The Association of suPAR with Cardiovascular Risk Factors in Young and Healthy Adults
Abstract
:1. Introduction
2. Methods
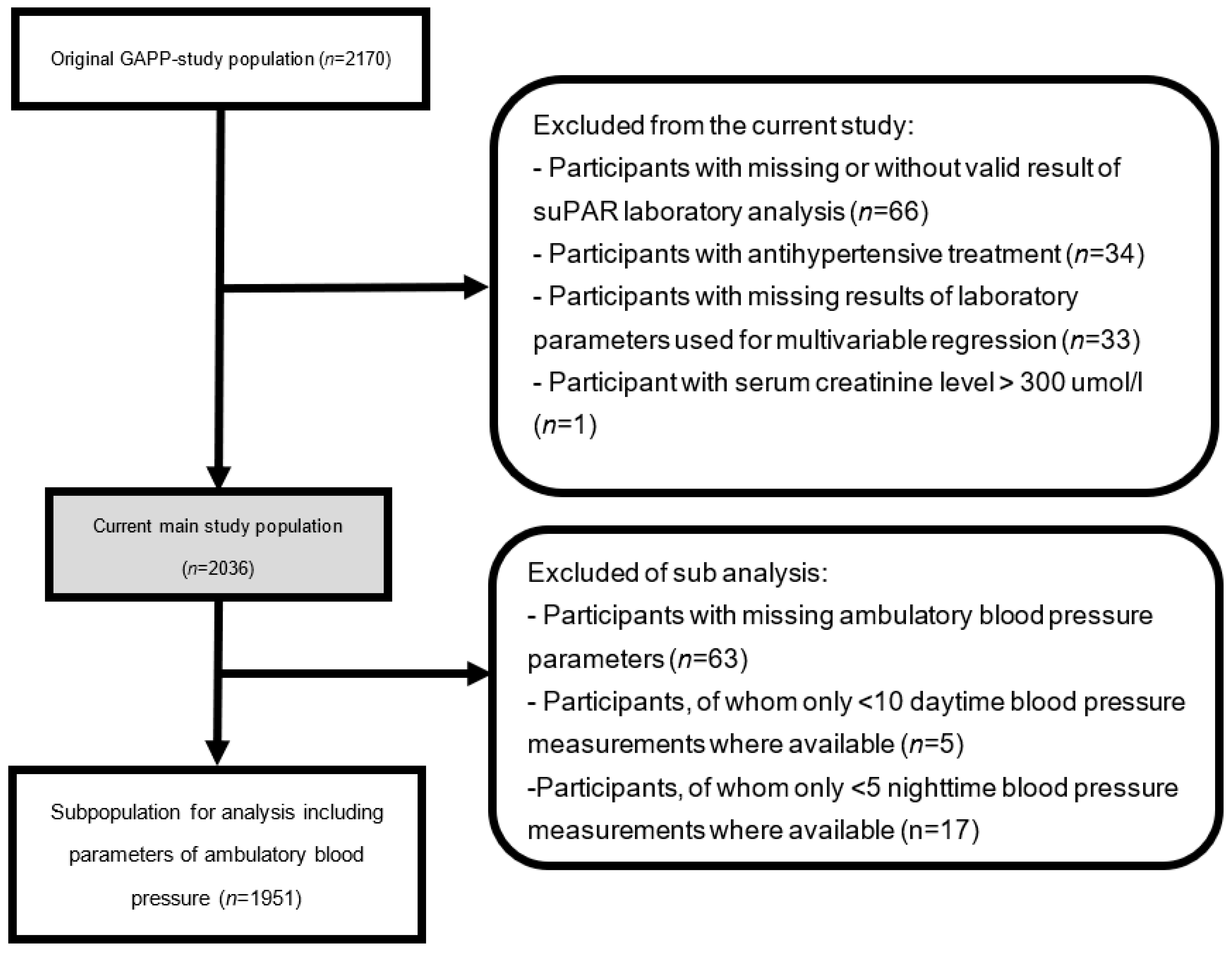
2.1. Study Population
2.2. Assessment of Laboratory Parameters
2.3. Cardiovascular Risk Profile Algorithms
2.4. Blood Pressure Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Westermann, D.; Neumann, J.T.; Sörensen, N.A.; Blankenberg, S. High-sensitivity assays for troponin in patients with cardiac disease. Nat. Rev. Cardiol. 2017, 14, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.; Eugen-Olsen, J.; Kofoed, K.; Iversen, J.; Haugaard, S.B. Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV-infected patients receiving highly active antiretroviral therapy. J. Med. Virol. 2008, 80, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Montuori, N.; Visconte, V.; Rossi, G.; Ragno, P. Soluble and cleaved forms of the urokinase-receptor: Degradation products or active molecules? Thromb. Haemost. 2005, 93, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W.; Marshall, C.J. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010, 11, 23–36. [Google Scholar] [CrossRef]
- Thunø, M.; Macho, B.; Eugen-Olsen, J. suPAR: The molecular crystal ball. Dis. Markers 2009, 27, 157–172. [Google Scholar] [CrossRef]
- Florquin, S.; van den Berg, J.G.; Olszyna, D.P.; Claessen, N.; Opal, S.M.; Weening, J.J.; Van Der Poll, T. Release of urokinase plasminogen activator receptor during urosepsis and endotoxemia. Kidney Int. 2001, 59, 2054–2061. [Google Scholar] [CrossRef]
- Yang, Q.; Cogswell, M.E.; Flanders, W.D.; Hong, Y.; Zhang, Z.; Loustalot, F.; Gillespie, C.; Merritt, R.; Hu, F.B. Trends in Cardiovascular Health Metrics and Associations with All-Cause and CVD Mortality among US Adults. JAMA 2012, 307, 1273–1283. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Conen, D.; Schön, T.; Aeschbacher, S.; Paré, G.; Frehner, W.; Risch, M.; Risch, L. Genetic and phenotypic determinants of blood pressure and other cardiovascular risk factors (GAPP). Swiss Med. Wkly. 2013, 143, w13728. [Google Scholar] [CrossRef] [PubMed]
- SN EN ISO/IEC 17025; Allgemeine Anforderung an die Kompetenz von Prüf- und Kalibrierlaboren. DIN: Berlin, Germany, 2017.
