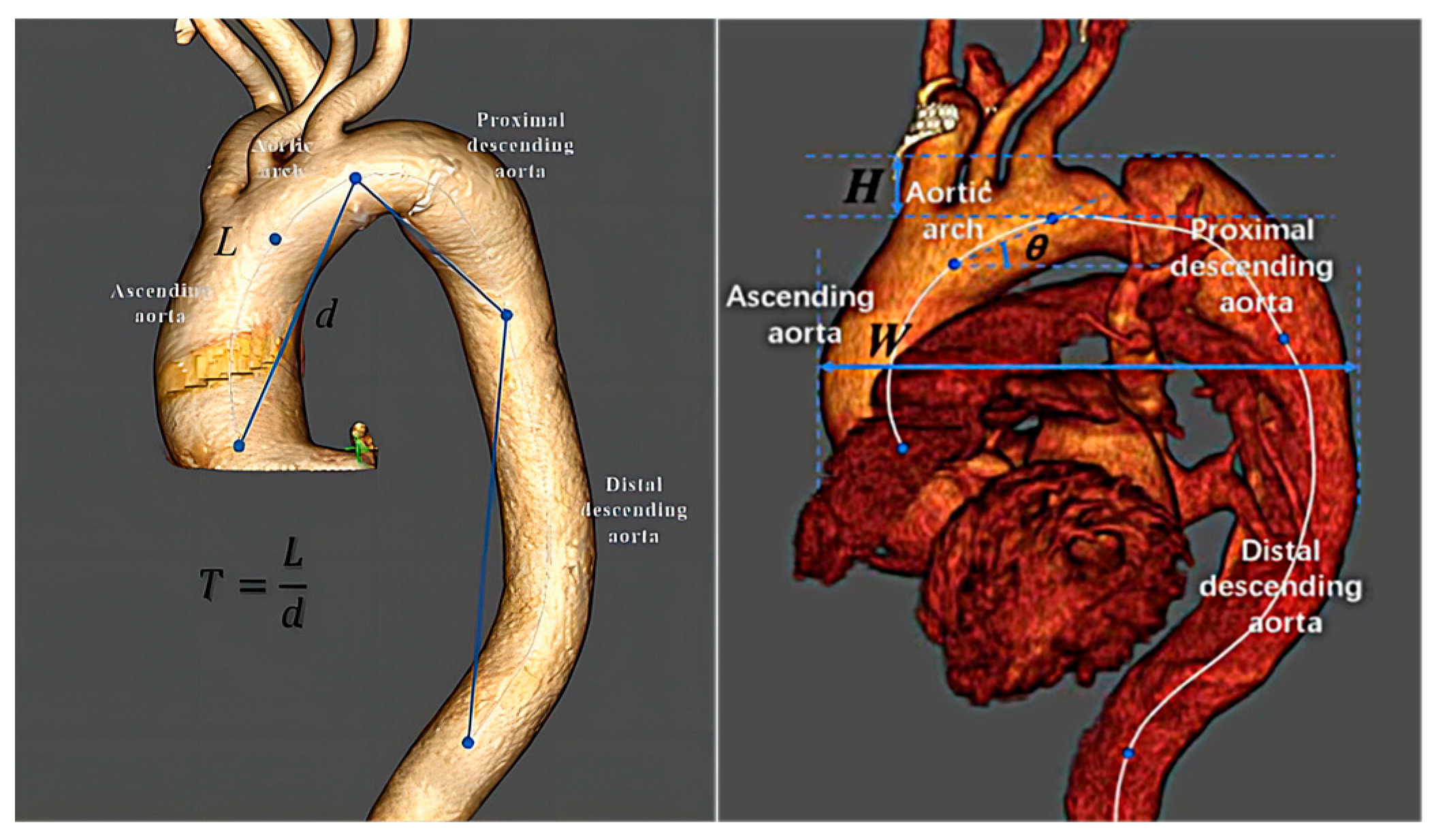
2.3. Predictor Variables
2.4. Statistical Description
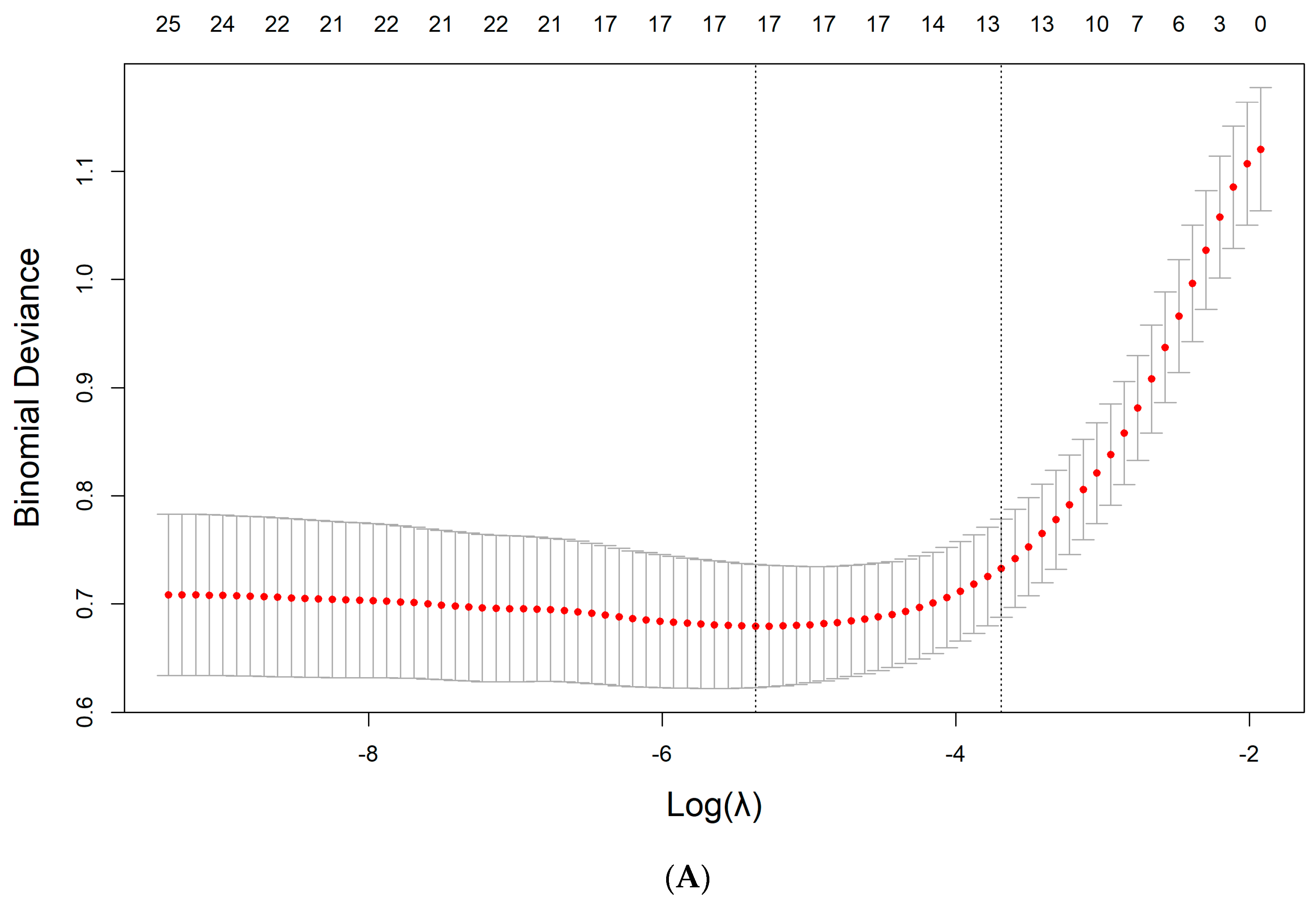
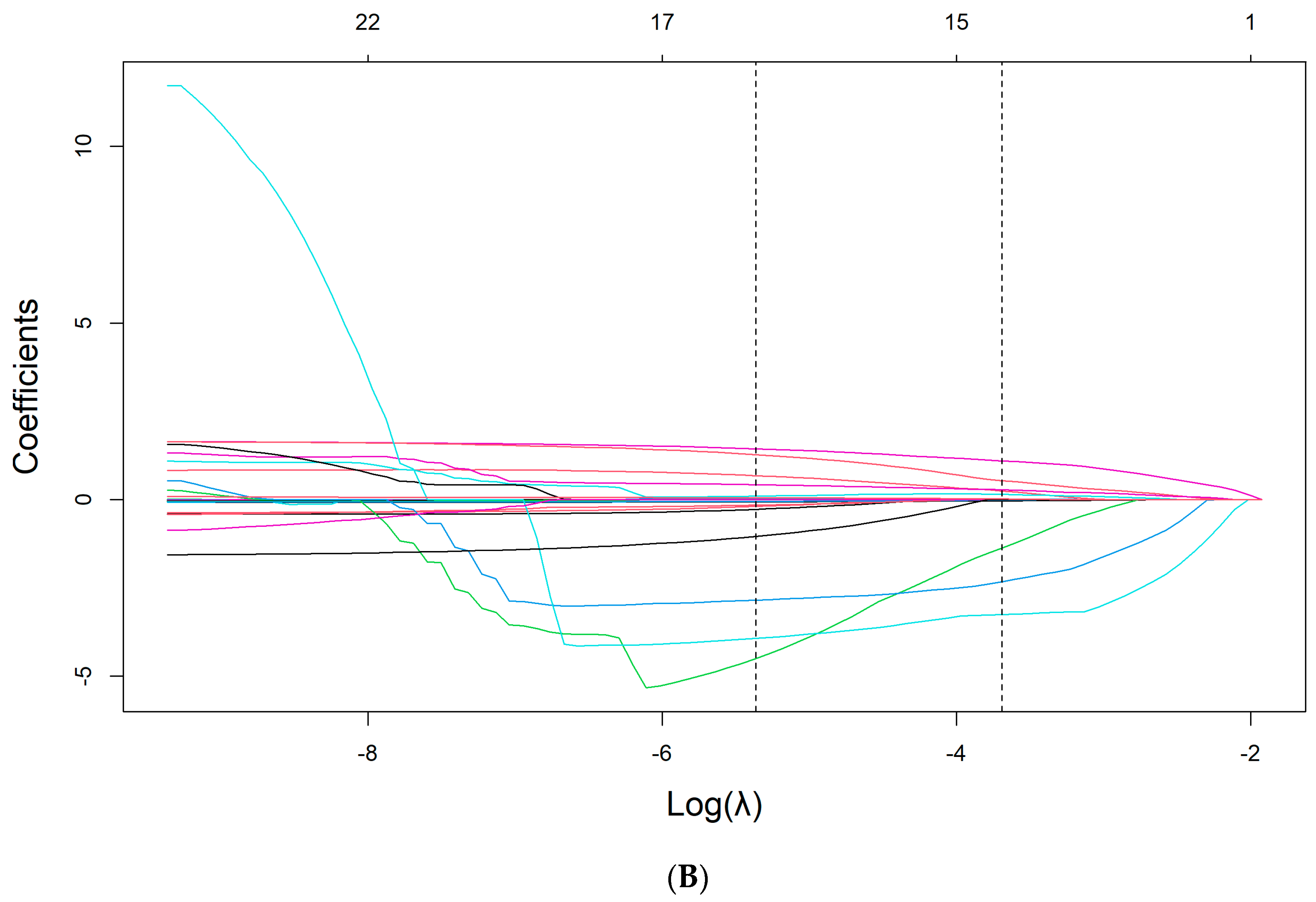
2.5. Derivation of the Model
2.6. Prediction Model Performance in the Derivation Set
2.7. Internal Validation of the Model
3. Results
3.1. Baseline Profiles
3.2. Aortic Geometry
3.3. Feature Selection
3.4. Prediction Model for TBAD
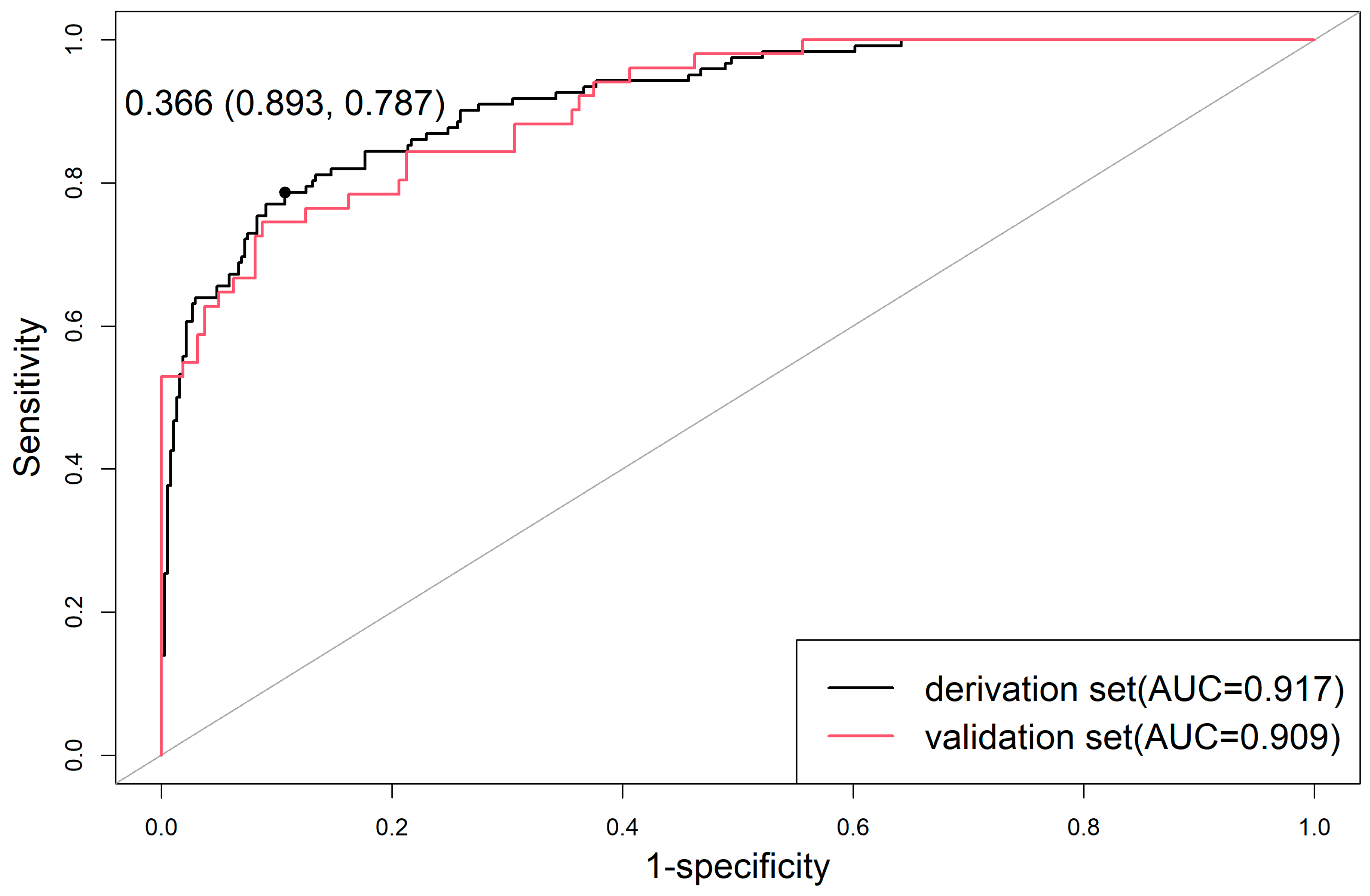
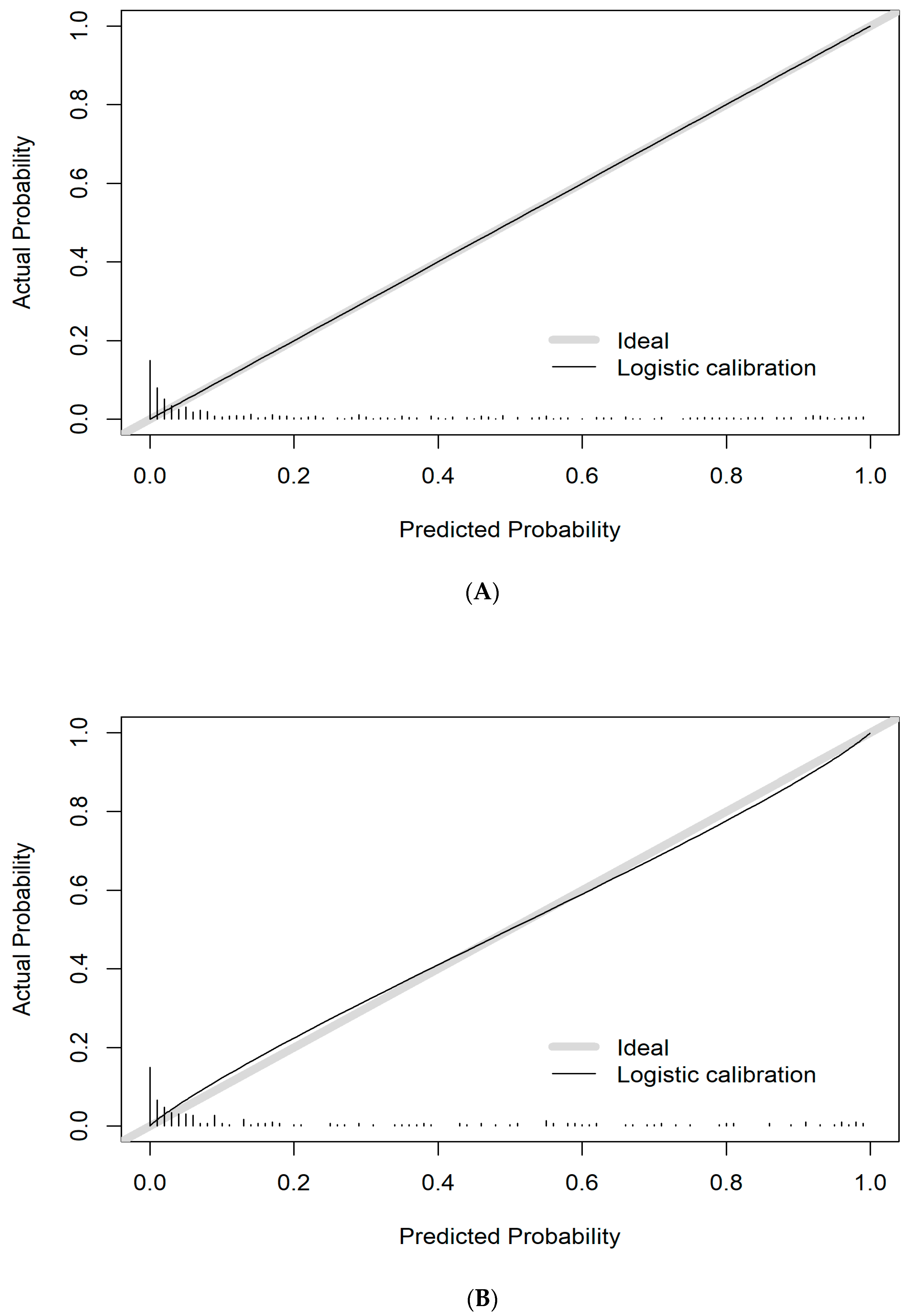
3.5. Performance of the Prediction Model for TBAD
3.6. Evaluation of Model Prediction Ability
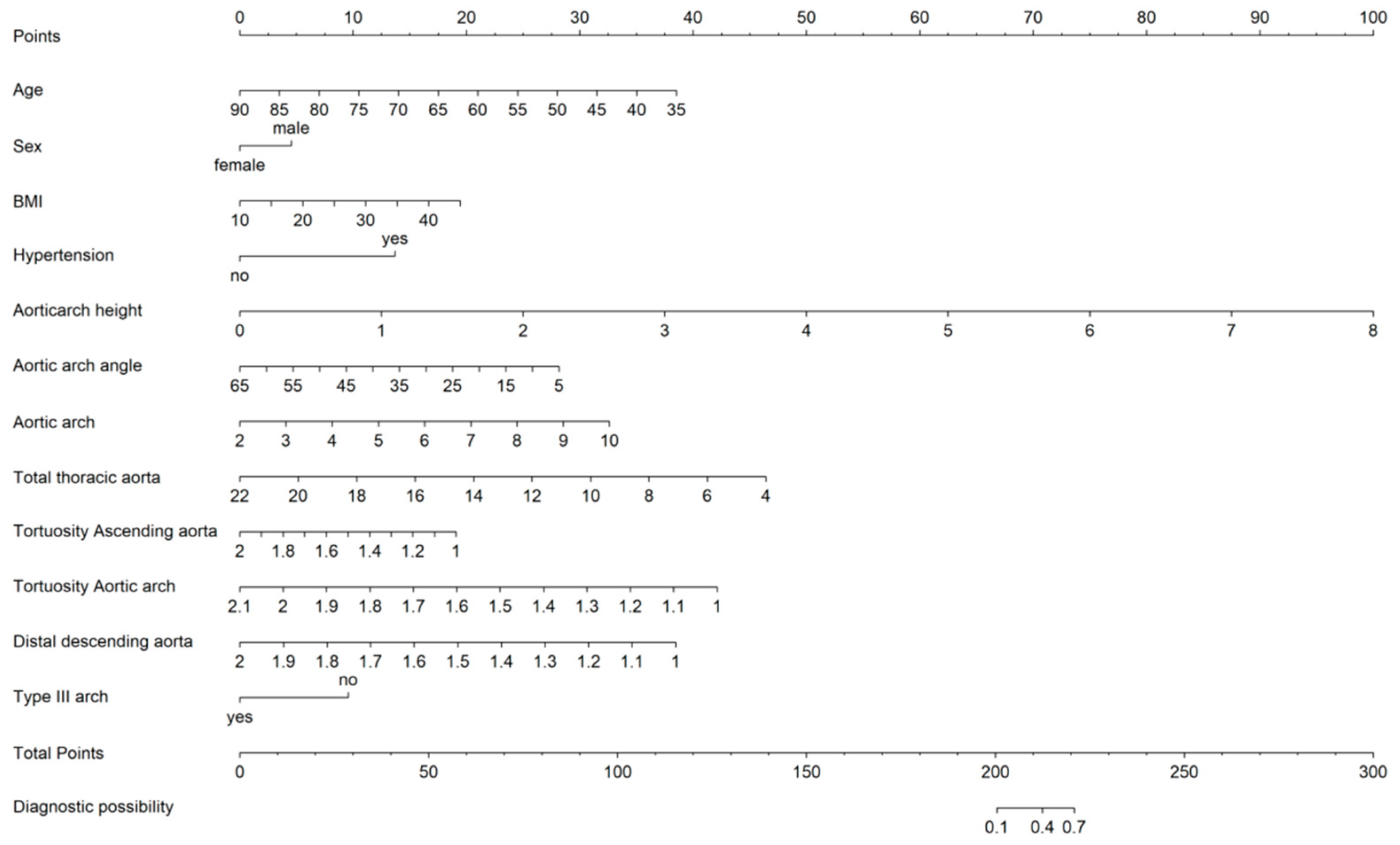
3.7. Clinical Use
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakkar, D.; Dake, M.D. Management of Type B Aortic Dissections: Treatment of Acute Dissections and Acute Complications from Chronic Dissections. Tech. Vasc. Interv. Radiol. 2018, 21, 124–130. [Google Scholar] [CrossRef]
- Riambau, V.; Böckler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G.; Czerny, M.; Fraedrich, G.; Haulon, S.; Jacobs, M.J.; et al. Editor’s Choice—Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef]
- Reutersberg, B.; Salvermoser, M.; Trenner, M.; Geisbüsch, S.; Zimmermann, A.; Eckstein, H.H.; Kuehnl, A. Hospital Incidence and In-Hospital Mortality of Surgically and Interventionally Treated Aortic Dissections: Secondary Data Analysis of the Nationwide German Diagnosis-Related Group Statistics from 2006 to 2014. J. Am. Heart Assoc. 2019, 8, e11402. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Nagao, K.; Hasegawa, S.; Kawashima, M.; Tsujimoto, T.; Izumi, S.; Matsumori, M.; Murakami, H.; Honda, T.; Mukohara, N. Fatal Complications of New-Onset Complicated Type B Aortic Dissection After Endovascular Abdominal Aortic Aneurysm Repair: Report of 2 Cases and Literature Review. Vasc. Endovasc. Surg. 2019, 53, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; Eagle, K.A. Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat. Rev. Cardiol. 2021, 18, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Heuts, S.; Adriaans, B.P.; Gerretsen, S.; Natour, E.; Vos, R.; Cheriex, E.C.; Crijns, H.J.G.M.; E Wildberger, J.; Maessen, J.G.; Schalla, S.; et al. Aortic elongation part II: The risk of acute type A aortic dissection. Heart 2018, 104, 1778–1782. [Google Scholar] [CrossRef]
- Higashigaito, K.; Sailer, A.M.; van Kuijk, S.; Willemink, M.J.; Hahn, J.D.; Hastie, T.J.; Miller, D.C.; Fischbein, M.P.; Fleischmann, D. Aortic growth and development of partial false lumen thrombosis are associated with late TBADverse events in type B aortic dissection. J. Thorac. Cardiovasc. Surg. 2021, 161, 1184–1190. [Google Scholar] [CrossRef]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; Kouchoukos, N.T.; Lytle, B.W.; Milewicz, D.M.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J. Am. Coll. Cardiol. 2010, 55, e27–e129. [Google Scholar] [CrossRef]
- Linda, A.P.; Thomas, T.T.; Eric, M.I. Aortic diameter ≥ 5.5 cm is not a good predictor of type a aortic dissection: Observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007, 116, 1120–1127. [Google Scholar]
- Qiu, P.; Liu, J.; Chen, Y.; Zha, B.; Ye, K.; Qin, J.; Hao, P.; Kang, J.; Zhang, C.; Zhu, H.; et al. Changes in aortic arch geometry and the risk for Stanford B dissection. J. Thorac. Dis. 2020, 12, 7193. [Google Scholar] [CrossRef]
