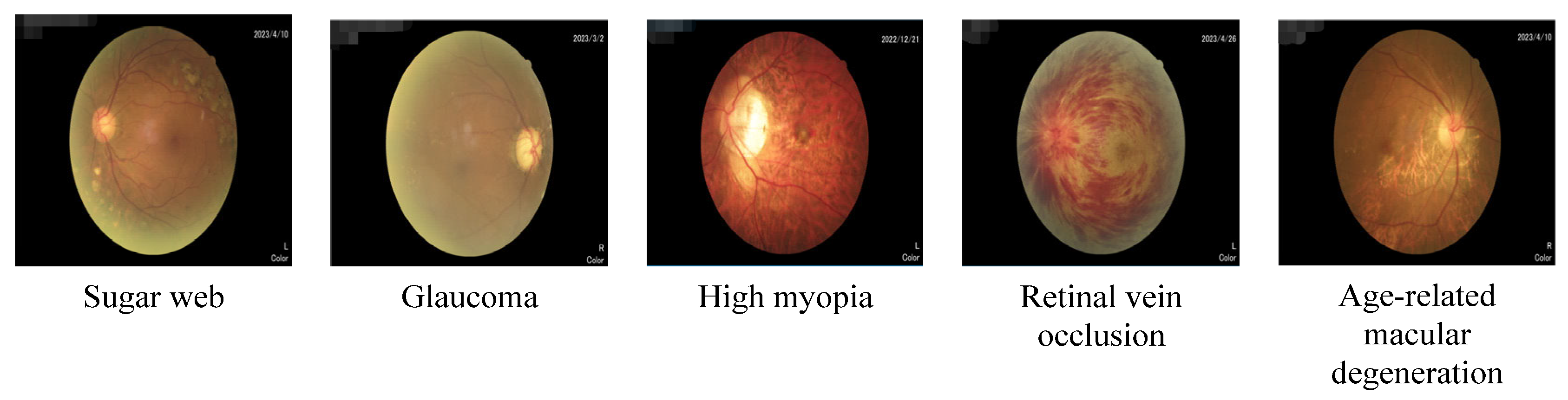
2.1. Introduction to Eye Diseases
With the development of modern society and the change in people’s lifestyles and environmental conditions, the incidence rate of eye diseases is increasing year by year. Many eye diseases, if not treated in a timely manner or treated improperly, may lead to serious visual impairment and even blindness. Therefore, it is essential to prevent eye disease, conduct regular eye examinations, and receive timely treatment. Sugar web, glaucoma, high myopia, RVO, and age-related macular degeneration are all common eye diseases, as shown in
Figure 1.
Diabetic patients are prone to diabetic retinopathy, that is, sugar web, and sugar web is one of the important causes of blindness. Diabetic retinopathy is one of the most common ocular complications in diabetic patients. Long-term high blood sugar levels cause damage to blood vessels in the eyes, leading to the occurrence of retinopathy. Diabetic retinopathy can be divided into two types: non-proliferative and proliferative.
Non-proliferative diabetic retinopathy includes microangioma, microvascular blockage, and leakage. Microhemangioma is the dilation and embrittlement of blood vessels, which are prone to rupture and bleeding; microvascular occlusion is stenosis or occlusion, resulting in retinal ischemia; and leakage is an increase in the permeability of the blood vessel wall, allowing fluid and proteins to seep into the retinal tissue.
Proliferative diabetic retinopathy refers to the growth of new blood vessels based on non-proliferative diseases. These new blood vessels are fragile and prone to rupture, which may lead to bleeding and retinal detachment. If the condition progresses severely, it may lead to severe visual impairment or even blindness.
Early diabetic retinopathy usually has no obvious symptoms. With the development of the disease, patients may have blurred vision, visual field loss, dark adaptation, ability decline, and other symptoms. Regular fundus examination is an important means to prevent and detect early diabetic retinopathy.
The methods of treating diabetic retinopathy include controlling blood sugar levels, controlling blood pressure, injecting anti-antigenic drugs, laser therapy, and surgical treatment. Early diagnosis and treatment can effectively slow down the progression of lesions and protect vision. Therefore, patients with diabetes should have regular eye examinations and actively manage diabetes to prevent and control the occurrence and progress of diabetic retinopathy.
