Observation of Anatomical Structures in the Human Larynx Using Micro-Computed Tomography with Lugol’s Solution Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Lugol’s Staining
2.2. Micro-Computed Tomography
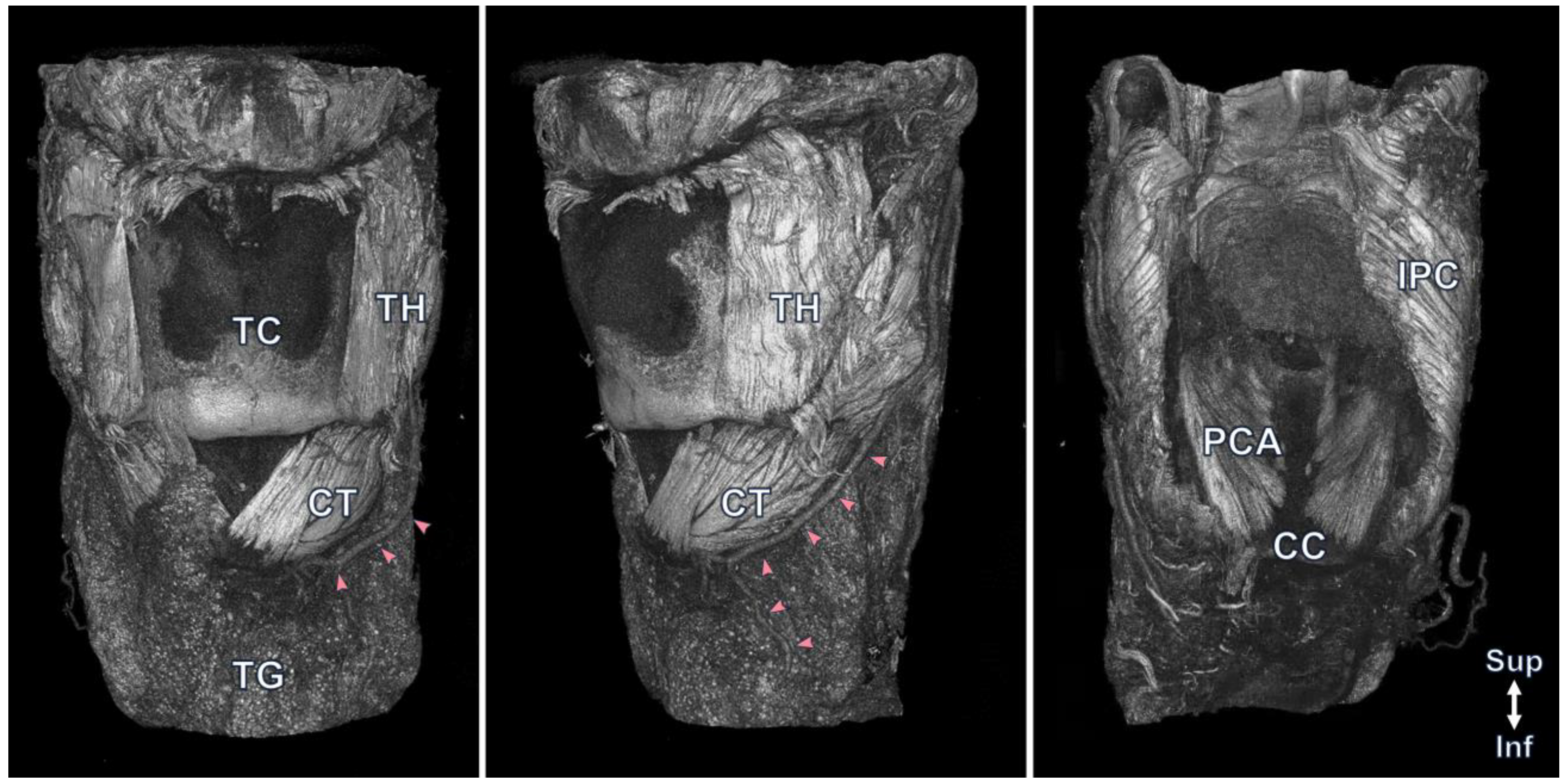
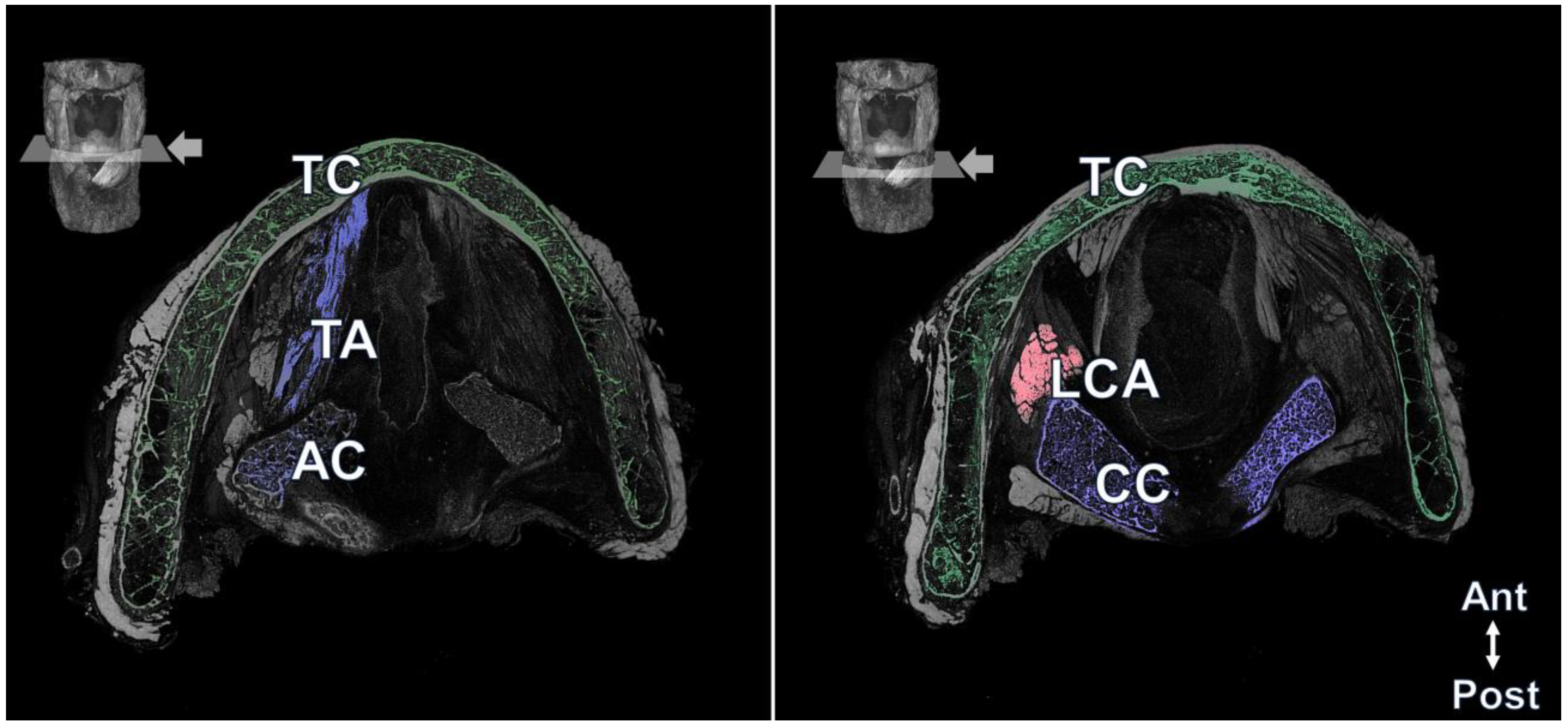
3. Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Constable, J.D.; Bathala, S.; Ahmed, J.J.; McGlashan, J.A. Non-recurrent laryngeal nerve with a coexisting contralateral nerve demonstrating extralaryngeal branching. Case Rep. 2017, 2017, bcr2016218280. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, F.A.; Imamura, R.; Tsuji, D.H.; Sennes, L.U. Surgical approach to the thyroarytenoid branch of the inferior laryngeal nerve through the thyroid cartilage. Acta Cirúrgica Bras. 2016, 31, 442–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Severin, F.; Rosu, A.-M.; Tiglis, M.; Checherita, L.-E.; Stegaru, G.; Cobzeanu, M.D.; Hainarosie, R.; Cobzeanu, B.M.; Palade, O.D. Multidisciplinary Therapeutic Management in Complex Cervical Trauma. Medicina 2023, 59, 596. [Google Scholar] [CrossRef] [PubMed]
- Cergan, R.; Dumitru, M.; Vrinceanu, D.; Neagos, A.; Jeican, I.I.; Ciuluvica, R.C. Ultrasonography of the larynx: Novel use during the SARS-CoV-2 pandemic. Exp. Ther. Med. 2021, 21, 273. [Google Scholar] [CrossRef]
- Costache, A.; Dumitru, M.; Anghel, I.; Cergan, R.; Anghel, A.G.; Sarafoleanu, C. Ultrasonographic anatomy of head and neck-a pictorial for the ENT specialist. Med. Ultrason. 2015, 17, 104–108. [Google Scholar] [CrossRef]
