Acute Coronary Syndrome with Non-Obstructive Plaque on Angiography and Features of Vulnerable Plaque on Intracoronary Optical Coherence Tomography
Abstract
1. Introduction
2. Objective
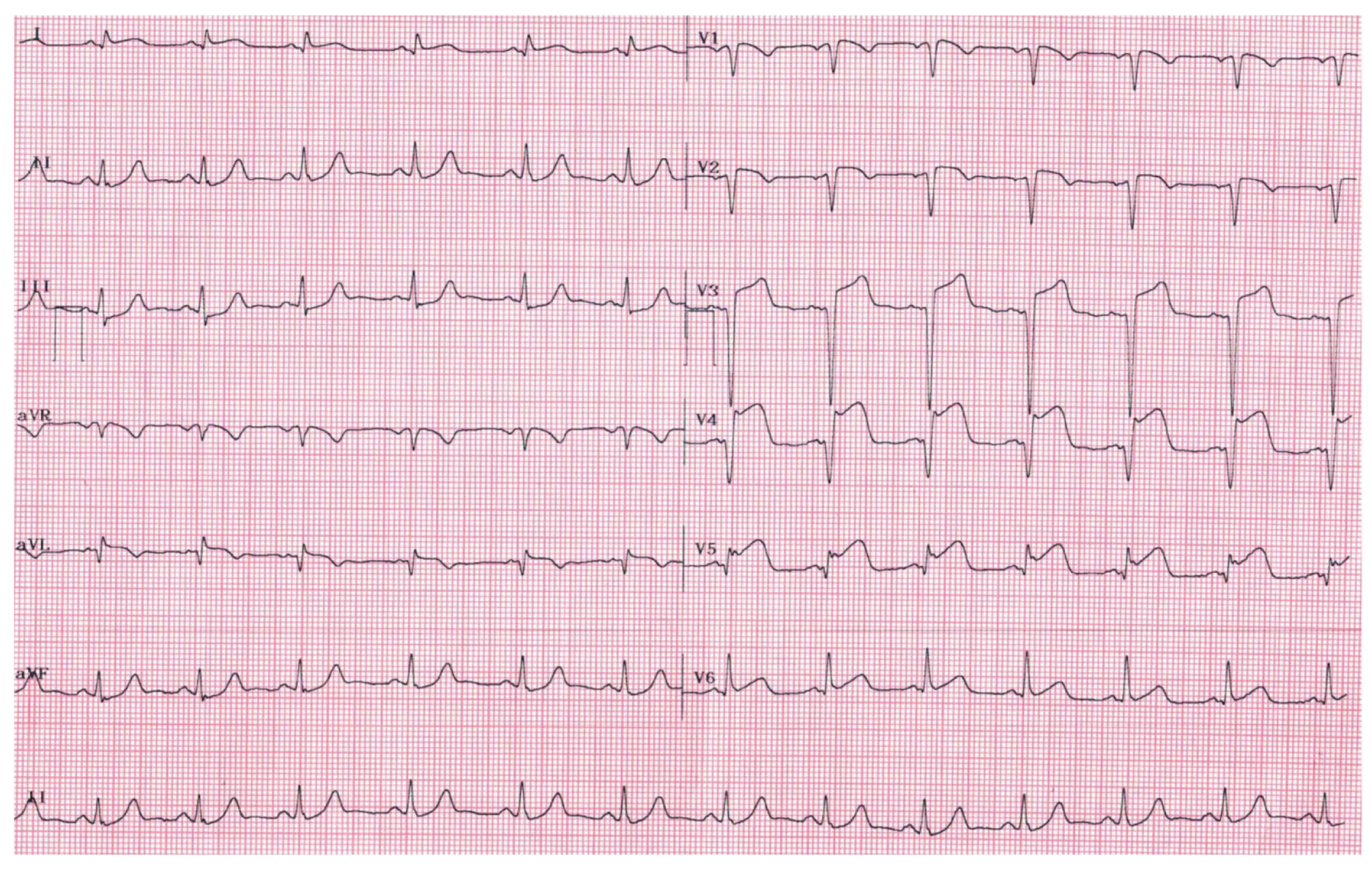
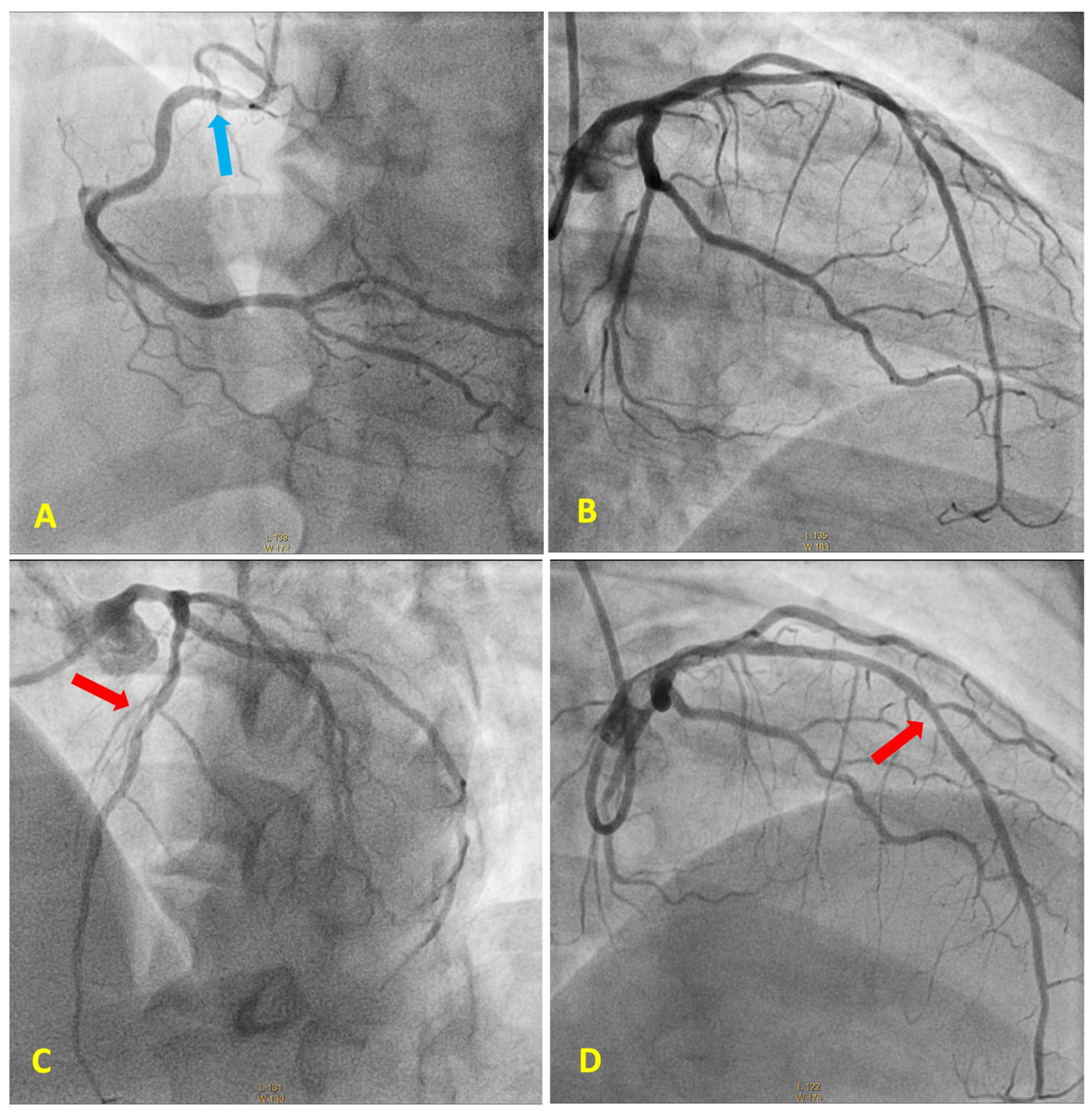
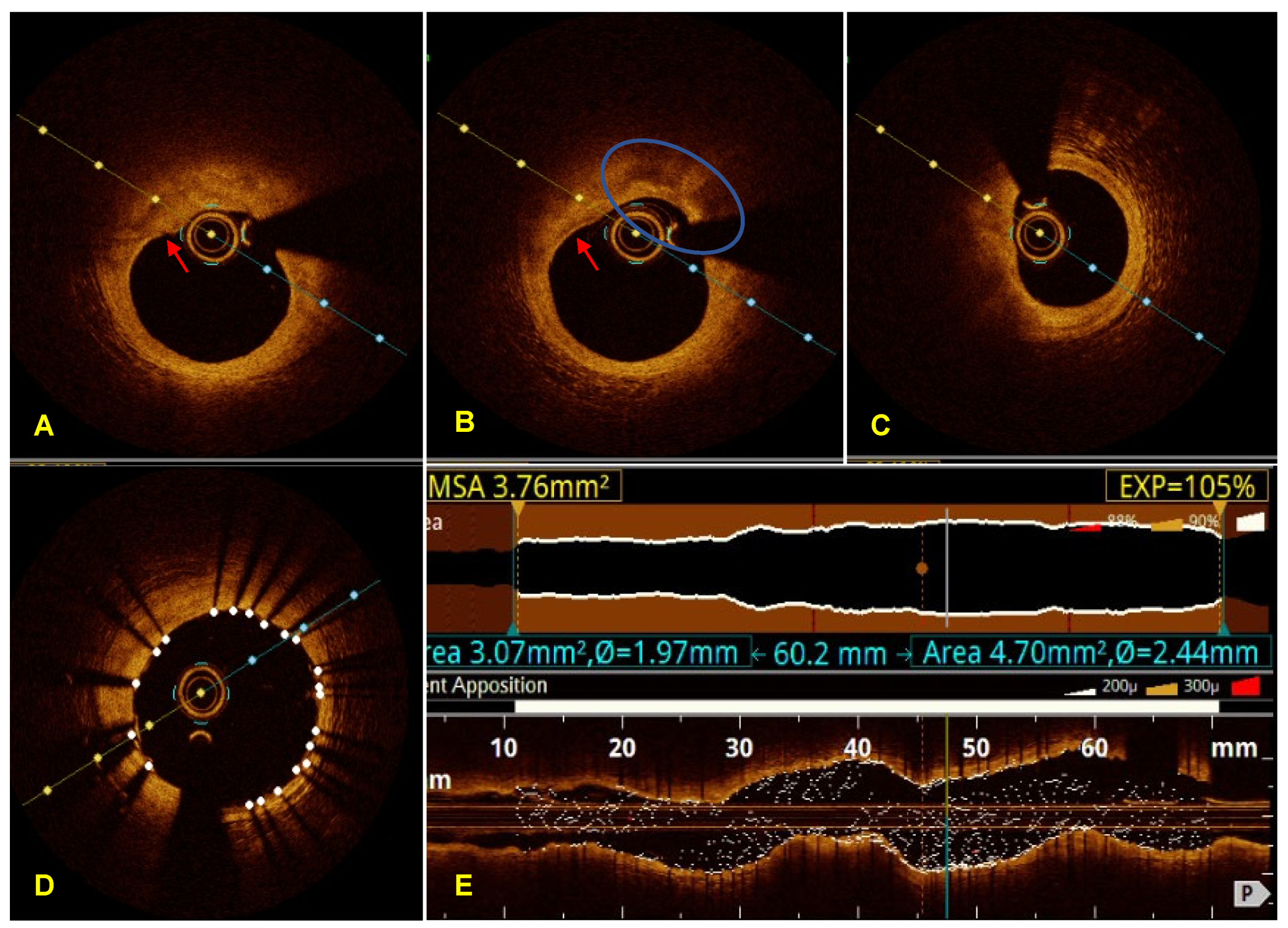
3. Case Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vedanthan, R.; Seligman, B.; Fuster, V. Global perspective on acute coronary syndrome: A burden on the young and poor. Circ. Res. 2014, 114, 1959–1975. [Google Scholar] [CrossRef] [PubMed]
- Gach, O.; El, H.Z.; Lancellotti, P. Acute coronary syndrome. Rev. Med. Liège 2018, 73, 243–250. [Google Scholar] [PubMed]
- Smith, J.N.; Negrelli, J.M.; Manek, M.B.; Hawes, E.M.; Viera, A.J. Diagnosis and management of acute coronary syndrome: An evidence-based update. J. Am. Board Fam. Med. 2015, 28, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Shrivastava, A.; Vijayvergiya, R.; Chhikara, S.; Datta, R.; Aziz, A.; Singh Meena, D.; Nath, R.K.; Kumar, J.R. Optical coherence tomography: An eye into the coronary artery. Front. Cardiovasc. Med. 2022, 9, 854554. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.Y.; Lee, S.J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J.; et al. Intravascular imaging-guided or angiography-guided complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef]
- Casella, G.; Klauss, V.; Ottani, F.; Siebert, U.; Sangiorgio, P.; Bracchetti, D. Impact of intravascular ultrasound-guided stenting on long-term clinical outcome: A meta-analysis of available studies comparing intravascular ultrasound-guided and angiographically guided stenting. Catheter. Cardiovasc. Interv. 2003, 59, 314–321. [Google Scholar] [CrossRef]
- Chieffo, A.; Latib, A.; Caussin, C.; Presbitero, P.; Galli, S.; Menozzi, A.; Varbella, F.; Mauri, F.; Valgimigli, M.; Arampatzis, C.; et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: The AVIO trial. Am. Heart J. 2013, 165, 65–72. [Google Scholar] [CrossRef]
