Lung Ultrasound Reduces Chest X-rays in Postoperative Care after Thoracic Surgery: Is There a Role for Artificial Intelligence?—Systematic Review
Abstract
1. Introduction
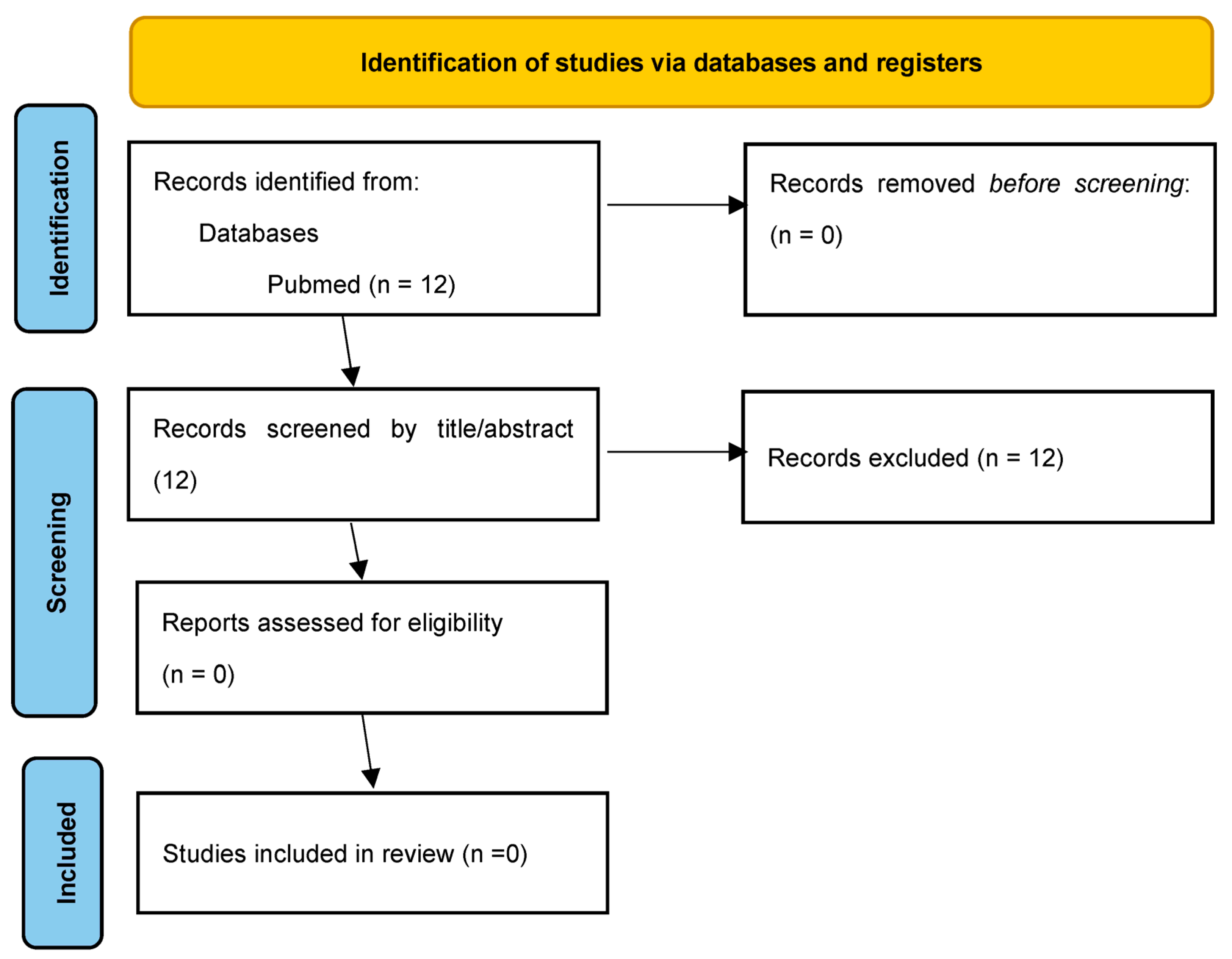
2. Materials and Methods
3. Results
3.1. LUS in Postoperative Care after Non-Cardiac Thoracic Surgery
3.1.1. Basic Characteristics of the Reviewed Trials
3.1.2. Summary of the Reviewed Trials Methods
3.1.3. Summary of the Reviewed Trials Results
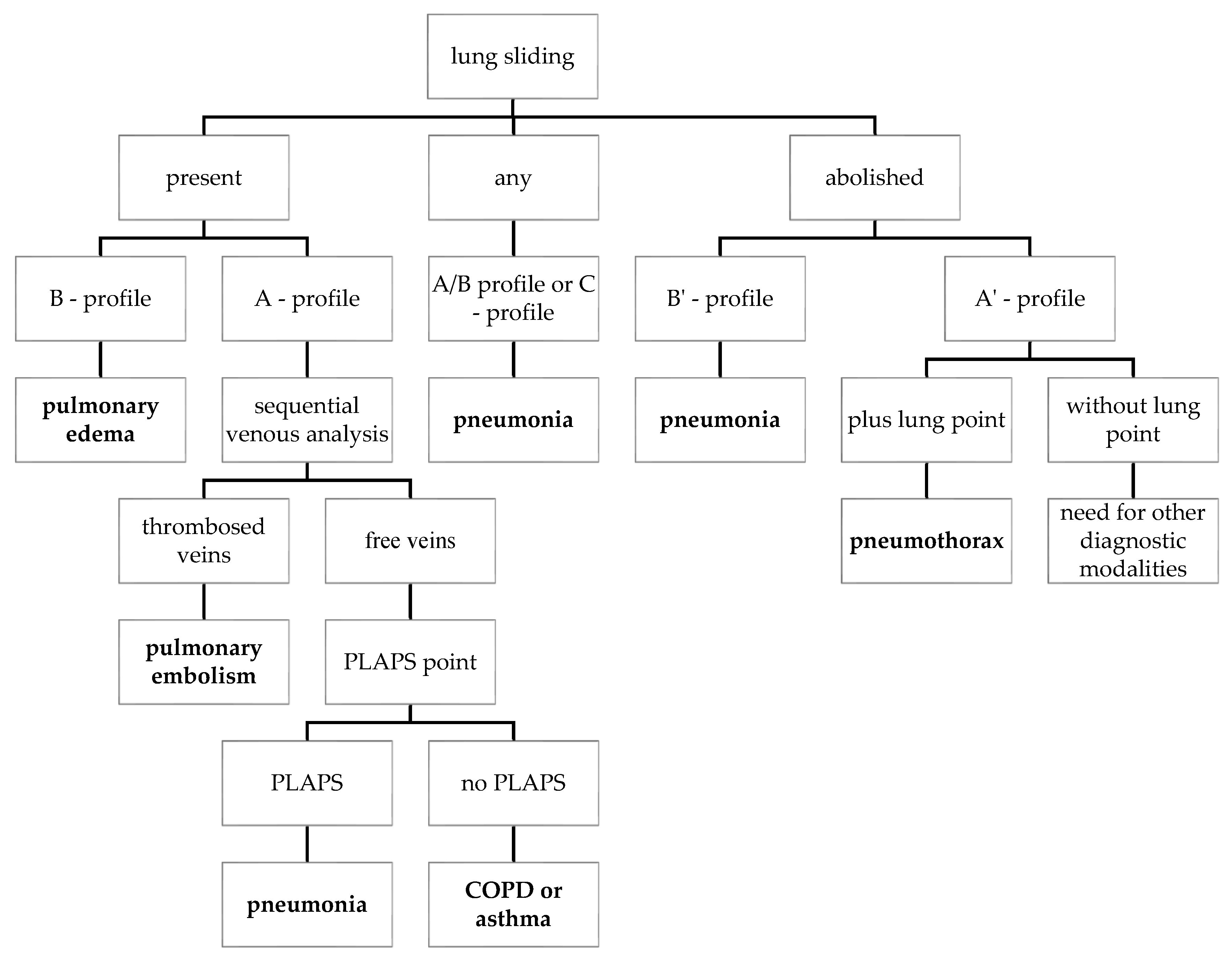
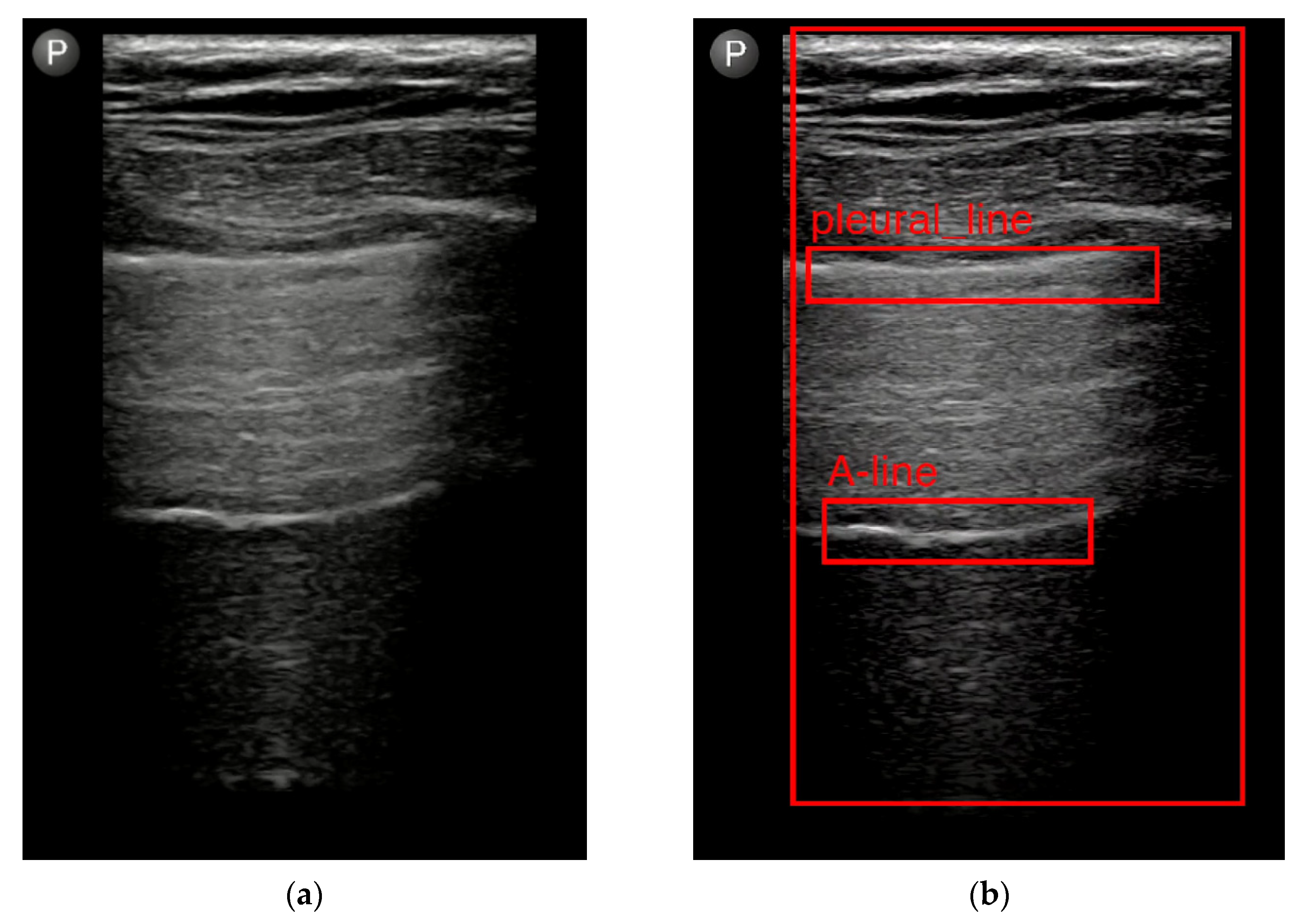
3.1.4. Possible Areas Where Evaluation of LUS Videos Using AI Could Be Helpful
3.1.5. Summary of the Reviewed Trials Conclusions
3.2. AI in LUS Images Evaluation in Postoperative Care after Non-Cardiac Thoracic Surgery
4. Discussion
4.1. LUS in Postoperative Care after Non-Cardiac Thoracic Surgery
4.2. AI in LUS Images Evaluation in COVID Patients
4.3. AI in LUS Data Evaluation in Postoperative Care after Non-Cardiac Thoracic Surgery
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lichtenstein, D.A.; Menu, Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995, 108, 1345–1348. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mézière, G.; Biderman, P.; Gepner, A.; Barré, O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Mezière, G.A.; Lagoueyte, J.F.; Biderman, P.; Goldstein, I.; Gepner, A. A-lines and B-lines: Lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 2009, 136, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Mezière, G.; Biderman, P.; Gepner, A. The "lung point": An ultrasound sign specific to pneumothorax. Intensive Care Med. 2000, 26, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Lascols, N.; Prin, S.; Mezière, G. The "lung pulse": An early ultrasound sign of complete atelectasis. Intensive Care Med. 2003, 29, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Lascols, N.; Mezière, G.; Gepner, A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004, 30, 276–281. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125, Correction in Chest 2013, 144, 721. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. BLUE-protocol and FALLS-protocol: Two applications of lung ultrasound in the critically ill. Chest 2015, 147, 1659–1670. [Google Scholar] [CrossRef]
- Frankel, H.L.; Kirkpatrick, A.W.; Elbarbary, M.; Blaivas, M.; Desai, H.; Evans, D.; Summerfield, D.T.; Slonim, A.; Breitkreutz, R.; Price, S.; et al. Guidelines for the Appropriate Use of Bedside General and Cardiac Ultrasonography in the Evaluation of Critically Ill Patients-Part I: General Ultrasonography. Crit. Care Med 2015, 43, 2479–2502. [Google Scholar] [CrossRef]
