Indeterminate Thyroid Nodules: From Cytology to Molecular Testing
Abstract
:1. Introduction
2. Cytological Classification Systems
3. Biomarkers in Indeterminate Thyroid Nodules
4. Molecular Testing and Indeterminate Thyroid Nodules
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaccarella, S.; Dal Maso, L. Challenges in Investigating Risk Factors for Thyroid Cancer. Lancet Diabetes Endocrinol. 2021, 9, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Vigneri, R.; Malandrino, P.; Vigneri, P. The Changing Epidemiology of Thyroid Cancer: Why Is Incidence Increasing? Curr. Opin. Oncol. 2015, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Celletti, I.; Fresilli, D.; De Vito, C.; Bononi, M.; Cardaccio, S.; Cozzolino, A.; Durante, C.; Grani, G.; Grimaldi, G.; Isidori, A.M.; et al. TIRADS, SRE and SWE in INDETERMINATE Thyroid Nodule Characterization: Which Has Better Diagnostic Performance? Radiol. Med. (Torino) 2021, 126, 1189–1200. [Google Scholar] [CrossRef]
- Nardi, F.; Basolo, F.; Crescenzi, A.; Fadda, G.; Frasoldati, A.; Orlandi, F.; Palombini, L.; Papini, E.; Zini, M.; Pontecorvi, A.; et al. Italian Consensus for the Classification and Reporting of Thyroid Cytology. J. Endocrinol. Investig. 2014, 37, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Z.; Baloch, Z.W.; Cochand-Priollet, B.; Schmitt, F.C.; Vielh, P.; VanderLaan, P.A. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023, 33, 9. [Google Scholar] [CrossRef]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer: American Thyroid Association Anaplastic Thyroid Cancer Guidelines Task Force. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef]
- Tastekin, E.; Canberk, Ş.; Schmitt, F.C. Follicular Growth Pattern Disease on Thyroid Fine-Needle Aspiration Biopsy. Balk. Med. J. 2022, 39, 230–238. [Google Scholar] [CrossRef]
- Rossi, E.D.; Pantanowitz, L.; Faquin, W.C. The Role of Molecular Testing for the Indeterminate Thyroid FNA. Genes 2019, 10, 736. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Trimboli, P.; Iorio, S.; Maiorino, M.I.; Longo, M.; Croce, L.; Pignatelli, M.F.; Ferrandes, S.; Cozzolino, I.; Montella, M.; et al. Repeat Thyroid FNAC: Inter-Observer Agreement among High- and Low-Volume Centers in Naples Metropolitan Area and Correlation with the EU-TIRADS. Front. Endocrinol. 2022, 13, 1001728. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, A.; Palermo, A.; Trimboli, P. Cancer Prevalence in the Subcategories of the Indeterminate Class III (AUS/FLUS) of the Bethesda System for Thyroid Cytology: A Meta-Analysis. J. Endocrinol. Investig. 2021, 44, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Crescenzi, A.; Giovanella, L. Performance of Italian Consensus for the Classification and Reporting of Thyroid Cytology (ICCRTC) in Discriminating Indeterminate Lesions at Low and High Risk of Malignancy. A Systematic Review and Meta-Analysis. Endocrine 2018, 60, 31–35. [Google Scholar] [CrossRef]
- Trimboli, P.; Ferrarazzo, G.; Cappelli, C.; Piccardo, A.; Castellana, M.; Barizzi, J. Thyroid Nodules with Indeterminate FNAC According to the Italian Classification System: Prevalence, Rate of Operation, and Impact on Risk of Malignancy. An Updated Systematic Review and Meta-Analysis. Endocr. Pathol. 2022, 33, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, L.; Poma, A.M.; Macerola, E.; Rago, T.; Vignali, P.; Romani, R.; Proietti, A.; Di Stefano, I.; Scuotri, G.; Ugolini, C.; et al. The I Talian C Onsensus for the C Lassification and R Eporting of T Hyroid C Ytology: Cytohistologic and Molecular Correlations on 37,371 Nodules from a Single Institution. Cancer Cytopathol. 2022, 130, 899–912. [Google Scholar] [CrossRef]
- WHO Classification of Tumors Editorial Board: Endocrine and Neuroendocrine Tumors, 5th ed.; WHO Classification of Tumors Series; International Agency for Research on Cancer: Lyon, France, 2022; Volume 8.