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Aeschbacher, S.; Bossard, M.; Ruperti Repilado, F.J.; Good, N.; Schoen, T.; Zimny, M.; Probst-Hensch, N.M.; Schmidt-Trucksäss, A.; Risch, M.; Risch, L.; et al. Healthy lifestyle and heart rate variability in young adults. Eur. J. Prev. Cardiol. 2016, 23, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Itoh, K.; Uezono, K.; Sasaki, H. A Simple Method for Estimating 24 h Urinary Sodium and Potassium Excretion from Second Morning Voiding Urine Specimen in Adults. Clin. Exp. Pharmacol. Physiol. 1993, 20, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, T.G.; Protogerou, A.D.; Vrachatis, D.; Konstantonis, G.; Aissopou, E.; Argyris, A.; Nasothimiou, E.; Gialafos, E.J.; Karamanou, M.; Tousoulis, D.; et al. Mean arterial pressure values calculated using seven different methods and their associations with target organ deterioration in a single-center study of 1878 individuals. Hypertens. Res. 2016, 39, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Meaney, E.; Alva, F.; Moguel, R.; Meaney, A.; Alva, J.; Webel, R. Formula and nomogram for the sphygmomanometric calculation of the mean arterial pressure. Heart 2000, 84, 64. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- Frary, C.E.; Biering-Sørensen, T.; Nochioka, K.; Blicher, M.K.; Olesen, T.B.; Stidsen, J.V.; Greve, S.V.; Vishram-Nielsen, J.K.K.; Rasmussen, S.L.; Eugen-Olsen, J.; et al. Sex- and age-related differences in the predictive capability of circulating biomarkers: From the MONICA 10 cohort. Scand. Cardiovasc. J. 2021, 55, 65–72. [Google Scholar] [CrossRef]
- Mehta, A.; Desai, S.; Ko, Y.; Liu, C.; Dhindsa, D.S.; Nayak, A.; Hooda, A.; Martini, M.A.; Ejaz, K.; Sperling, L.S.; et al. Sex Differences in Circulating Soluble Urokinase-Type Plasminogen Activator Receptor (suPAR) Levels and Adverse Outcomes in Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e015457. [Google Scholar] [CrossRef]
- Haupt, T.H.; Kallemose, T.; Ladelund, S.; Rasmussen, L.J.H.; Thorball, C.W.; Andersen, O.; Pisinger, C.; Eugen-Olsen, J. Risk Factors Associated with Serum Levels of the Inflammatory Biomarker Soluble Urokinase Plasminogen Activator Receptor in a General Population. Biomark. Insights 2014, 9, 91–100. [Google Scholar] [CrossRef]