Glaucoma is a disease caused by high intraocular pressure. If not treated in a timely manner, it can lead to a serious decline in vision and seriously affect the quality of life. Color fundus images are one of the commonly used tools for diagnosing glaucoma. Although color fundus images cannot directly detect glaucoma, they can provide important information that helps with glaucoma screening and diagnosis.
Color fundus images are considered a sufficient marker for glaucoma, mainly for the following reasons:
Glaucoma is a group of diseases with the common characteristics of optic papillary atrophy and depression, visual field defect, and vision loss. Pathological increase in intraocular pressure and insufficient optic nerve blood supply are the primary risk factors. The optic nerve tolerance to pressure damage is also related to the occurrence and development of glaucoma. Color fundus images can visually display the condition of the fundus, including the optic disc, retina, and blood vessels. By observing fundus images, doctors can evaluate the shape and color of the optic disc, as well as the status of the retina and blood vessels to determine whether there is damage caused by glaucoma.
Color fundus images can help doctors detect early lesions in glaucoma. Early glaucoma lesions may not show obvious symptoms, but fundus images can display subtle changes such as deformation of the optic disc, color changes, retinal damage, etc., providing the possibility of early diagnosis.
Color fundus imaging is a non-invasive and painless examination method that is more convenient and comfortable for patients. By capturing fundus images, a large amount of information can be quickly obtained, reducing patients’ discomfort and inconvenience.
It should be noted that although color fundus images can serve as a marker for glaucoma, comprehensive judgment still needs to be made in conjunction with other clinical manifestations and examination results. Only the professional diagnosis of a doctor can determine whether one has glaucoma. If you suspect that you may have glaucoma, you should promptly consult a professional doctor for diagnosis and treatment.
High myopia is a serious myopia with a degree of myopia exceeding 600 degrees. If left untreated for a long time, complications such as retinal detachment may also occur.
RVO is a disease caused by retinal vascular occlusion, mainly manifested as sudden loss of vision or blindness, which requires timely treatment to avoid serious consequences.
Senile macular degeneration is a common degenerative eye disease that often occurs in the eyes of elderly people. Macular degeneration can lead to decreased central vision, which seriously affects the quality of life. At present, the main methods for treating age-related macular degeneration include laser therapy and surgical treatment.
Although color fundus photographs have been widely used in the detection of diabetic retinopathy, they still need the interpretation and judgment of professional doctors. Therefore, if people have diabetes or a family history of diabetes, it is recommended to conduct a fundus examination regularly to detect and treat diabetic retinopathy as soon as possible.
2.2. HEs Automatic Detection
HEs are the most prominent fundus lesions in the early stages of glucose reticulopathy and their automatic identification is a key aspect of the supplementary diagnostic system for glucose reticulopathy. Therefore, efficient automatic recognition of HEs for patients with diabetic retinopathy is of great clinical significance for its large base and high incidence rate. Currently, the automatic detection of HEs is mostly based on machine learning methods, which select a set of features based on experience, establish a classifier, and then use shallow learning methods to locate and recognize HEs in FIs.
In recent years, deep learning algorithms represented by CNN have made significant breakthroughs in image processing. Compared with shallow learning, deep learning exhibits many advantages, as it does not require manual setting of features to be extracted and has strong generalization ability. Therefore, this article will use deep learning technology to conduct deep feature learning on HEs and establish a classification model for HEs.
- (1)
Convolutional neural network
CNN (Convolutional neural network) is an algorithm for deep learning. Deep learning is a machine learning method that learns and trains via multi-layer neural networks. As a special type of neural network for deep learning, CNN is mainly used to process data with spatial structure, such as images, speech, etc.
CNN extracts features from images and classifies them via components such as convolutional layers, pooling layers, and fully connected layers. The convolutional layer extracts local features of the image via convolution operations; the pooling layer reduces the dimensionality of features via downsampling operations; and the fully connected layer performs tasks such as classification or regression via multiple neurons. The network structure of CNN can be adjusted and designed based on the complexity of tasks and the characteristics of data.