- Komutrattananont, P.; Mahakkanukrauh, P.; Das, S. Morphology of the human aorta and age-related changes: Anatomical facts. Anat. Cell Biol. 2019, 52, 109–114. [Google Scholar] [CrossRef]
- Redheuil, A.; Yu, W.C.; Mousseaux, E.; Harouni, A.A.; Kachenoura, N.; Wu, C.O.; Bluemke, D.; Lima, J.A. Age-related changes in aortic arch geometry: Relationship with proximal aortic function and left ventricular mass and remodeling. J. Am. Coll. Cardiol. 2011, 58, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Adriaans, B.P.; Heuts, S.; Gerretsen, S.; Cheriex, E.C.; Vos, R.; Natour, E.; Maessen, J.G.; Nia, P.S.; Crijns, H.J.; Wildberger, J.E.; et al. Aortic elongation part I: The normal aortic ageing process. Heart 2018, 104, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Q.; Liang, C.-H.; He, L.; Tian, J.; Liang, C.-S.; Chen, X.; Ma, Z.-L.; Liu, Z.-Y. Development and Validation of a RTBADiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J. Clin. Oncol. 2016, 34, 2157–2164. [Google Scholar] [CrossRef]
- Debray, T.P.; Collins, G.S.; Riley, R.D.; Snell, K.I.; Van Calster, B.; Reitsma, J.B.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar]
- Kramer, A.A.; Zimmerman, J.E. Assessing the calibration of mortality benchmarks in critical care: The Hosmer–Lemeshow test revisited. Crit. Care Med. 2007, 35, 2052–2056. [Google Scholar] [CrossRef]
- Howard, D.P.; Banerjee, A.; Fairhead, J.F.; Perkins, J.; Silver, L.E.; Rothwell, P.M. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013, 127, 2031–2037. [Google Scholar] [CrossRef]
- De Martino, A.; Morganti, R.; Falcetta, G.; Scioti, G.; Milano, A.D.; Pucci, A.; Bortolotti, U. Acute aortic dissection and pregnancy: Review and meta-analysis of incidence, presentation, and pathologic substrates. J. Card. Surg. 2019, 34, 1591–1597. [Google Scholar] [CrossRef]