- Lyngbæk, S.; Marott, J.L.; Sehestedt, T.; Hansen, T.W.; Olsen, M.H.; Andersen, O.; Linneberg, A.; Haugaard, S.B.; Eugen-Olsen, J.; Hansen, P.R.; et al. Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham Risk Score. Int. J. Cardiol. 2013, 167, 2904–2911. [Google Scholar] [CrossRef] [PubMed]
- Lyngbæk, S.; Sehestedt, T.; Marott, J.L.; Hansen, T.W.; Olsen, M.H.; Andersen, O.; Linneberg, A.; Madsbad, S.; Haugaard, S.B.; Eugen-Olsen, J.; et al. CRP and SuPAR Are Differently Related to Anthropometry and Subclinical Organ Damage. Int. J. Cardiol. 2013, 167, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Eugen-Olsen, J.; Andersen, O.; Linneberg, A.; Ladelund, S.; Hansen, T.; Langkilde, A.; Petersen, J.; Pielak, T.; Møller, L.N.; Jeppesen, J.; et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J. Intern. Med. 2010, 268, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Sherif, E.M.; El Maksood, A.A.A.; Youssef, O.I.; Salah El-Din, N.Y.; Khater, O.K.M. Soluble Urokinase Plasminogen Activator Receptor in Type 1 Diabetic Children, Relation to Vascular Complications. J. Diabetes Complicat. 2019, 33, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, M.Z.; Diederichsen, S.Z.; Mickley, H.; Steffensen, F.H.; Lambrechtsen, J.; Sand, N.P.R.; Christensen, K.L.; Olsen, M.H.; Diederichsen, A.; Grønhøj, M.H. Prognostic value of suPAR and hs-CRP on cardiovascular disease. Atherosclerosis 2018, 271, 245–251. [Google Scholar] [CrossRef]
- Pinho-Gomes, A.C.; Peters, S.A.E.; Thomson, B.; Woodward, M. Sex Differences in Prevalence, Treatment and Control of Cardiovascular Risk Factors in England. Heart 2020. [Google Scholar] [CrossRef]
- Sehestedt, T.; Lyngbæk, S.; Eugen-Olsen, J.; Jeppesen, J.; Andersen, O.; Hansen, T.; Linneberg, A.; Jørgensen, T.; Haugaard, S.; Olsen, M. Soluble urokinase plasminogen activator receptor is associated with subclinical organ damage and cardiovascular events. Atherosclerosis 2011, 216, 237–243. [Google Scholar] [CrossRef]
| Total Study Population | n = 2036 | ||
|---|---|---|---|
| Sex | Men (n = 929) | Women (n = 1107) | |
| Age, years | 37 (31–40) | 37 (31–40) | 0.667 |
| BMI, kg/m2 | 25.8 ± 3.3 | 23.4 ± 3.8 | <0.001 |
| Regular physical activity, n (%) | 683 (74%) | 733 (66%) | <0.001 |
| Irregular physical activity, n (%) | 246 (26%) | 374 (34%) | |
| Alcohol consumption, g/day | 1.4 (0.0–3.0) | 0.0 (0.0–0.8) | <0.001 |
| Smoking (current), n (%) | 237 (26%) | 207 (19%) | <0.001 |