CNN is a deep learning method that can automatically learn features from images and achieve excellent performance in tasks such as classification and recognition. In smart medical systems based on color retinal FIs of lesions, CNN can be used as a tool for image recognition and classification, automatically detecting and diagnosing important information such as the location, size, and type of lesions, and providing corresponding diagnostic results and recommendations. Therefore, the application of CNN in the intelligent medical system to focus color retinal FIs can greatly improve the early diagnosis and treatment of eye diseases such as diabetic retinopathy. For smart medical system-aided diagnosis based on color retinal FIs of lesions, CNN can help ophthalmologists quickly and accurately locate and identify lesion lesions.
When using CNN for the recognition and analysis of lesion color retinal FIs, the image is first input into a convolutional layer and filtered via convolutional verification to extract a series of feature maps. Next, downsampling is performed in the pooling layer to reduce the size and complexity of the feature map while preserving the most significant features. Finally, features are classified and predicted in the fully connected layer.
The input layer is a two-dimensional sample image, and each convolution layer is composed of multiple feature maps. The feature map
obtained from the previous layer is convolved with the learnable convolution kernel
, and the convolution result is generated into feature map
via the nonlinear activation function
. The specific form is as follows:
In the formula,
represents the output of the k-th feature map of layer 1;
corresponds to the offset of
;
represents convolution operation;
is a convolutional kernel that can be convolved with multiple feature maps of layers (1–1); and
represents the set of input feature maps corresponding to
. The commonly used activation functions of CNN include the Sigmaid function (Formula (2)), Tanh function (Formula (3)), ReLU function (Formula (4)), etc., which can simulate the response of neurons to excitation.
The sampling layer implements the downsampling processing of each feature map. The common methods in this layer are pooling the maximum value and pooling the average value. The expressions of the two are
In the formula, is the window function input for the sampling layer. Finally, the fully connected layer performs regression, classification, and other processing on the previously extracted features via layer-by-layer transformation and mapping. Like the output layer, the trained feature maps are summarized into feature vectors to train the HE classification model. CNN is suitable