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.R.M.; Baig, M.; Mohamoud, H.S.A.; Ulhaq, Z.; Hoessli, D.C.; Khogeer, G.S.; Al-Sayed, R.R.; Al-Aama, J.Y. BRAF Gene: From Human Cancers to Developmental Syndromes. Saudi J. Biol. Sci. 2015, 22, 359–373. [Google Scholar] [CrossRef]
- Rojas, A.M.; Fuentes, G.; Rausell, A.; Valencia, A. The Ras Protein Superfamily: Evolutionary Tree and Role of Conserved Amino Acids. J. Cell Biol. 2012, 196, 189–201. [Google Scholar] [CrossRef]
- Cope, N.; Candelora, C.; Wong, K.; Kumar, S.; Nan, H.; Grasso, M.; Novak, B.; Li, Y.; Marmorstein, R.; Wang, Z. Mechanism of BRAF Activation through Biochemical Characterization of the Recombinant Full-Length Protein. ChemBioChem 2018, 19, 1988–1997. [Google Scholar] [CrossRef]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A Comprehensive Survey of Ras Mutations in Cancer. Cancer Res. 2012, 72, 2457–2467. [Google Scholar] [CrossRef]
- Romei, C.; Elisei, R. RET/PTC Translocations and Clinico-Pathological Features in Human Papillary Thyroid Carcinoma. Front. Endocrinol. 2012, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Moccia, M.; Federico, G.; Carlomagno, F. RET Gene Fusions in Malignancies of the Thyroid and Other Tissues. Genes 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Simões-Pereira, J.; Moura, M.M.; Marques, I.J.; Rito, M.; Cabrera, R.A.; Leite, V.; Cavaco, B.M. The Role of EIF1AX in Thyroid Cancer Tumourigenesis and Progression. J. Endocrinol. Investig. 2019, 42, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Asya, O.; Yumuşakhuylu, A.C.; Bağcı, P.; Kaya, H.; Gönen, A.; Gündoğdu, Y.; Muradov, T.; Şahin, A.; Oysu, Ç. Relationship of PPARG Overexpression with Prognostic Parameters in Papillary Thyroid Carcinoma. Acta Otorhinolaryngol. Ital. 2022, 42, 34–40. [Google Scholar] [CrossRef]
- McIver, B.; Grebe, S.K.G.; Eberhardt, N.L. The PAX8 / PPARγ Fusion Oncogene as a Potential Therapeutic Target in Follicular Thyroid Carcinoma. Curr. Drug Targets Immune Endocr. Metab. Disord. 2004, 4, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, F.; Kelly, L.M.; Liu, P.; Zhong, S.; Dacic, S.; Wang, X.; Singhi, A.D.; Dhir, R.; Chiosea, S.I.; Kuan, S.-F.; et al. THADA Fusion Is a Mechanism of IGF2BP3 Activation and IGF1R Signaling in Thyroid Cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 2307–2312. [Google Scholar] [CrossRef]