- Akutsu, K. Etiology of aortic dissection. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 271–276. [Google Scholar] [CrossRef]
- Adriaans, B.P.; Wildberger, J.E.; Westenberg, J.J.; Lamb, H.J.; Schalla, S. Predictive imaging for thoracic aortic dissection and rupture: Moving beyond diameters. Eur. Radiol. 2019, 29, 6396–6404. [Google Scholar] [CrossRef]
- Poullis, M.P.; Warwick, R.; Oo, A.; Poole, R.J. Ascending aortic curvature as an independent risk factor for type A dissection, and ascending aortic aneurysm formation: A mathematical model. Eur. J. Cardiothorac. Surg. 2008, 33, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Elefteriades, J.A. Natural history of thoracic aortic aneurysms: Indications for surgery, and surgical versus nonsurgical risks. Ann. Thorac. Surg. 2002, 74, S1877–S1880; discussion S1892–S1898. [Google Scholar] [CrossRef]
- Ohyama, Y.; Redheuil, A.; Kachenoura, N.; Ambale Venkatesh, B.; Lima, J.A. Imaging Insights on the Aorta in Aging. Circ. Cardiovasc. Imaging 2018, 11, e005617. [Google Scholar] [CrossRef] [PubMed]
- Writing Group Members; Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey Jr, D.E.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010, 121, e266–e369. [Google Scholar] [PubMed]
- Waterford, S.D.; Di Eusanio, M.; Ehrlich, M.P.; Reece, T.B.; Desai, N.D.; Sundt, T.M.; Myrmel, T.; Gleason, T.G.; Forteza, A.; de Vincentiis, C.; et al. Postoperative myocardial infarction in acute type A aortic dissection: A report from the International Registry of Acute Aortic Dissection. J. Thorac. Cardiovasc. Surg. 2017, 153, 521–527. [Google Scholar] [CrossRef]