for processing complex data such as high-dimensional data and images, and can help doctors quickly and accurately locate and identify various lesions, such as macular holes, edema, frontal disc melanoma, etc. Therefore, there is a close relationship between CNN and color retinal FIs of lesions, which is an important component of diagnostic technology in smart medical systems.
- (2)
Evaluation indicators
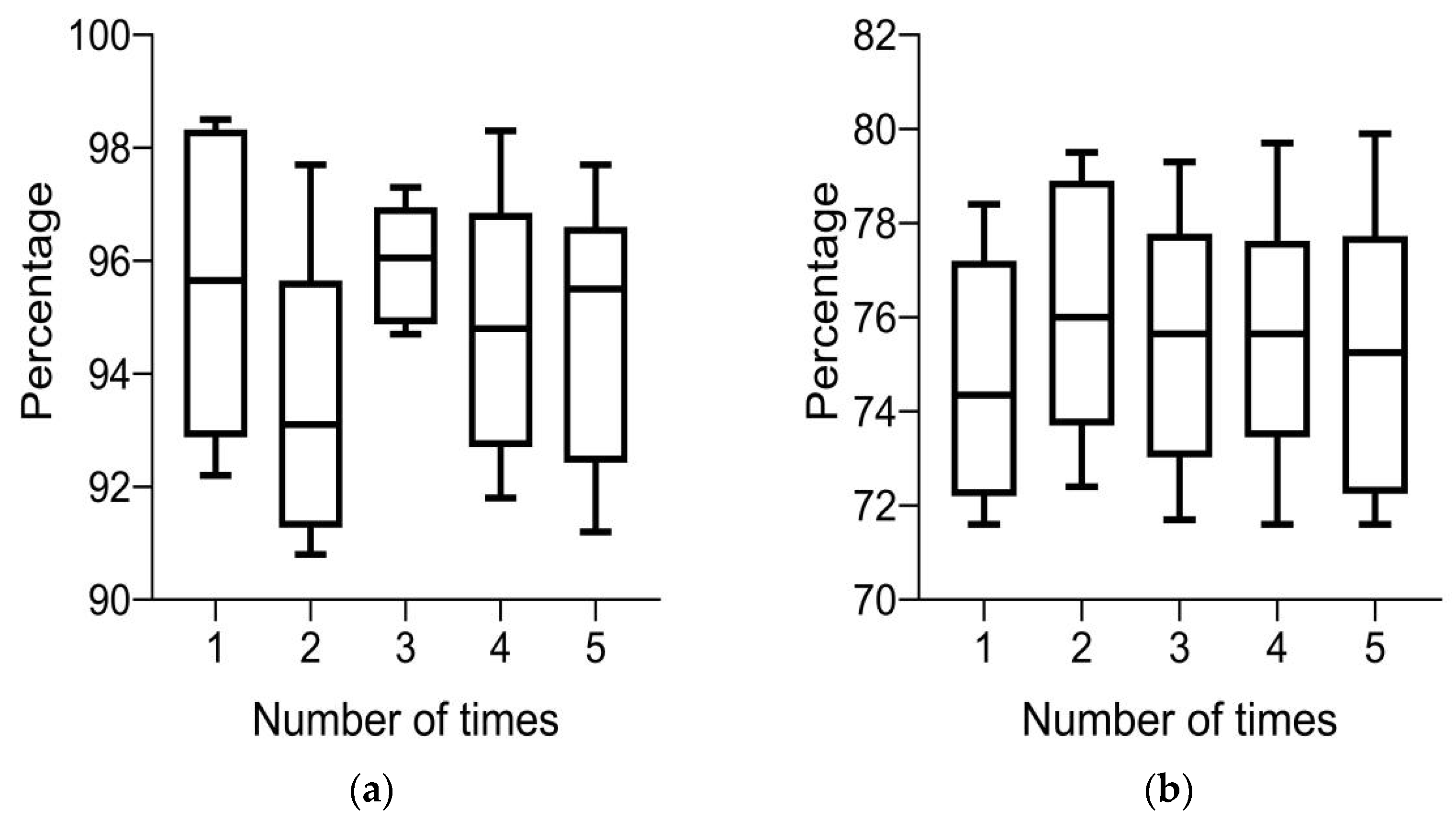
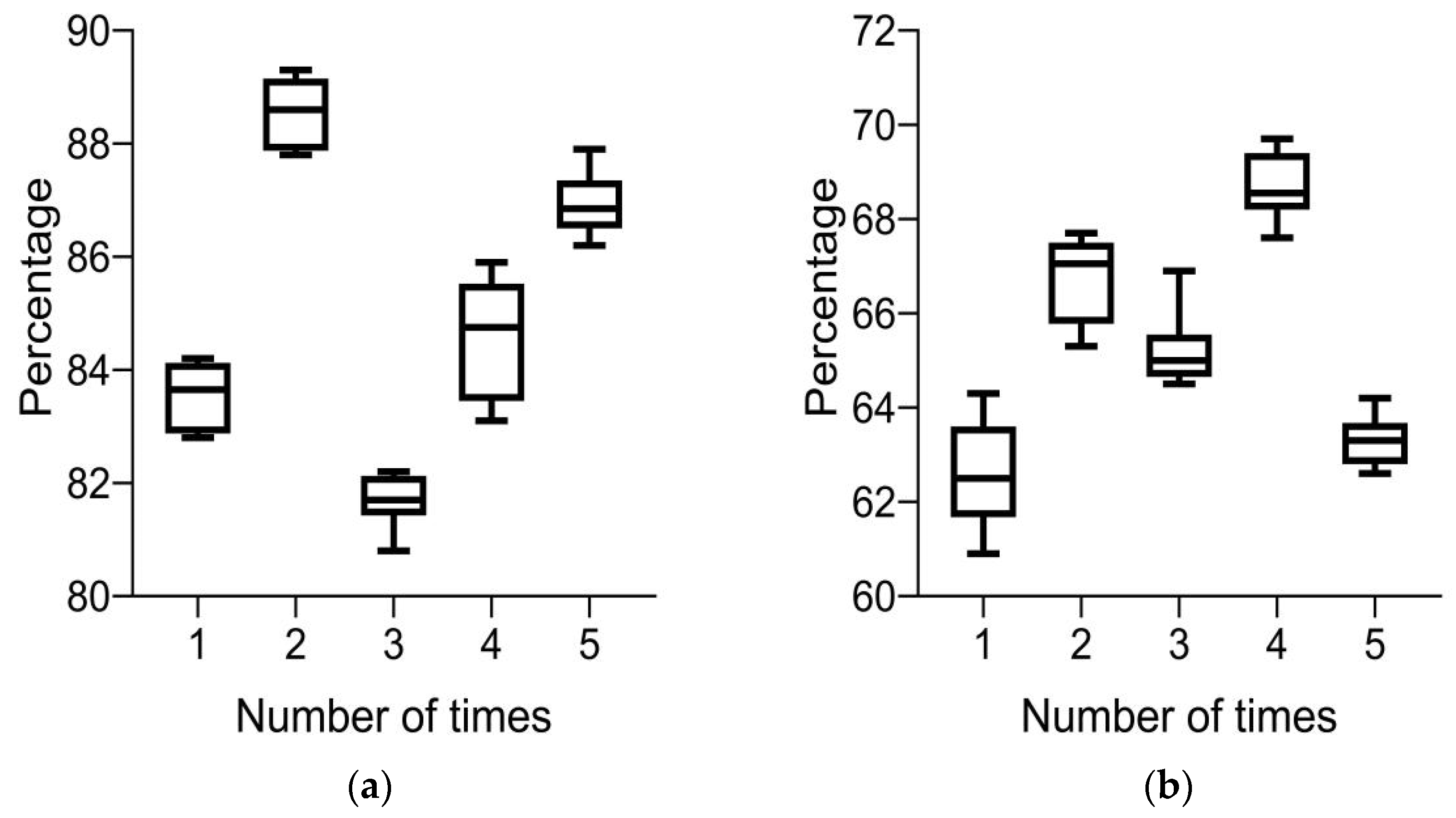
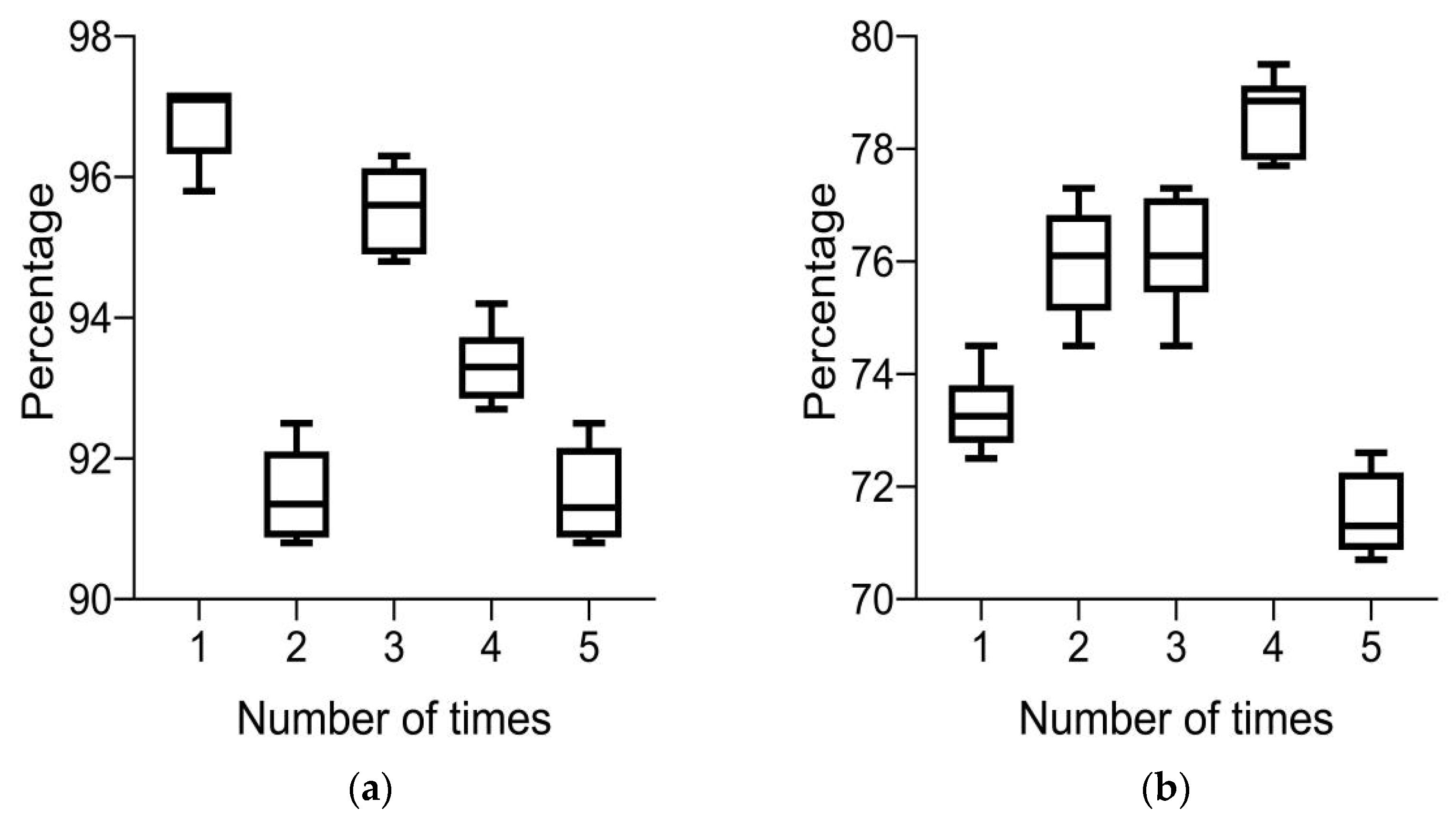
Currently, there are two main performance evaluations for HE automatic recognition algorithms: one is based on image level evaluation indicators, and the other is based on lesion area evaluation indicators. There are three main evaluation indicators for evaluating automatic detection methods: accuracy, recall, and precision. The calculation formulas are as follows:
In Formula (8), TP, FP, FN, and TN are true positive, false positive, false negative, and true negative, respectively. In the image-based assessment index, the unit of this assessment index is the number of whole FIs, whereas in the lesion level-based assessment index, the unit of this assessment index is the number of areas. This index reflects the probability of hard exudate areas appearing in FIs and can better evaluate the effectiveness of the algorithm.