| Smoking (past), n (%) | 222 (24%) | 251 (23%) | |
| Smoking (never), n (%) | 470 (50%) | 649 (58%) | |
| Mean office SBP, mmHg | 127 ± 11 | 113 ± 10 | <0.001 |
| Mean office DBP, mmHg | 82 ± 8 | 75 ± 8 | <0.001 |
| Mean arterial pressure, mmHg | 101 ± 9 | 91 ± 8 | <0.001 |
| Fasting glucose, mmol/L | 5.0 ± 0.5 | 4.7 ± 0.4 | <0.001 |
| HbA1c, % | 5.4 ± 0.4 | 5.4 ± 0.4 | 0.013 |
| Cholesterol, mmol/L | 5.1 ± 1.0 | 4.8 ± 0.8 | <0.001 |
| LDL-C, mmol/L | 3.3 ± 0.9 | 2.7 ± 0.7 | <0.001 |
| HDL-C, mmol/L | 1.3 ± 0.3 | 1.7 ± 0.4 | <0.001 |
| suPAR, ng/mL | 1.5 ± 0.6 | 1.7 ± 0.1 | <0.001 |
| hsCRP, mg/L | 1.70± 3.8 | 1.97 ± 4.7 | 0.152 |
| eGFR, mL/min/1.73 m2 | 116 ± 14 | 140 ± 18 | <0.001 |
| Healthy lifestyle score | 4 ± 1.2 | 5 ± 1.1 | <0.001 |
| Framingham score | 2 ± 2 | 3 ± 1.4 | <0.001 |
| Subgroup (n = 1951) | Subgroup (n = 897) | Subgroup (n = 1054) | |
| Mean 24 h SBP, mmHg | 130 ± 10 | 117 ± 9 | <0.001 |
| Mean daytime SBP, mmHg | 134 ± 11 | 121 ± 9 | <0.001 |
| Mean nighttime SBP, mmHg | 114 ± 11 | 104 ± 10 | <0.001 |
| Mean 24 h DBP, mmHg | 82 ± 8 | 75 ± 7 | <0.001 |
| Mean daytime DBP, mmHg | 85 ± 8 | 79 ± 7 | <0.001 |
| Mean nighttime DBP, mmHg | 69 ± 8 | 64 ± 7 | <0.001 |
| Male Study Population | n = 929 | ||||
|---|---|---|---|---|---|
| Quartiles Based on suPAR Levels | Quartile 1 (n = 233) | Quartile 2 (n = 232) | Quartile 3 (n = 232) | Quartile 4 (n = 232) | p-Value |
| suPAR Range (ng/L), men | 1.10 | 1.10–1.40 | 1.40–1.70 | 1.70 | |
| Age, years | 37 (32–41) | 36 (30–40) | 37 (32–40) | 36 (31–40) | 0.851 |
| BMI, kg/m2 | 25.5 2.8 | 25.6 3.2 | 26.1 3.4 | 26.1 3.6 | 0.014 |
| Regular physical activity, min/week | 168 (72%) | 159 (69%) | 175 (75%) | 181 (78%) | 0.257 |
| Irregular physical activity, min/week | 65 (28%) | 73 (31%) | 57 (25%) | 51 (22%) | |
| Alcohol consumption, g/day | 1.7 (0.6–3.0) | 1.4 (0.0–3.1) | 1.4 (0.0–2.5) | 0.9 (0.0–2.4) | 0.329 |
| Smoking (current), n (%) | 46 (20%) | 43 (18%) | 47 (20%) | 101 (44%) | |
| Smoking (past), n (%) | 51 (22%) | 60 (26%) | 55 (24%) | 56(24%) | <0.001 |
| Smoking (never), n (%) | 136 (58%) | 129 (56%) | 130 (56%) | 75 (32%) | |
| Mean office SBP, mmHg | 127 10 | 128 11 | 128 12 | 127 10.9 | 0.402 |
| Mean office SBP, mmHg | 82 | 82 8 | 83 8 | 82 8 | 0.943 |
| Mean arterial pressure, mmHg | 101 8 | 101 9 | 101 9 | 101 9 | 0.632 |