- Mehta, R.H.; Suzuki, T.; Hagan, P.G.; Bossone, E.; Gilon, D.; Llovet, A.; Maroto, L.C.; Cooper, J.V.; Smith, D.E.; Armstrong, W.F.; et al. Predicting death in patients with acute type A aortic dissection. Circulation 2002, 105, 200–206. [Google Scholar] [CrossRef]
- Tolenaar, J.L.; Froehlich, W.; Jonker, F.H.; Upchurch, R.G., Jr.; Rampoldi, V.; Tsai, T.T.; Bossone, E.; Evangelista, A.; O’Gara, P.; Pape, L.; et al. Predicting in-hospital mortality in acute type B aortic dissection: Evidence from International Registry of Acute Aortic Dissection. Circulation 2014, 130 (Suppl. S1), S45–S50. [Google Scholar] [CrossRef]
- Guo, T.; Fang, Z.; Yang, G.; Zhou, Y.; Ding, N.; Peng, W.; Gong, X.; He, H.; Pan, X.; Chai, X. Machine learning models for predicting in-hospital mortality in acute aortic dissection patients. Front. Cardiovasc. Med. 2021, 8, 727773. [Google Scholar] [CrossRef]
| Studies n = 173 | Controls n = 534 | p | |
|---|---|---|---|
| Male (n, %) | 142 (82.08) | 312 (58.43) | <0.001 |
| Age (y) | 60.23 ± 12.33 | 65.24 ± 12.21 | <0.001 |
| BMI (kg/m2) | 25.22 ± 3.55 | 23.52 ± 3.83 | <0.001 |
| BSA (m2) | 1.73 ± 0.14 | 1.66 ± 0.16 | <0.001 |
| Hypertension (n, %) | 134 (77.46) | 198 (37.08) | <0.001 |
| Diabetes (n, %) | 37 (21.39) | 84 (15.73) | 0.109 |
| Smoking (n, %) | 61 (35.26) | 169 (31.65) | 0.431 |
| All n = 707 | Derivation Set n = 496 | Validation Set n = 211 | p | |
|---|---|---|---|---|
| Male (n, %) | 454 (64.21) | 323 (65.12) | 131 (62.09) | 0.494 |
| Age (y) | 64.01 ± 12.42 | 63.86 ± 12.43 | 64.36 ± 12.42 | 0.628 |
| BMI (kg/m2) | 23.94 ± 3.83 | 24.01 ± 3.85 | 23.77 ± 3.79 | 0.460 |
| BSA (m2) | 1.68 ± 0.16 | 1.68 ± 0.16 | 1.66 ± 0.16 | 0.145 |
| Hypertension (n, %) | 332 (46.96) | 234 (47.18) | 98 (46.45) | 0.924 |