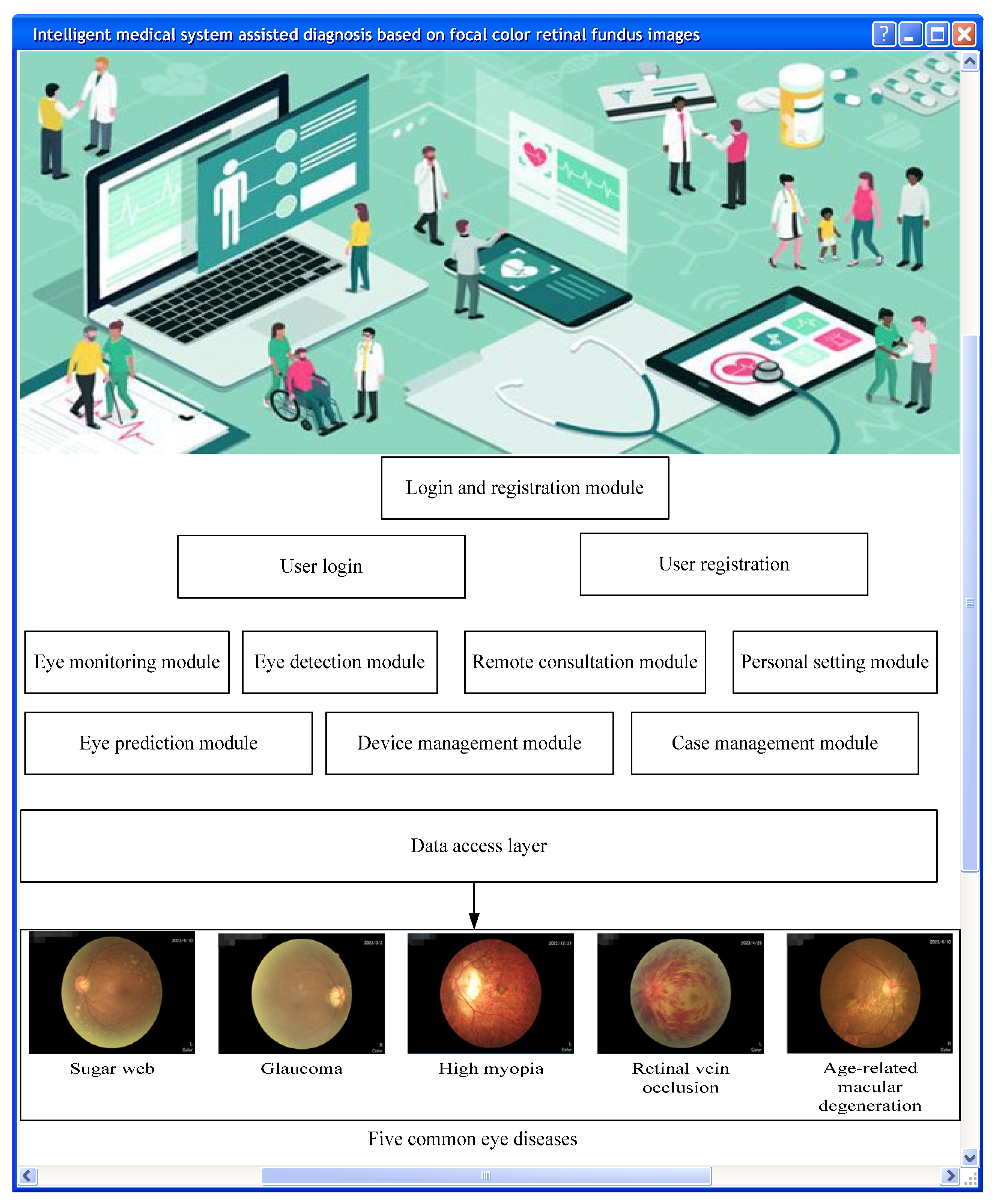
2.3. Smart Medical System
The smart medical system is an advanced medical service system that utilizes technologies such as artificial intelligence, BD, and cloud computing to achieve medical informatization and intelligence [
6,
7]. It can achieve disease diagnosis, treatment, and prevention more quickly and accurately via the effective integration and analysis of medical data, knowledge, and experience.
Smart medical systems can be applied to multiple medical fields, such as imaging, diagnosis, remote medicine, intelligent monitoring, drug research and development, etc. [
8,
9]. Among them, medical image diagnosis systems based on BD and artificial intelligence technology have become an important branch of smart medical systems. It can automatically detect, classify, and locate different medical images, improving the work efficiency and diagnostic accuracy of doctors [
10,
11]. In addition, smart healthcare systems can also achieve the sharing and allocation of medical resources by connecting hospitals, doctors, and patients. Compared with traditional healthcare, they have advantages such as more convenience, efficiency, low cost, and sustainability.
In short, smart medical systems are medical service systems with broad application prospects that integrate medical informatization and intelligence, providing patients with more convenient, efficient, and accurate medical services [
12,
13]. The system’s logical architecture design is shown in
Figure 2.
Color retinal FIs of lesions are one of the important criteria for ophthalmologists to diagnose and treat diseases. They can directly reflect information such as eye tissue structure and pathological changes and therefore have essential clinical value. The smart medical system is a new technology system for the diagnosis and treatment of ophthalmic diseases. By integrating and applying advanced computer technology and data processing algorithms, it provides accurate, fast, and intelligent diagnosis, detection, and analysis services for ophthalmologists [
14,
15].
In smart medical systems, color retinal FIs of lesions are an important component [
16]. By utilizing computer vision and artificial intelligence technology to analyze these images, automated disease diagnosis and auxiliary decision making can be achieved, improving diagnostic accuracy and efficiency. The emergence of such smart medical systems would greatly help the work of ophthalmologists by alleviating the shortage of physician resources and improving the efficiency and accuracy of medical care, which is expected to bring new opportunities and challenges for the treatment and prevention of ophthalmic diseases [
17,
18].