- Demi, L.; Wolfram, F.; Klersy, C.; De Silvestri, A.; Ferretti, V.V.; Muller, M.; Miller, D.; Feletti, F.; Wełnicki, M.; Buda, N.; et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. J. Ultrasound Med. 2023, 42, 309–344. [Google Scholar] [CrossRef]
- Blaivas, M.; Lyon, M.; Duggal, S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg. Med. 2005, 12, 844–849. [Google Scholar] [CrossRef]
- Knudtson, J.L.; Dort, J.M.; Helmer, S.D.; Smith, R.S. Surgeon-performed ultrasound for pneumothorax in the trauma suite. J. Trauma 2004, 56, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Testa, A.; Sher, S.; Pignataro, G.; La Sala, M.; Silveri, N.G. Occult traumatic pneumothorax: Diagnostic accuracy of lung ultrasonography in the emergency department. Chest 2008, 133, 204–211. [Google Scholar] [CrossRef]
- Touw, H.R.; Parlevliet, K.L.; Beerepoot, M.; Schober, P.; Vonk, A.; Twisk, J.W.; Elbers, P.W.; Boer, C.; Tuinman, P.R. Lung ultrasound compared with chest X-ray in diagnosing postoperative pulmonary complications following cardiothoracic surgery: A prospective observational study. Anaesthesia 2018, 73, 946–954. [Google Scholar] [CrossRef]
- Goudie, E.; Bah, I.; Khereba, M.; Ferraro, P.; Duranceau, A.; Martin, J.; Thiffault, V.; Liberman, M. Prospective trial evaluating sonography after thoracic surgery in postoperative care and decision making. Eur. J. Cardiothorac. Surg. 2012, 41, 1025–1030. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiappetta, M.; Meacci, E.; Cesario, A.; Smargiassi, A.; Inchingolo, R.; Petracca Ciavarella, L.; Lopatriello, S.; Contegiacomo, A.; Congedo, M.T.; Margaritora, S. Postoperative chest ultrasound findings and effectiveness after thoracic surgery: A pilot study. Ultrasound Med. Biol. 2018, 44, 1960–1967. [Google Scholar] [CrossRef]
- Patella, M.; Saporito, A.; Puligheddu, C.; Mongelli, F.; La Regina, D.; Pini, R.; Inderbitzi, R.; Cafarotti, S. Lung Ultrasound to Detect Residual Pneumothorax after Chest Drain Removal in Lung Resections. Ann. Thorac. Surg. 2018, 105, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Smargiassi, A.; Inchingolo, R.; Chiappetta, M.; Ciavarella, L.P.; Lopatriello, S.; Corbo, G.M.; Margaritora, S.; Richeldi, L. Agreement between chest ultrasonography and chest X-ray in patients who have undergone thoracic surgery: Preliminary results. Multidiscip. Respir. Med. 2019, 14, 9. [Google Scholar] [CrossRef]
- Galetin, T.; Defosse, J.; Schieren, M.; Marks, B.; Lopez-Pastorini, A.; Koryllos, A.; Kosse, N.; Wappler, F.; Stoelben, E. Sensitivity of chest ultrasound for postoperative pneumothorax in comparison to chest X-ray after lung resecting surgery. Eur. J. Cardiothorac. Surg. 2020, 57, 846–853. [Google Scholar] [CrossRef]