| Diabetes (n, %) | 121 (17.11) | 88 (17.74) | 33 (15.64) | 0.569 |
| Smoking (n, %) | 230 (32.53) | 161 (32.46) | 69 (32.7) | 1.000 |
| Studies n = 173 | Controls n = 534 | p | |
|---|---|---|---|
| Length | |||
| Ascending aorta | 62.49 ± 11.25 | 61.8 ± 11.09 | 0.480 |
| Aortic arch | 27.68 ± 6.81 | 25.54 ± 7.47 | 0.001 |
| Proximal descending aorta | 74.92 ± 16.83 | 68.14 ± 15.67 | <0.001 |
| Distal descending aorta | 140.6 ± 19.81 | 152.05 ± 23.44 | <0.001 |
| Total thoracic aorta | 305.69 ± 33.63 | 307.53 ± 38.04 | 0.437 |
| Aortic arch height, mm | 2.49 ± 1.04 | 1.84 ± 0.89 | <0.001 |
| Aortic arch width, mm | 8.09 ± 1.63 | 7.2 ± 1.40 | <0.001 |
| Aortic arch angle | 31.78 ± 10.78 | 33.62 ± 9.40 | 0.045 |
| Distance | |||
| Ascending aorta | 7.24 ± 1.17 | 6.70 ± 0.95 | <0.001 |
| Aortic arch | 6.16 ± 1.29 | 5.27 ± 1.07 | <0.001 |
| Distal descending aorta | 12.23 ± 1.49 | 12.27 ± 1.59 | 0.801 |
| Total thoracic aorta | 11.40 ± 1.49 | 11.63 ± 1.78 | 0.240 |
| Tortuosity | |||
| Ascending aorta | 1.25 ± 0.10 | 1.31 ± 0.13 | <0.001 |
| Aortic arch | 1.22 ± 0.12 | 1.29 ± 0.15 | <0.001 |
| Distal descending aorta | 1.15 ± 0.10 | 1.24 ± 0.14 | <0.001 |
| Total thoracic aorta | 2.72 ± 0.45 | 2.69 ± 0.48 | 0.442 |
| Type III arch (n, %) | 38 (21.97) | 98 (18.35) | 0.349 |
| All n = 707 | Derivation Set n = 496 | Validation Set n = 211 | p | |
|---|---|---|---|---|
| Length | ||||
| Ascending aorta | 61.97 ± 11.12 | 62.2 ± 11.25 | 61.41 ± 10.82 | 0.978 |
| Aortic arch | 26.06 ± 7.37 | 25.88 ± 7.59 | 26.50 ± 6.82 | 0.282 |
| Proximal descending aorta | 69.80 ± 16.21 | 69.73 ± 16.11 | 69.96 ± 16.48 | 0.868 |
| Distal descending aorta | 149.25 ± 23.12 | 149.75 ± 23.43 | 148.07 ± 22.38 | 0.369 |