| Fasting glucose, mmol/L | 5.0 0.4 | 5.0 0.4 | 5.0 0.5 | 5.0 0.5 | 0.103 |
| HbA1c, % | 5.4 0.4 | 5.4 0.4 | 5.4 0.4 | 5.5 0.4 | 0.222 |
| Cholesterol, mmol/L | 5.1 0.9 | 5.1 1.0 | 5.0 1.0 | 4.9 0.9 | 0.034 |
| LDL-C, mmol/L | 3.3 0.8 | 3.3 0.9 | 3.2 0.9 | 3.2 0.9 | 0.408 |
| HDL-C, mmol/L | 1.4 0.3 | 1.4 0.3 | 1.3 0.3 | 1.2 0.3 | <0.001 |
| hsCRP, mg/L | 0.6 (0.4–1.2) | 0.9 (0.5–1.6) | 0.9 (0.5–1.9) | 1.3 (0.9–2.3) | <0.001 |
| eGFR, mL/min/1.73 m2 | 117 14 | 117 13 | 115 14 | 114 13 | 0.011 |
| Healthy lifestyle score | 3.7 ± 1.2 | 3.6 ± 1.3 | 3.6 ± 1.2 | 3.3 ± 1.2 | <0.001 |
| Framingham score | 1.1 ± 1.8 | 1.4 ± 1.7 | 1.6 ± 1.7 | 1.9 ± 1.7 | <0.001 |
| Subgroup (n = 897) | |||||
| Quartiles based on suPAR levels | Quartile 1 (n = 225) | Quartile 2 (n = 224) | Quartile 3 (n = 224) | Quartile 4 (n = 224) | |
| Mean 24 h SBP, mmHg | 129 ± 9 | 130 ± 10 | 130 ± 10 | 130 ± 10 | 0.727 |
| Mean daytime SBP, mmHg | 133 ± 9 | 134 ± 11 | 134 ± 11 | 134 ± 11 | 0.617 |
| Mean nighttime SBP, mmHg | 114 ± 10 | 114 ± 11 | 115 ± 11 | 114 ± 11 | 0.864 |
| Mean 24 h DBP, mmHg | 82 ± 6 | 82 ± 8 | 82 ± 8 | 81 ± 8 | 0.993 |
| Mean daytime DBP, mmHg | 85 ± 7 | 85 ± 9 | 85 ± 9 | 85 ± 8 | 0.960 |
| Mean nighttime DBP, mmHg | 69 ± 7 | 69 ± 8 | 70 ± 8 | 69 ± 9 | 0.984 |
| Male Study Population | n = 929 | |||||
|---|---|---|---|---|---|---|
| Quartiles Based on suPAR Levels | Continuous (n = 929) | Quartile 1 (n = 233) | Quartile 2 (n = 232) | Quartile 3 (n = 232) | Quartile 4 (n = 232) | p-Value |
| BMI, kg/m2 | −0.056 | reference | 0.014 | 0.040 | −0.009 | 0.115 |
| Irregular physical activity, % | 0.780 | reference | −0.037 | 0.029 | 0.059 | 0.014 |
| Alcohol consumption, g/day | 0.055 | reference | −0.024 | −0.052 | −0.055 | 0.085 |
| Current smoking, % | 0.267 | reference | −0.107 ** | −0.035 | 0.231 ** | <0.001 |
| Past smoking, % | 0.047 | reference | −0.039 | 0.002 | 0.059 | 0.164 |
| Mean office SBP, mmHg | −0.020 | reference | −0.011 | 0.054 | −0.004 | 0.561 |
| Mean office DBP, mmHg | 0.030 | reference | 0.010 | 0.035 | −0.018 | 0.380 |
| Mean office MAP, mmHg | 0.032 | reference | ≥0.001 | 0.049 | −0.012 | 0.351 |
| Fasting glucose, mmol/L | −0.003 | reference | 0.007 | 0.011 | 0.037 | 0.932 |
| HbA1c, % | 0.004 | reference | −0.046 | 0.004 | 0.012 | 0.910 |