- Galetin, T.; Schieren, M.; Marks, B.; Defosse, J.; Stoelben, E. Sensitivity of lung ultrasound for the detection of pneumothorax one day after pulmonary resection—A prospective observational study. Eur. Surg. 2021, 53, 23–28. [Google Scholar] [CrossRef]
- Galetin, T.; Merres, J.; Schieren, M.; Marks, B.; Haffke, Y.; Defosse, J.; Wappler, F.; Koryllos, A.; Stoelben, E. Most patient conditions do not a priori debilitate the sensitivity of thoracic ultrasound in thoracic surgery-a prospective comparative study. J. Cardiothorac. Surg. 2021, 16, 75. [Google Scholar] [CrossRef]
- Malík, M.; Dzian, A.; Skaličanová, M.; Hamada, L.; Zeleňák, K.; Grendár, M. Chest Ultrasound Can Reduce the Use of Roentgenograms in Postoperative Care After Thoracic Surgery. Ann. Thorac. Surg. 2021, 112, 897–904. [Google Scholar] [CrossRef]
- Dzian, A.; Malík, M.; Hamada, L.; Skaličanová, M.; Zeleňák, K.; Števík, M.; Grendár, M. Lung ultrasound could reduce X-ray after major lung resection. Bratisl. Lek. Listy 2021, 122, 871–875. [Google Scholar] [CrossRef]
- Bosch, L.; Mathe, O.; Robin, J.J.; Serres, I.; Labaste, F.; Masquère, P.; Grigoli, M.; Brouchet, L.; Conil, J.M.; Minville, V. Assessment of lung ultrasound for early detection of respiratory complications in thoracic surgery. Braz. J. Anesthesiol. 2022, 72, 128–134. [Google Scholar] [CrossRef]
- Messina, G.; Bove, M.; Noro, A.; Opromolla, G.; Natale, G.; Ferrara, V.; Della Corte, C.M.; Di Liello, R.; Martone, M.; Mirra, R.; et al. The use of ultrasound in the evaluation of postoperative pneumothorax and lung re-expansion in patients after lung resection. Ann. Ital. Chir. 2022, 92, 294–299. [Google Scholar]
- Jakobson, D.; Cohen, O.; Cherniavsky, E.; Batumsky, M.; Fuchs, L.; Yellin, A. Ultrasonography can replace chest X-rays in the postoperative care of thoracic surgical patients. PLoS ONE 2022, 17, e0276502. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, X.; Wang, C.; Wu, Z. Prognostic value of the early lung ultrasound B-line score for postoperative pulmonary insufficiency in patients undergoing thoracic surgery: An observational study. Eur. J. Med. Res 2023, 28, 160. [Google Scholar] [CrossRef]
- Deng, B.; Tan, Q.Y.; Zhao, Y.P.; Wang, R.W.; Jiang, Y.G. Suction or non-suction to the underwater seal drains following pulmonary operation: Meta-analysis of randomised controlled trials. Eur. J. Cardiothorac. Surg. 2010, 38, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.M.; Emmerton-Coughlin, H.M.; Malthaner, R. Management of chest tubes after pulmonary resection: A systematic review and meta-analysis. Can. J. Surg. 2012, 55, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.; Manickavasagar, M.; Burdett, C.; Treasure, T.; Fiorentino, F.; Barua, A.; Batchelor, T.; Fewtrell, J.; Fitzmaurice, G.; Eaton, D.; et al. Suction on chest drains following lung resection: Evidence and practice are not aligned. Eur. J. Cardiothorac. Surg. 2016, 49, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Zardo, P.; Busk, H.; Kutshka, I. Chest tube management: State of the art. Curr. Opin Anaesthesiol. 