| Total thoracic aorta | 307.08 ± 36.99 | 307.57 ± 37.34 | 305.94 ± 36.21 | 0.590 |
| Aortic arch height, mm | 2.00 ± 0.97 | 2.02 ± 1.00 | 1.97 ± 0.89 | 0.526 |
| Aortic arch width, mm | 7.42 ± 1.51 | 7.44 ± 1.51 | 7.37 ± 1.51 | 0.585 |
| Aortic arch angle | 33.17 ± 9.78 | 32.97 ± 9.91 | 33.63 ± 9.48 | 0.406 |
| Distance | ||||
| Ascending aorta | 6.83 ± 1.03 | 6.83 ± 1.06 | 6.83 ± 0.97 | 0.911 |
| Aortic arch | 5.49 ± 1.19 | 5.49 ± 1.19 | 5.47 ± 1.20 | 0.833 |
| Distal descending aorta | 12.26 ± 1.57 | 12.32 ± 1.60 | 12.11 ± 1.50 | 0.096 |
| Total thoracic aorta | 11.58 ± 1.71 | 11.67 ± 1.74 | 11.35 ± 1.63 | 0.054 |
| Tortuosity | ||||
| Ascending aorta | 1.29 ± 0.13 | 1.29 ± 0.14 | 1.29 ± 0.11 | 0.790 |
| Aortic arch | 1.28 ± 0.15 | 1.27 ± 0.15 | 1.28 ± 0.15 | 0572 |
| Distal descending aorta | 1.22 ± 0.14 | 1.22 ± 0.14 | 1.23 ± 0.13 | 0.493 |
| Total thoracic aorta | 2.7 ± 0.47 | 2.68 ± 0.46 | 2.75 ± 0.51 | 0.108 |
| Type III arch | 136 (19.24) | 96 (19.35) | 40 (18.96) |
| Variables | Estimate | Std. Error | Z Value | p Value | OR | 95% CI | VIF | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Age | −0.075 | 0.015 | −4.857 | 0.000 | 0.928 | 0.899 | 0.956 | 1.75 |
| Sex (Male) | 0.839 | 0.364 | 2.307 | 0.021 | 2.314 | 1.147 | 4.797 | 1.17 |
| BMI | 0.070 | 0.044 | 1.604 | 0.109 | 1.072 | 0.985 | 1.168 | 1.11 |
| Hypertension | 1.533 | 0.325 | 4.712 | 0.000 | 4.631 | 2.483 | 8.929 | 1.24 |
| Aortic arch height, mm | 1.698 | 0.283 | 6.005 | 0.000 | 5.461 | 3.213 | 9.760 | 3.56 |
| Aortic arch angle | −0.070 | 0.023 | −3.005 | 0.003 | 0.933 | 0.890 | 0.975 | 2.12 |
| Distance of aortic arch | 0.495 | 0.159 | 3.112 | 0.002 | 1.640 | 1.208 | 2.260 | 1.52 |