- Skibiel, C.; Ren, S.; Reid, L. Clinicopathologic Features of Thyroid Neoplasm with THADA-IGF2BP3 Fusion. Am. J. Clin. Pathol. 2021, 156, S53–S54. [Google Scholar] [CrossRef]
- Cipriani, N.A.; Johnson, D.N.; Sarne, D.H.; Angelos, P.; Reeves, W.; Antic, T. The Significance of RAS-Like Mutations and MicroRNA Profiling in Predicting Malignancy in Thyroid Biopsy Specimens. Endocr. Pathol. 2022, 33, 446–456. [Google Scholar] [CrossRef]
- Poma, A.M.; Macerola, E.; Proietti, A.; Vignali, P.; Sparavelli, R.; Torregrossa, L.; Matrone, A.; Basolo, A.; Elisei, R.; Santini, F.; et al. Clinical–Pathological Features and Treatment Outcome of Patients With Hobnail Variant Papillary Thyroid Carcinoma. Front. Endocrinol. 2022, 13, 842424. [Google Scholar] [CrossRef]
- Jin, A.; Xu, J.; Wang, Y. The Role of TERT Promoter Mutations in Postoperative and Preoperative Diagnosis and Prognosis in Thyroid Cancer. Medicine (Baltimore) 2018, 97, e11548. [Google Scholar] [CrossRef]
- Macerola, E.; Poma, A.M.; Vignali, P.; Basolo, A.; Ugolini, C.; Torregrossa, L.; Santini, F.; Basolo, F. Molecular Genetics of Follicular-Derived Thyroid Cancer. Cancers 2021, 13, 1139. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, B.A.; Umbricht, C.B.; Zeiger, M.A. Telomerase Reverse Transcriptase (TERT) Regulation in Thyroid Cancer: A Review. Front. Endocrinol. 2020, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. P53 Signaling in Cancer Progression and Therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Marotta, V.; Bifulco, M.; Vitale, M. Significance of RAS Mutations in Thyroid Benign Nodules and Non-Medullary Thyroid Cancer. Cancers 2021, 13, 3785. [Google Scholar] [CrossRef]
- Kakudo, K. Different Threshold of Malignancy for RAS-like Thyroid Tumors Causes Significant Differences in Thyroid Nodule Practice. Cancers 2022, 14, 812. [Google Scholar] [CrossRef]
- Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A.; Sipos, J.A.; Figge, J.J.; Mandel, S.; Haugen, B.R.; Burman, K.D.; et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules With Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2019, 5, 204. [Google Scholar] [CrossRef]
- Jinih, M.; Foley, N.; Osho, O.; Houlihan, L.; Toor, A.A.; Khan, J.Z.; Achakzai, A.A.; Redmond, H.P. BRAF V600E Mutation as a Predictor of Thyroid Malignancy in Indeterminate Nodules: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. EJSO 2017, 43, 1219–1227. [Google Scholar] [CrossRef]