| Cholesterol, mmol/L | −0.048 | reference | 0.001 | −0.029 | −0.082 * | 0.149 |
| LDL-C, mmol/L | −0.030 | reference | 0.023 | 0.001 | −0.030 | 0.381 |
| HDL-C, mmol/L | −0.155 | reference | −0.044 | −0.103 * | −0.150 * | <0.001 |
| Healthy lifestyle score | −0.129 | reference | 0.035 | −0.035 | −0.091 ** | 0.001 |
| Framingham score | 0.161 | reference | −0.027 | 0.039 | 0.124 ** | <0.001 |
| Subgroup (n = 897) | ||||||
| Quartiles based on suPAR levels | Continuous (n = 897) | Quartile 1 (n = 225) | Quartile 2 (n = 224) | Quartile 3 (n = 224 | Quartile 4 (n = 224) | |
| Mean 24 h BP, mmHg | −0.019 | reference | −0.003 | 0.003 | 0.027 | 0.561 |
| Mean daytime BP, mmHg | −0.017 | reference | −0.007 | 0.011 | 0.010 | 0.623 |
| Mean nighttime BP, mmHg | −0.035 | reference | 0.027 | −0.030 | 0.049 | 0.295 |
| Mean 24 h BP, mmHg | −0.025 | reference | 0.005 | 0.008 | 0.029 | 0.491 |
| Mean daytime BP, mmHg | −0.027 | reference | 0.008 | 0.011 | 0.009 | 0.453 |
| Mean nighttime BP, mmHg | −0.019 | reference | 0.005 | −0.013 | 0.063 | 0.594 |
| Female Study Population | n = 1107 | ||||
|---|---|---|---|---|---|
| Quartiles based on suPAR levels | Quartile 1 (n = 277) | Quartile 2 (n = 277) | Quartile 3 (n = 277) | Quartile 4 (n = 276) | p-Value |
| suPAR range (ng/L), women | 1.30 | 1.30–1.60 | 1.60–2.00 | 2.00 | |
| Age, years | 37 (31–41) | 37 (31–40) | 36 (31–40) | 37 (31–40) | 0.720 |
| BMI, kg/m2 | 23.0 3.8 | 23.1 3.6 | 23.1 3.5 | 24.2 4.1 | <0.001 |
| Regular physical activity, min/week | 176 (64%) | 183 (66%) | 190 (69%) | 184 (67%) | 0.108 |
| Irregular physical activity, min/week | 101 (36%) | 94 (34%) | 87 (31%) | 92 (33%) | |
| Alcohol consumption, g/day | 0.0 (0.0–1.3) | 0.0 (0.0–0.6) | 0.0 (0.0–0.9) | 0.0 (0.0–0.6) | 0.176 |
| Smoking (current), n (%) | 38 (14%) | 35 (19%) | 49 (18%) | 85 (31%) | |
| Smoking (past), n (%) | 78 (28%) | 67 (26%) | 56 (20%) | 50 (18%) | <0.001 |
| Smoking (never), n (%) | 161 (58%) | 175 (56%) | 172 (62%) | 141 (51%) | |
| Mean office SBP, mmHg | 113 ± 10 | 113 ± 10 | 114 ± 10 | 114 ± 11 | 0.784 |
| Mean office DBP, mmHg | 74.3 ± 8 | 75 ± 8 | 75 ± 8 | 75 ± 8 | 0.220 |
| Mean arterial pressure, mmHg | 90 ± 8 | 90 ± 8 | 91 ± 8 | 91 ± 9 | 0.406 |
| Fasting glucose, mmol/L | 4.6 ± 0.4 | 4.7 ± 0.4 | 4.7 ± 0.5 | 4.8 ± 0.5 | 0.005 |