2015, 28, 45–49. [Google Scholar] [CrossRef] [PubMed]
- French, D.G.; Dilena, M.; Laplante, S.; Shamji, F.; Sundaresan, S.; Villeneuve, J.; Seely, A.; Maziak, D.; Gilbert, S. Optimizing postoperative care protocols in thoracic surgery: Best evidence and new technology. J. Thorac. Dis. 2016, 8 (Suppl. S1), S3–S11. [Google Scholar] [PubMed]
- Mets, O.; Spronk, P.E.; Binnekade, J.; Stoker, J.; de Mol, B.A.; Schultz, M.J. Elimination of daily routine chest radiographs does not change on-demand radiography practice in post-cardiothoracic surgery patients. J. Thorac. Cardiovasc. Surg. 2007, 134, 139–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galata, C.; Cascant Ortolano, L.; Shafiei, S.; Hetjens, S.; Müller, L.; Stauber, R.H.; Stamenovic, D.; Roessner, E.D.; Karampinis, I. Are Routine Chest X-rays Necessary following Thoracic Surgery? A Systematic Literature Review and Meta-Analysis. Cancers 2022, 14, 4361. [Google Scholar] [CrossRef]
- Reeb, J.; Falcoz, P.E.; Olland, A.; Massard, G. Are daily routine chest radiographs necessary after pulmonary surgery in adult patients? Interact. Cardiovasc. Thorac. Surg. 2013, 17, 995–998. [Google Scholar] [CrossRef]
- Gilbert, S.; McGuire, A.L.; Maghera, S.; Sundaresan, S.R.; Seely, A.J.; Maziak, D.E.; Shamji, F.M.; Villeneuve, P.J. Randomized trial of digital versus analog pleural drainage in patients with or without a pulmonary air leak after lung resection. J. Thorac. Cardiovasc. Surg. 2015, 150, 1243–1249. [Google Scholar] [CrossRef]
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.J.; Gonzalez, M.; Ljungqvist, O.; Petersen, R.H.; Popescu, W.M.; Slinger, P.D.; et al. Guidelines for enhanced recovery after lung surgery: Recommendations of the enhanced recovery after surgery (ERAS®) society and the European societyof thoracic surgeons (ESTS). Eur. J. Cardiothorac. Surg. 2019, 55, 91–115. [Google Scholar] [CrossRef]
- Grapatsas, K.; Leivaditis, V.; Ehle, B.; Papaporfyriou, A. Can Chest Ultrasound Replace Chest X-ray in Thoracic Surgery? Tomography 2022, 8, 2083–2092. [Google Scholar] [CrossRef]
- Massion, P.P.; Antic, S.; Ather, S.; Arteta, C.; Brabec, J.; Chen, H.; Declerck, J.; Dufek, D.; Hickes, W.; Kadir, T.; et al. Assessing the accuracy of a deep learning method to risk stratify indeterminate pulmonary nodules. Am. J. Respir. Crit. Care Med. 2020, 202, 241–249. [Google Scholar] [CrossRef]