- Trimboli, P.; Treglia, G.; Condorelli, E.; Romanelli, F.; Crescenzi, A.; Bongiovanni, M.; Giovanella, L. BRAF-Mutated Carcinomas among Thyroid Nodules with Prior Indeterminate FNA Report: A Systematic Review and Meta-Analysis. Clin. Endocrinol. (Oxf.) 2016, 84, 315–320. [Google Scholar] [CrossRef]
- Giordano, T.J. Genomic Hallmarks of Thyroid Neoplasia. Annu. Rev. Pathol. 2018, 13, 141–162. [Google Scholar] [CrossRef]
- Wong, R.; Farrell, S.G.; Grossmann, M. Thyroid Nodules: Diagnosis and Management. Med. J. Aust. 2018, 209, 92–98. [Google Scholar] [CrossRef]
- Silaghi, C.A.; Lozovanu, V.; Georgescu, C.E.; Georgescu, R.D.; Susman, S.; Năsui, B.A.; Dobrean, A.; Silaghi, H. Thyroseq v3, Afirma GSC, and microRNA Panels Versus Previous Molecular Tests in the Preoperative Diagnosis of Indeterminate Thyroid Nodules: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 649522. [Google Scholar] [CrossRef] [PubMed]
- Bardet, S.; Goardon, N.; Lequesne, J.; Vaur, D.; Ciappuccini, R.; Leconte, A.; Monpeyssen, H.; Saguet-Rysanek, V.; Clarisse, B.; Lasne-Cardon, A.; et al. Diagnostic and Prognostic Value of a 7-Panel Mutation Testing in Thyroid Nodules with Indeterminate Cytology: The SWEETMAC Study. Endocrine 2021, 71, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Bellevicine, C.; Migliatico, I.; Sgariglia, R.; Nacchio, M.; Vigliar, E.; Pisapia, P.; Iaccarino, A.; Bruzzese, D.; Fonderico, F.; Salvatore, D.; et al. Evaluation of BRAF, RAS, RET/PTC, and PAX8/PPARg Alterations in Different Bethesda Diagnostic Categories: A Multicentric Prospective Study on the Validity of the 7-gene Panel Test in 1172 Thyroid FNAs Deriving from Different Hospitals in South Italy. Cancer Cytopathol. 2020, 128, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Paschke, R.; Cantara, S.; Crescenzi, A.; Jarzab, B.; Musholt, T.J.; Sobrinho Simoes, M. European Thyroid Association Guidelines Regarding Thyroid Nodule Molecular Fine-Needle Aspiration Cytology Diagnostics. Eur. Thyroid J. 2017, 6, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Muzza, M.; Pogliaghi, G.; Palazzo, S.; Vannucchi, G.; Vicentini, L.; Persani, L.; Gazzano, G.; Fugazzola, L. The Thyroid Risk Score (TRS) for Nodules with Indeterminate Cytology. Endocr. Relat. Cancer 2021, 28, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Hysek, M.; Hellgren, L.S.; Stenman, A.; Darai-Ramqvist, E.; Ljung, E.; Schliemann, I.; Condello, V.; Larsson, C.; Zedenius, J.; Jatta, K.; et al. Digital Droplet PCR TERT Promoter Mutational Screening in Fine Needle Aspiration Cytology of Thyroid Lesions: A Highly Specific Technique for Pre-operative Identification of High-risk Cases. Diagn. Cytopathol. 2023, 51, 331–340. [Google Scholar] [CrossRef]
- Policardo, F.; Tralongo, P.; Arciuolo, D.; Fiorentino, V.; Cardasciani, L.; Pierconti, F.; Carlino, A.; Curatolo, M.; Pontecorvi, A.; Fadda, G.; et al. P53 Expression in Cytology Samples May Represent a Marker of Early-stage Cancer. Cancer Cytopathol. 2023, 131, 392–401. [Google Scholar] [CrossRef]
- Boufraqech, M.; Klubo-Gwiezdzinska, J.; Kebebew, E. MicroRNAs in the Thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 603–619. [Google Scholar] [CrossRef]
- Macerola, E.; Poma, A.M.; Proietti, A.; Rago, T.; Romani, R.; Vignali, P.; Ugolini, C.; Torregrossa, L.; Basolo, A.; Santini, F.; et al. Down-Regulation of miR-7-5p and miR-548ar-5p Predicts Malignancy in Indeterminate Thyroid Nodules Negative for BRAF and RAS Mutations. Endocrine 2022, 76, 677–686. [Google Scholar] [CrossRef]
- Macerola, E.; Poma, A.M.; Vignali, P.; Proietti, A.; Torregrossa, L.; Ugolini, C.; Basolo, A.; Matrone, A.; Elisei, R.; Santini, F.; et al. MicroRNA Expression Profiling of RAS-Mutant Thyroid Tumors with Follicular Architecture: MicroRNA Signatures to Discriminate Benign from Malignant Lesions. J. Endocrinol. Investig. 2023. [Google Scholar] [CrossRef]
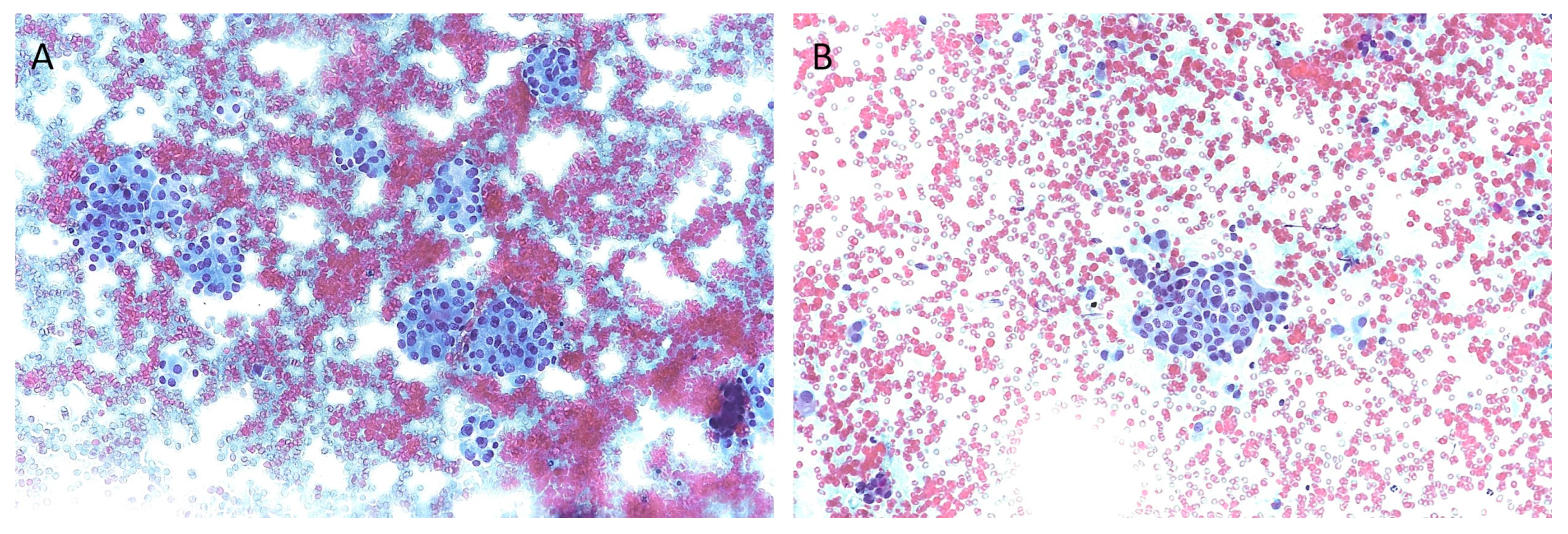
| The Italian Consensus for the Classification and Reporting of Thyroid Cytology (ICCRTC) | The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) | ||||
|---|---|---|---|---|---|
| Class | Diagnostic category | Risk of malignancy (%) | Class | Diagnostic category | Risk of malignancy (%) |
| TIR1 | Inadequate and/or non-representative | Not defined | I | Non-diagnostic samples | 13 |
| TIR1C | Non-diagnostic cystic | Low | |||
| TIR2 | Non-malignant/benign lesions | <3 | II | Benign lesions | 4 |
| TIR3A | Low-risk indeterminate lesions | <10 | III | Atypia of undetermined significance (AUS) | 22 |
| TIR3B | High-risk indeterminate lesions | 30 | IV | Follicular neoplasm (FN) | 30 |