| HbA1c, % | 5,3 ± 0.4 | 5.3 ± 0.3 | 5.4 ± 0.4 | 5.5 ± 0.4 | <0.001 |
| Cholesterol, mmol/L | 4.9 ± 0.8 | 4.7 ± 0.8 | 4.7 ± 0.8 | 4.7 ± 0.8 | 0.001 |
| LDL-C, mmol/L | 2.8 ± 0.7 | 2.6 ± 0.7 | 2.7 ± 0.8 | 2.7 ± 0.7 | 0.858 |
| HDL-C, mmol/L | 1.8 ± 0.4 | 1.7 ± 0.4 | 1.7 ± 0.4 | 1.6 ± 0.4 | <0.001 |
| Triglyceride, mmol/L | 0.7 (0.6–1.0) | 0.7 (0.5–0.9) | 0.7 (0.6–0.9) | 0.8 (0.6–1.1) | 0.283 |
| hsCRP, mg/L | 0.9 (0.5–2.0) | 0.8 (0.4–1.9) | 0.90 (0.5–1.8) | 1.2 (0.6–2.8) | 0.048 |
| eGFR, mL/min/1.73 m2 | 141 ± 17 | 141 ± 18 | 140 ± 19 | 137 ± 16 | 0.009 |
| Healthy lifestyle score | 4.6 ± 1.1 | 4.6 ± 1.1 | 4.6 ± 1.1 | 4.3 ± 1.2 | 0.005 |
| Framingham score | 2.1 ± 1.1 | 2.1 ± 1.1 | 2.1 ± 1.1 | 2.1 ± 1.1 | 0.096 |
| Subgroup (n = 1054) | |||||
| Quartiles based on suPAR levels | Quartile 1 (n = 264) | Quartile 2 (n = 264) | Quartile 3 (n = 263) | Quartile 4 (n = 263) | p-Value |
| Mean 24 h BP, mmHg | 118 ± 10 | 116 ± 8 | 117 ± 9 | 118 ± 9 | 0.560 |
| Mean daytime BP, mmHg | 122 ± 10 | 120 ± 8 | 120 ± 9 | 121 ±10 | 0.562 |
| Mean nighttime BP, mmHg | 105 ± 11 | 104 ± 9 | 104 ± 9 | 105 ± 10 | 0.746 |
| Mean 24 h BP, mmHg | 76 ± 7 | 75 ± 6 | 75 ± 7 | 76 ± 7 | 0.732 |
| Mean daytime BP, mmHg | 79 ± 7 | 78 ± 7 | 78 ± 7 | 79 ± 7 | 0.824 |
| Mean nighttime BP, mmHg | 64 ± 7 | 64 ± 7 | 63 ± 6 | 64 ± 7 | 0.680 |
| Female Study Population | n = 1107 | |||||
|---|---|---|---|---|---|---|
| Quartiles Based on sST2 Levels | Continuous (n = 1107) | Quartile 1 (n = 277) | Quartile 2 (n = 277) | Quartile 3 (n = 277) | Quartile 4 (n = 276) | p-Value |
| BMI, kg/m2 | 0.031 | reference | 0.002 | 0.007 | 0.044 | 0.365 |
| Irregular physical activity, % | 0.053 | Reference | 0.013 | 0.062 | −0.015 | 0.075 |
| Alcohol consumption, g/day | −0.038 | reference | −0.002 | <0.001 | −0.041 | 0.216 |
| Current smoking, % | 0.076 | reference | −0.095 ** | −0.014 | 0.144 ** | 0.017 |
| Past smoking, % | −0.009 | reference | −0.017 | −0.037 | −0.020 | 0.770 |
| Mean office SBP, mmHg | 0.014 | reference | −0.004 | 0.005 | −0.014 | 0.657 |
| Mean office DBP, mmHg | 0.060 | reference | 0.036 | 0.041 | 0.026 | 0.056 |
| Mean Office MAP, mmHg | 0.042 | reference | 0.018 | 0.025 | 0.008 | 0.186 |
| Fasting glucose, mmol/L | 0.011 | reference | −0.012 | 0.026 | 0.049 | 0.718 |
| HbA1c, % | 0.081 | reference | 0.028 | 0.068 | 0.137 ** | 0.008 |