- Ali, Z.A.; Maehara, A.; Généreux, P.; Shlofmitz, R.A.; Fabbiocchi, F.; Nazif, T.M.; Guagliumi, G.; Meraj, P.M.; Alfonso, F.; Samady, H.; et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): A randomised controlled trial. Lancet 2016, 388, 2618–2628. [Google Scholar] [CrossRef]
- Ali, Z.A.; Karimi Galougahi, K.; Mintz, G.S.; Maehara, A.; Shlofmitz, R.A.; Mattesini, A. Intracoronary optical coherence tomography: State of the art and future directions. EuroIntervention 2021, 17, e105–e123. [Google Scholar] [CrossRef]
- Johnson, T.W.; Räber, L.; Di Mario, C.; Bourantas, C.V.; Jia, H.; Mattesini, A.; Gonzalo, N.; de la Torre Hernandez, J.M.; Prati, F.; Koskinas, K.C.; et al. Clinical use of intracoronary imaging. Part 2: Acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 2019, 15, 434–451. [Google Scholar] [CrossRef]
- Muller, J.E.; Tofler, G.H.; Stone, P.H. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 1989, 79, 733–743. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.M.; Garcia-Garcia, H.M.; de Boer, S.P.; Kardys, I.; Heo, J.H.; Akkerhuis, K.M.; Oemrawsingh, R.M.; van Domburg, R.T.; Ligthart, J.; Witberg, K.T.; et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Eur. Heart J. 2014, 35, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Erlinge, D.; Maehara, A.; Ben-Yehuda, O.; Bøtker, H.E.; Maeng, M.; Kjøller-Hansen, L.; Engstrøm, T.; Matsumura, M.; Crowley, A.; Dressler, O.; et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (Prospect II): A prospective natural history study. Lancet 2021, 397, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Ali, Z.A.; Held, C.; Matsumura, M.; Kjøller-Hansen, L.; Bøtker, H.E.; Maeng, M.; Engstrøm, T.; Wiseth, R.; et al. Percutaneous coronary intervention for vulnerable coronary atherosclerotic plaque. J. Am. Coll. Cardiol. 2020, 76, 2289–2301. [Google Scholar] [CrossRef]