- Choi, H.K.; Ghobrial, M.; Mazzone, P.J. Models to estimate the probability of malignancy in patients with pulmonary nodules. Ann. Am. Thorac. Soc. 2018, 15, 1117–1126. [Google Scholar] [CrossRef]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019, 25, 954–961, Correction in Nat. Med. 2019, 25, 1319. [Google Scholar] [CrossRef] [PubMed]
- Binczyk, F.; Prazuch, W.; Bozek, P.; Polanska, J. Radiomics and artificial intelligence in lung cancer screening. Transl. Lung Cancer Res. 2021, 10, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Lichter, Y.; Topilsky, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal-Oz, A.; Vine, J.; Goren, O.; Cohen, B.; et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020, 46, 1873–1883, Correction in Intensive Care Med. 2020, 46, 2128-2129. [Google Scholar] [CrossRef]
- Roy, S.; Menapace, W.; Oei, S.; Luijten, B.; Fini, E.; Saltori, C.; Huijben, I.; Chennakeshava, N.; Mento, F.; Sentelli, A. Deep learning for classification and localization of COVID-19 markers in point-of-care lung ultrasound. IEEE Trans. Med. Imaging 2020, 39, 2676–2687. [Google Scholar] [CrossRef]
- Ebadi, S.E.; Krishnaswamy, D.; Bolouri, S.E.S.; Zonoobi, D.; Greiner, R.; Meuser-Herr, N.; Jaremko, J.L.; Kapur, J.; Noga, M.; Punithakumar, K. Automated detection of pneumonia in lung ultrasound using deep video classification for COVID 19. Inform. Med. Unlocked 2021, 25, 100687. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Zhou, B.; Sohn, J.J.; Zhou, J.; Jacob, J.T.; Higgins, K.A.; Bradley, J.D.; Liu, T. Review of Machine Learning in Lung Ultrasound in COVID-19 Pandemic. J. Imaging 2022, 8, 65. [Google Scholar] [CrossRef]
- Arntfield, R.; VanBerlo, B.; Alaifan, T.; Phelps, N.; White, M.; Chaudhary, R.; Ho, J.; Wu, D. Development of a convolutional neural network to differentiate among the etiology of similar appearing pathological B lines on lung ultrasound: A deep learning study. BMJ Open 2021, 11, e045120. [Google Scholar] [CrossRef] [PubMed]
- Jaščur, M.; Bundzel, M.; Malík, M.; Dzian, A.; Ferenčík, N.; Babič, F. Detecting the Absence of Lung Sliding in Lung Ultrasounds Using Deep Learning. Appl. Sci. 2021, 11, 6976. [Google Scholar] [CrossRef]
- Hliboký, M.; Magyar, J.; Bundzel, M.; Malík, M.; Števík, M.; Vetešková, Š.; Dzian, A.; Szabóová, M.; Babič, F. Artifact Detection in Lung Ultrasound: An Analytical Approach. Electronics 2023, 12, 1551. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Baumann, M.H.; Strange, C.; Heffner, J.E.; Light, R.; Kirby, T.J.; Klein, J.; Luketich, J.; Panacek, E.A.; Sahn, S. Management of spontaneous pneumothorax: An American College of Chest Physicians Delphi consensus statement. Chest 2001, 119, 590–602. [Google Scholar] [CrossRef]