| TIR4 | Suspicious for malignancy | 60–80 | V | Suspicious for malignancy | 74 |
| TIR5 | Malignant lesions | >95 | VI | Malignant lesions | 97 |
| Gene | Protein | Role of Molecular Markers Alterations |
|---|---|---|
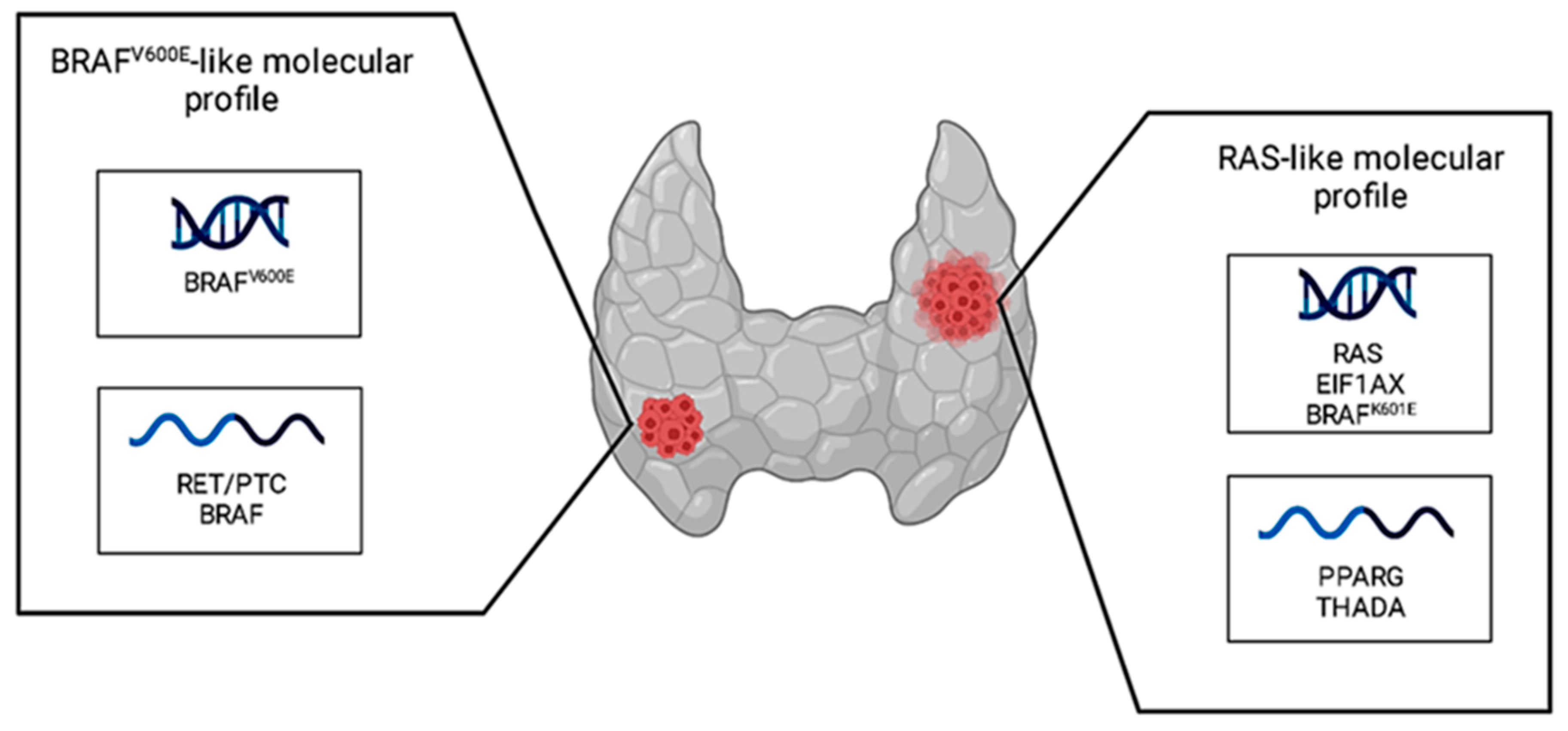
| BRAF | BRAF | Mutations in these proteins implicate constitutive MAPK pathway activity, resulting in hyperproliferative developmental disorder, leading to tumorigenesis. |
RAS family:
| RAS family:
| |
| CCDC6::RET | RET/PTC1 | This rearrangement results in a constitutive dimerization and activation of the RET thyrosin-kinase domain, promoting cell growth, differentiation, proliferation, and survival. |
| NCOA4::RET | RET/PTC3 | |
| EIF1AX | eIF1A | Mutations in this eukaryotic translation initiation factor 1A interfere with global protein translation. |
| PAX8::PPARG | PAX8/PPARG | This rearrangement generates a chimeric oncogenic protein, which promotes cell growth and reduces the rate of apoptosis. |
| THADA::IGF2BP3 | THADA/IGF2BP3 | This rearrangement implicates a high expression of the IGF2BP3 protein, resulting in a deregulated activation of MAPK and PI3K signaling. |
| TERT promoter | TERT | TERT promoter mutations implicate an upregulation of telomerase transcription, avoiding senescence and apoptosis. |
| TP53 | p53 | Mutations in TP53 genes cause protein loss or non-functioning/truncated protein forms, promoting cell survival and proliferation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignali, P.; Macerola, E.; Poma, A.M.; Sparavelli, R.; Basolo, F. Indeterminate Thyroid Nodules: From Cytology to Molecular Testing. Diagnostics 2023, 13, 3008. https://doi.org/10.3390/diagnostics13183008
Vignali P, Macerola E, Poma AM, Sparavelli R, Basolo F. Indeterminate Thyroid Nodules: From Cytology to Molecular Testing. Diagnostics. 2023; 13(18):3008. https://doi.org/10.3390/diagnostics13183008
Chicago/Turabian StyleVignali, Paola, Elisabetta Macerola, Anello Marcello Poma, Rebecca Sparavelli, and Fulvio Basolo. 2023. "Indeterminate Thyroid Nodules: From Cytology to Molecular Testing" Diagnostics 13, no. 18: 3008. https://doi.org/10.3390/diagnostics13183008
APA StyleVignali, P., Macerola, E., Poma, A. M., Sparavelli, R., & Basolo, F. (2023). Indeterminate Thyroid Nodules: From Cytology to Molecular Testing. Diagnostics, 13(18), 3008. https://doi.org/10.3390/diagnostics13183008