| Cholesterol, mmol/L | −0.094 | reference | −0.141 ** | −0.120 ** | −0.168 ** | 0.001 |
| LDL-C, mmol/L | −0.051 | reference | −0.075 * | −0.036 | −0.063 | 0.102 |
| HDL-C, mmol/L | −0.114 | reference | −0.092 ** | −0.123 | −0.214 | <0.001 |
| Healthy lifestyle score | −0.037 | reference | 0.056 | 0.015 | −0.104 ** | 0.226 |
| Framingham score, points | 0.034 | reference | 0.001 | 0.022 | 0.0034 | 0.261 |
| Subgroup (n = 1054) | ||||||
| Quartiles based on suPAR levels | Continuous (n = 1054) | Quartile 1 (n = 264) | Quartile 2 (n = 264) | Quartile 3 (n = 263) | Quartile 4 (n = 263) | p-Value |
| Mean 24 h BP, mmHg | −0.006 | reference | 0.052 | −0.031 | −0.026 | 0.849 |
| Mean daytime BP, mmHg | −0.003 | reference | 0.054 | −0.039 | −0.026 | 0.915 |
| Mean nighttime BP, mmHg | −0.003 | reference | 0.025 | 0.005 | −0.026 | 0.909 |
| Mean 24 h BP, mmHg | 0.023 | reference | 0.022 | −0.011 | −0.008 | 0.439 |
| Mean daytime BP, mmHg | 0.025 | reference | 0.026 | −0.027 | −0.001 | 0.407 |
| Mean nighttime BP, mmHg | 0.024 | reference | −0.009 | −0.054 | −0.038 | 0.424 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wohlwend, N.F.; Grossmann, K.; Aeschbacher, S.; Weideli, O.C.; Telser, J.; Risch, M.; Conen, D.; Risch, L. The Association of suPAR with Cardiovascular Risk Factors in Young and Healthy Adults. Diagnostics 2023, 13, 2938. https://doi.org/10.3390/diagnostics13182938
Wohlwend NF, Grossmann K, Aeschbacher S, Weideli OC, Telser J, Risch M, Conen D, Risch L. The Association of suPAR with Cardiovascular Risk Factors in Young and Healthy Adults. Diagnostics. 2023; 13(18):2938. https://doi.org/10.3390/diagnostics13182938
Chicago/Turabian StyleWohlwend, Niklas Fabio, Kirsten Grossmann, Stefanie Aeschbacher, Ornella C. Weideli, Julia Telser, Martin Risch, David Conen, and Lorenz Risch. 2023. "The Association of suPAR with Cardiovascular Risk Factors in Young and Healthy Adults" Diagnostics 13, no. 18: 2938. https://doi.org/10.3390/diagnostics13182938
APA StyleWohlwend, N. F., Grossmann, K., Aeschbacher, S., Weideli, O. C., Telser, J., Risch, M., Conen, D., & Risch, L. (2023). The Association of suPAR with Cardiovascular Risk Factors in Young and Healthy Adults. Diagnostics, 13(18), 2938. https://doi.org/10.3390/diagnostics13182938