- Gambardella, C.; Offi, C.; Romano, R.M.; De Palma, M.; Ruggiero, R.; Candela, G.; Puziello, A.; Docimo, L.; Grasso, M.; Docimo, G. Transcutaneous laryngeal ultrasonography: A reliable, non-invasive and inexpensive preoperative method in the evaluation of vocal cords motility—A prospective multicentric analysis on a large series and a literature review. Updates Surg. 2020, 72, 885–892. [Google Scholar] [CrossRef]
- Chen, T.; Chodara, A.M.; Sprecher, A.J.; Fang, F.; Song, W.; Tao, C.; Jiang, J.J. A new method of reconstructing the human laryngeal architecture using micro-MRI. J. Voice 2012, 26, 555–562. [Google Scholar] [CrossRef]
- Elders, B.B.; Hermelijn, S.M.; Tiddens, H.A.; Pullens, B.; Wielopolski, P.A.; Ciet, P. Magnetic resonance imaging of the larynx in the pediatric population: A systematic review. Pediatr. Pulmonol. 2019, 54, 478–486. [Google Scholar] [CrossRef]
- Cho, T.-H.; Kwon, H.-J.; Jehoon, O.; Cho, J.; Kim, S.H.; Yang, H.-M. The pathway of injectate spread during thoracic intertransverse process (ITP) block: Micro-computed tomography findings and anatomical evaluations. J. Clin. Anesth. 2022, 77, 110646. [Google Scholar] [CrossRef]
- De Bournonville, S.; Vangrunderbeeck, S.; Kerckhofs, G. Contrast-enhanced microCT for virtual 3D anatomical pathology of biological tissues: A literature review. Contrast Media Mol. Imaging 2019, 2019, 8617406. [Google Scholar] [CrossRef]
- Swart, P.; Wicklein, M.; Sykes, D.; Ahmed, F.; Krapp, H.G. A quantitative comparison of micro-CT preparations in Dipteran flies. Sci. Rep. 2016, 6, 39380. [Google Scholar] [CrossRef] [PubMed]