| Distance of total thoracic aorta | −0.299 | 0.103 | −2.911 | 0.004 | 0.741 | 0.603 | 0.904 | 1.29 |
| Tortuosity of ascending aorta | −5.453 | 1.646 | −3.312 | 0.001 | 0.004 | 0.000 | 0.094 | 1.17 |
| Tortuosity of aortic arch | −3.253 | 1.376 | −2.364 | 0.018 | 0.039 | 0.002 | 0.529 | 1.28 |
| Tortuosity of distal descending aorta | −5.056 | 1.563 | −3.235 | 0.001 | 0.006 | 0.000 | 0.122 | 1.09 |
| Type III arch (n, %) | −1.513 | 0.545 | −2.775 | 0.006 | 0.220 | 0.073 | 0.626 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Huang, S.; Zhao, D.; Qiu, P.; Hu, J.; Liu, X.; Lu, X.; Feng, L.; Hu, M.; Cheng, Y. Establishing and Validating a Morphological Prediction Model Based on CTA to Evaluate the Incidence of Type-B Dissection. Diagnostics 2023, 13, 3130. https://doi.org/10.3390/diagnostics13193130
Fu Y, Huang S, Zhao D, Qiu P, Hu J, Liu X, Lu X, Feng L, Hu M, Cheng Y. Establishing and Validating a Morphological Prediction Model Based on CTA to Evaluate the Incidence of Type-B Dissection. Diagnostics. 2023; 13(19):3130. https://doi.org/10.3390/diagnostics13193130
Chicago/Turabian StyleFu, Yan, Siyi Huang, Deyin Zhao, Peng Qiu, Jiateng Hu, Xiaobing Liu, Xinwu Lu, Lvfan Feng, Min Hu, and Yong Cheng. 2023. "Establishing and Validating a Morphological Prediction Model Based on CTA to Evaluate the Incidence of Type-B Dissection" Diagnostics 13, no. 19: 3130. https://doi.org/10.3390/diagnostics13193130
APA StyleFu, Y., Huang, S., Zhao, D., Qiu, P., Hu, J., Liu, X., Lu, X., Feng, L., Hu, M., & Cheng, Y. (2023). Establishing and Validating a Morphological Prediction Model Based on CTA to Evaluate the Incidence of Type-B Dissection. Diagnostics, 13(19), 3130. https://doi.org/10.3390/diagnostics13193130