- Lesser, T.; Doenst, T.; Lehmann, T.; Mukdessi, J. Lung Biopsy Without Pleural Drainage. Dtsch. Arztebl. Int. 2019, 116, 329–334. [Google Scholar]
- Galetin, T.; Stoelben, E. Sensitivity of lung ultrasound for postsurgical pneumothorax. Ann. Thorac. Surg. 2019, 108, 960–961. [Google Scholar] [CrossRef]
- Ding, W.; Shen, Y.; Yang, J.; He, X.; Zhang, M. Diagnosis of pneumothorax by radiography and ultrasonography: A meta-analysis. Chest 2011, 140, 859–866. [Google Scholar] [CrossRef]
- Yousefifard, M.; Baikpour, M.; Ghelichkhani, P.; Asady, H.; Shahsavari Nia, K.; Moghadas Jafari, A.; Hosseini, M.; Safari, S. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emergency 2016, 4, 1–10. [Google Scholar] [PubMed]
- Staquet, M.; Rozencweig, M.; Lee, Y.J.; Muggia, F.M. Methodology for the assessment of new dichotomous diagnostic tests. J. Chronic Dis. 1981, 34, 599–610. [Google Scholar] [CrossRef]
- Emerson, S.C.; Waikar, S.S.; Fuentes, C.; Bonventre, J.V.; Betensky, R.A. Biomarker validation with an imperfect reference: Issues and bounds. Stat. Methods Med. Res. 2018, 27, 2933–2945. [Google Scholar] [CrossRef] [PubMed]
- Vetrugno, L.; Meroi, F.; Orso, D.; D’Andrea, N.; Marin, M.; Cammarota, G.; Mattuzzi, L.; Delrio, S.; Furlan, D.; Foschiani, J.; et al. Can Lung Ultrasound Be the Ideal Monitoring Tool to Predict the Clinical Outcome of Mechanically Ventilated COVID-19 Patients? An Observational Study. Healthcare 2022, 10, 568. [Google Scholar] [CrossRef] [PubMed]
- Meroi, F.; Orso, D.; Vetrugno, L.; Bove, T. Lung Ultrasound Score in Critically Ill COVID-19 Patients: A Waste of Time or a Time-Saving Tool? Acad. Radiol. 2021, 28, 1323–1324. [Google Scholar] [CrossRef]
- Sadik, F.; Dastider, A.G.; Fattah, S.A. SpecMEn-DL: Spectral mask enhancement with deep learning models to predict COVID-19 from lung ultrasound videos. Health Inf. Sci. Syst. 2021, 9, 28. [Google Scholar] [CrossRef]
- Awasthi, N.; Dayal, A.; Cenkeramaddi, L.R.; Yalavarthy, P.K. Mini-COVIDNet: Efficient Lightweight Deep Neural Network for Ultrasound Based Point-of-Care Detection of COVID-19. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2021, 68, 2023–2037. [Google Scholar] [CrossRef]