- Dunmore-Buyze, P.J.; Tate, E.; Xiang, F.l.; Detombe, S.A.; Nong, Z.; Pickering, J.G.; Drangova, M. Three-dimensional imaging of the mouse heart and vasculature using micro-CT and whole-body perfusion of iodine or phosphotungstic acid. Contrast Media Mol. Imaging 2014, 9, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Gignac, P.M.; Kley, N.J. Iodine-enhanced micro-CT imaging: Methodological refinements for the study of the soft-tissue anatomy of post-embryonic vertebrates. J. Exp. Zool. Part B Mol. Dev. Evol. 2014, 322, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Hur, M.-S.; Lee, S.; Kang, T.M.; Oh, C.-S. The three muscle layers in the pyloric sphincter and their possible function during antropyloroduodenal motility. Sci. Rep. 2021, 11, 20094. [Google Scholar] [CrossRef]
- Hur, M.S.; O, J.; Yang, H.M.; Kwon, H.J.; Lee, S.; Lim, H.S.; Lim, S.Y.; Oh, C.S. Heights and spatial relationships of the facial muscles acting on the nasolabial fold by dissection and three-dimensional microcomputed tomography. PLoS ONE 2020, 15, e0237043. [Google Scholar] [CrossRef]
- Jehoon, O.; Kwon, H.J.; Cho, T.H.; Woo, S.H.; Rhee, Y.H.; Yang, H.M. Micro-computed tomography with contrast enhancement: An excellent technique for soft tissue examination in humans. PLoS ONE 2021, 16, e0254264. [Google Scholar]
- Cannito, M.P.; Kahane, J.C.; Chorna, L. Vocal aging and adductor spasmodic dysphonia: Response to botulinum toxin injection. Clin. Interv. Aging 2008, 3, 131–151. [Google Scholar] [CrossRef]
- Remacle, M.; Lawson, G. Results with collagen injection into the vocal folds for medialization. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 148–152. [Google Scholar] [CrossRef]