- Kedhi, E.; Berta, B.; Roleder, T.; Hermanides, R.S.; Fabris, E.; IJsselmuiden, A.J.J.; Kauer, F.; Alfonso, F.; von Birgelen, C.; Escaned, J.; et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: The Combine OCT–FFR trial. Eur. Heart J. 2021, 42, 4671–4679. [Google Scholar] [CrossRef]
- Prati, F.; Romagnoli, E.; Gatto, L.; La Manna, A.; Burzotta, F.; Ozaki, Y.; Marco, V.; Boi, A.; Fineschi, M.; Fabbiocchi, F.; et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: The CLIMA study. Eur. Heart J. 2020, 41, 383–391. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Iannaccone, M.; De Filippo, O.; Leone, A.M.; Niccoli, G.; Zilio, F.; Ugo, F.; Cerrato, E.; Fineschi, M.; Mancone, M.; et al. Optical coherence tomography compared with fractional flow reserve guided approach in acute coronary syndromes: A propensity matched analysis. Int. J. Cardiol. 2017, 244, 5458. [Google Scholar] [CrossRef]
- Hakeem, A.; Edupuganti, M.M.; Almomani, A.; Pothineni, N.V.; Payne, J.; Abualsuod, A.M.; Bhatti, S.; Ahmed, Z.; Uretsky, B.F. Long-term prognosis of deferred acute coronary syndrome lesions based on nonischemic fractional flow reserve. J. Am. Coll. Cardiol. 2016, 68, 118191. [Google Scholar] [CrossRef]
- Masrani Mehta, S.; Depta, J.P.; Novak, E.; Patel, J.S.; Patel, Y.; Raymer, D.; Facey, G.; Zajarias, A.; Lasala, J.M.; Singh, J.; et al. Association of lower fractional flow reserve values with higher risk of adverse cardiac events for lesions deferred revascularization among patients with acute coronary syndrome. J. Am. Heart Assoc. 2015, 4, e002172. [Google Scholar] [CrossRef] [PubMed]