- Dastider, A.G.; Sadik, F.; Fattah, S.A. An integrated autoencoder-based hybrid CNN-LSTM model for COVID-19 severity prediction from lung ultrasound. Comput. Biol. Med. 2021, 132, 104296. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; He, Q.; Liao, H.; Luo, J. Quantitative analysis of pleural line and B-lines in lung ultrasound images for severity assessment of COVID-19 pneumonia. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2022, 69, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Mezière, G.A. The BLUE-points: Three standardized points used in the BLUE-protocol for ultrasound assessment of the lung in acute respiratory failure. Crit. Ultrasound. J. 2011, 3, 109–110. [Google Scholar] [CrossRef]
- VanBerlo, B.; Wu, D.; Li, B.; Rahman, M.A.; Hogg, G.; VanBerlo, B.; Tschirhart, J.; Ford, A.; Ho, J.; McCauley, J.; et al. Accurate assessment of the lung sliding artefact on lung ultrasonography using a deep learning approach. Comput. Biol. Med. 2022, 148, 105953. [Google Scholar] [CrossRef] [PubMed]
- Born, J.; Wiedemann, N.; Cossio, M.; Buhre, C.; Brändle, G.; Leidermann, K.; Aujayeb, A.; Moor, M.; Rieck, B.; Borgwardt, K. Accelerating Detection of Lung Pathologies with Explainable Ultrasound Image Analysis. Appl. Sci. 2021, 11, 672. [Google Scholar] [CrossRef]

| Study | Patients/ Examinations | Study Design | Surgical Procedure | Results | |||
|---|---|---|---|---|---|---|---|
| PTX Sen/Spe/ PPV/NPV in % | PE Sen/Spe/ PPV/NPV in % | Percentage of Agreement/ Cohen’s Kappa | Percentage of Saved CXR | ||||
| 1. Goudie, 2012 [15] | 120/352 | LUS when CXR | whole spectrum | 21.2/94.7/ 52.7/81.8 | 83.1/59.3/ 36.1/92.7 | NA | NA |
| 2. Chiappetta, 2018 [16] | 24/24 | LUS and CXR first 48 h after surgery | lung resections (wedge resections, lobectomies) mediastinal tumours resections/biopsy | NA | NA | NA | 67% after open procedures, 85% after mini-invasive procedures |
| 3. Patella, 2018 [17] | 50/50 | LUS and CXR 2 h after chest tube removal | lung resections (wedge resections, lobectomies, segmentectomies) | NA/NA/ 71/100 | NA | NA | 86% |
| 4. Smargiassi, 2019 [18] | 24/24 | LUS and CXR first 48 h after surgery | details NA (mini-invasive approach: 16; thoracotomy: 6; robotic thymectomy: 2) | NA | NA | LUS vs. CXR: PTX: 79%/50% PE: 70%/39% LC: 50%/6% SCE: 58%/21% DP: 91%/70% | NA |
| 5. Galetin, 2020 [19] | 123/123 | LUS and CXR within 1 day after chest tube removal | lung resections and/or chest wall resection | 32/85/54/69 | NA | Conformity between LUS and CXR-based therapy 97% | NA |
| For PTX ≥ 3 cm: 100/82/19/100 | |||||||
| 6. Galetin, 2021 [20] | 68/68 | LUS and CXR on the first day after thoracic surgery and after chest tube removal | lung resection, chest wall resection, decortication | 48/81–100/ NA/76 | NA | Conformity between LUS and CXR-based therapy 96% | NA |
| 7. Malík, 2021 [22] | 297/545 | LUS and CXR postoperatively and prior to chest tube removal after its clamping | whole spectrum | 1st exam: 59.4/95.9/ 67.9/94.2 | 1st exam: 44.4/92.6/ 66.7/83.3 | LUS vs. CXR 1st exam: PTX 91.3%/58.4% PE 80.5%/41.6% | 61.6% |