- Anghel, A.G.; Anghel, I.; Dumitru, M.; Soreanu, C.C. Respiratory and phonatory impairment due to iatrogenic vocal fold paralysis and paresis. A retrospective study of 188 patients. Rom. J. Leg. Med. 2012, 20, 287–290. [Google Scholar] [CrossRef]
- Lee, Y.C.; Pei, Y.C.; Lu, Y.A.; Chung, H.F.; Li, H.Y.; Lee, L.A.; Fang, T.J. Long-Lasting Effect after Single Hyaluronate Injection for Unilateral Vocal Fold Paralysis: Does Concentration Matter? Biomolecules 2021, 11, 1580. [Google Scholar] [CrossRef]
- DeFatta, R.A.; Chowdhury, F.R.; Sataloff, R.T. Complications of injection laryngoplasty using calcium hydroxylapatite. J. Voice 2012, 26, 614–618. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, K.-H.; Lee, S.; Lee, J.-H.; Lee, H.-J. Observation of Anatomical Structures in the Human Larynx Using Micro-Computed Tomography with Lugol’s Solution Enhancement. Diagnostics 2023, 13, 3005. https://doi.org/10.3390/diagnostics13183005
Yi K-H, Lee S, Lee J-H, Lee H-J. Observation of Anatomical Structures in the Human Larynx Using Micro-Computed Tomography with Lugol’s Solution Enhancement. Diagnostics. 2023; 13(18):3005. https://doi.org/10.3390/diagnostics13183005
Chicago/Turabian StyleYi, Kyu-Ho, Siyun Lee, Ji-Hyun Lee, and Hyung-Jin Lee. 2023. "Observation of Anatomical Structures in the Human Larynx Using Micro-Computed Tomography with Lugol’s Solution Enhancement" Diagnostics 13, no. 18: 3005. https://doi.org/10.3390/diagnostics13183005
APA StyleYi, K.-H., Lee, S., Lee, J.-H., & Lee, H.-J. (2023). Observation of Anatomical Structures in the Human Larynx Using Micro-Computed Tomography with Lugol’s Solution Enhancement. Diagnostics, 13(18), 3005. https://doi.org/10.3390/diagnostics13183005