- Wa, M.E.L.K.; De Silva, K.; Collet, C.; Perera, D. FLOWER-MI and the root of the problem with non-culprit revascularisation. Open Heart 2021, 8, e001763. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Maehara, A.; Kwong, R.Y.; Sedlak, T.; Saw, J.; Smilowitz, N.R.; Mahmud, E.; Wei, J.; Marzo, K.; Matsumura, M.; et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation 2021, 143, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Narula, J. Emergence of plaque erosion as an important clinical entity. JACC Cardiovasc. Imaging 2015, 8, 623625. [Google Scholar] [CrossRef][Green Version]
- Holmes, D.R., Jr.; Lerman, A.; Moreno, P.R.; King, S.B.; Sharma, S.K. Diagnosis and management of STEMI arising from plaque erosion. JACC Cardiovasc. Imaging 2013, 6, 290296. [Google Scholar] [CrossRef][Green Version]
- Partida, R.A.; Libby, P.; Crea, F.; Jang, I.-K. Plaque erosion: A new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur. Heart J. 2018, 39, 20702076. [Google Scholar] [CrossRef]
- Jia, H.; Dai, J.; Hou, J.; Xing, L.; Ma, L.; Liu, H.; Xu, M.; Yao, Y.; Hu, S.; Yamamoto, E.; et al. Effective anti-thrombotic therapy without stenting: Intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur. Heart J. 2017, 38, 792800. [Google Scholar] [CrossRef]
- van Nunen, L.X.; Zimmermann, F.M.; Tonino, P.A.; Barbato, E.; Baumbach, A.; Engstrøm, T.; Klauss, V.; MacCarthy, P.A.; Manoharan, G.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet 2015, 386, 18531860. [Google Scholar] [CrossRef]
- Subban, V.; Raffel, O.C. Optical coherence tomography: Fundamentals and clinical utility. Cardiovasc. Diagn. Ther. 2020, 10, 13891414. [Google Scholar] [CrossRef] [PubMed]
- Sörensson, P.; Ekenbäck, C.; Lundin, M.; Agewall, S.; Brolin, E.B.; Caidahl, K.; Cederlund, K.; Collste, O.; Daniel, M.; Jensen, J.; et al. Early comprehensive cardiovascular magnetic resonance imaging in patients with myocardial infarction with nonobstructive coronary arteries. JACC Cardiovasc. Imaging 2021, 14, 17741783. [Google Scholar] [CrossRef] [PubMed]