| 2nd exam: 50.0/94.8/ 56.5/93.4 | 2nd exam: 60.9/91.3/ 81.2/79.2 | LUS vs. CXR 2nd exam: PTX 89.5%/47.2% PE 79.8%/54.9% | |||||
| 8. Dzian, 2021 [23] | 48/87 | LUS and CXR postoperatively and prior to chest tube removal after its clamping | major lung resections (lobectomies/ bilobectomies) | 1st exam: 45.5–58.5/91.1–100/77.8–100/72.1–78.7 | 1st exam: 0–86.2/82.6–88.4/0–33.1/92.5–99 | LUS vs. CXR 1st exam: PTX 92.3%/77.5% PE 76.7%/3.6 | 77% |
| 2nd exam: 29.7–59.4/79.5–100/50–100/62.2–78.2 | 2nd exam: 32.6–36.9/68.5–100/88.3–100/12.2–17.8 | LUS vs. CXR 2nd exam: PTX 78.8%/39.7% PE 81.1%/61.1% | |||||
| 9. Messina, 2022 [25] | 157/525 | Daily LUS and CXR until chest tube removal | major lung resections (lobectomies) | 86/100/ 94/94 | NA | Conformity between LUS and CXR-based therapy 97% | NA |
| 10. Jakobson, 2022 [26] | 80/215 | 3x LUS and CXR (postoperatively, prior to chest tube removal and 4 h after chest tube removal) | lung resections (anatomical and non-anatomical resections), decortications | NA | NA | LUS/CXR agreement—absolute diagnostic/therapeutic: PTX: 72%/94% PE: 38%/80% LC: 100%/100% | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malík, M.; Dzian, A.; Števík, M.; Vetešková, Š.; Al Hakim, A.; Hliboký, M.; Magyar, J.; Kolárik, M.; Bundzel, M.; Babič, F. Lung Ultrasound Reduces Chest X-rays in Postoperative Care after Thoracic Surgery: Is There a Role for Artificial Intelligence?—Systematic Review. Diagnostics 2023, 13, 2995. https://doi.org/10.3390/diagnostics13182995
Malík M, Dzian A, Števík M, Vetešková Š, Al Hakim A, Hliboký M, Magyar J, Kolárik M, Bundzel M, Babič F. Lung Ultrasound Reduces Chest X-rays in Postoperative Care after Thoracic Surgery: Is There a Role for Artificial Intelligence?—Systematic Review. Diagnostics. 2023; 13(18):2995. https://doi.org/10.3390/diagnostics13182995
Chicago/Turabian StyleMalík, Marek, Anton Dzian, Martin Števík, Štefánia Vetešková, Abdulla Al Hakim, Maroš Hliboký, Ján Magyar, Michal Kolárik, Marek Bundzel, and František Babič. 2023. "Lung Ultrasound Reduces Chest X-rays in Postoperative Care after Thoracic Surgery: Is There a Role for Artificial Intelligence?—Systematic Review" Diagnostics 13, no. 18: 2995. https://doi.org/10.3390/diagnostics13182995
APA StyleMalík, M., Dzian, A., Števík, M., Vetešková, Š., Al Hakim, A., Hliboký, M., Magyar, J., Kolárik, M., Bundzel, M., & Babič, F. (2023). Lung Ultrasound Reduces Chest X-rays in Postoperative Care after Thoracic Surgery: Is There a Role for Artificial Intelligence?—Systematic Review. Diagnostics, 13(18), 2995. https://doi.org/10.3390/diagnostics13182995