- Mileva, N.; Paolisso, P.; Gallinoro, E.; Fabbricatore, D.; Munhoz, D.; Bergamaschi, L.; Belmonte, M.; Panayotov, P.; Pizzi, C.; Barbato, E.; et al. Diagnostic and prognostic role of cardiac magnetic resonance in MINOCA: Systematic review and meta-analysis. JACC Cardiovasc. Imaging 2023, 16, 376389. [Google Scholar] [CrossRef] [PubMed]
- Parwani, P.; Kang, N.; Safaeipour, M.; Mamas, M.A.; Wei, J.; Gulati, M.; Naidu, S.S.; Merz, N.B. Contemporary diagnosis and management of patients with MINOCA. Curr. Cardiol. Rep. 2023, 25, 561570. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dall’Orto, C.C.; Ferreira Lopes, R.P.; Eurípedes, L.V.; Pinto Filho, G.V.; da Silva, M.R. Acute Coronary Syndrome with Non-Obstructive Plaque on Angiography and Features of Vulnerable Plaque on Intracoronary Optical Coherence Tomography. Diagnostics 2023, 13, 3118. https://doi.org/10.3390/diagnostics13193118
Dall’Orto CC, Ferreira Lopes RP, Eurípedes LV, Pinto Filho GV, da Silva MR. Acute Coronary Syndrome with Non-Obstructive Plaque on Angiography and Features of Vulnerable Plaque on Intracoronary Optical Coherence Tomography. Diagnostics. 2023; 13(19):3118. https://doi.org/10.3390/diagnostics13193118
Chicago/Turabian StyleDall’Orto, Clarissa Campo, Rubens Pierry Ferreira Lopes, Lara Vilela Eurípedes, Gilvan Vilella Pinto Filho, and Marcos Raphael da Silva. 2023. "Acute Coronary Syndrome with Non-Obstructive Plaque on Angiography and Features of Vulnerable Plaque on Intracoronary Optical Coherence Tomography" Diagnostics 13, no. 19: 3118. https://doi.org/10.3390/diagnostics13193118
APA StyleDall’Orto, C. C., Ferreira Lopes, R. P., Eurípedes, L. V., Pinto Filho, G. V., & da Silva, M. R. (2023). Acute Coronary Syndrome with Non-Obstructive Plaque on Angiography and Features of Vulnerable Plaque on Intracoronary Optical Coherence Tomography. Diagnostics, 13(19), 3118. https://doi.org/10.3390/diagnostics13193118





