MRI-Based Radiomics Analysis of Levator Ani Muscle for Predicting Urine Incontinence after Robot-Assisted Radical Prostatectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
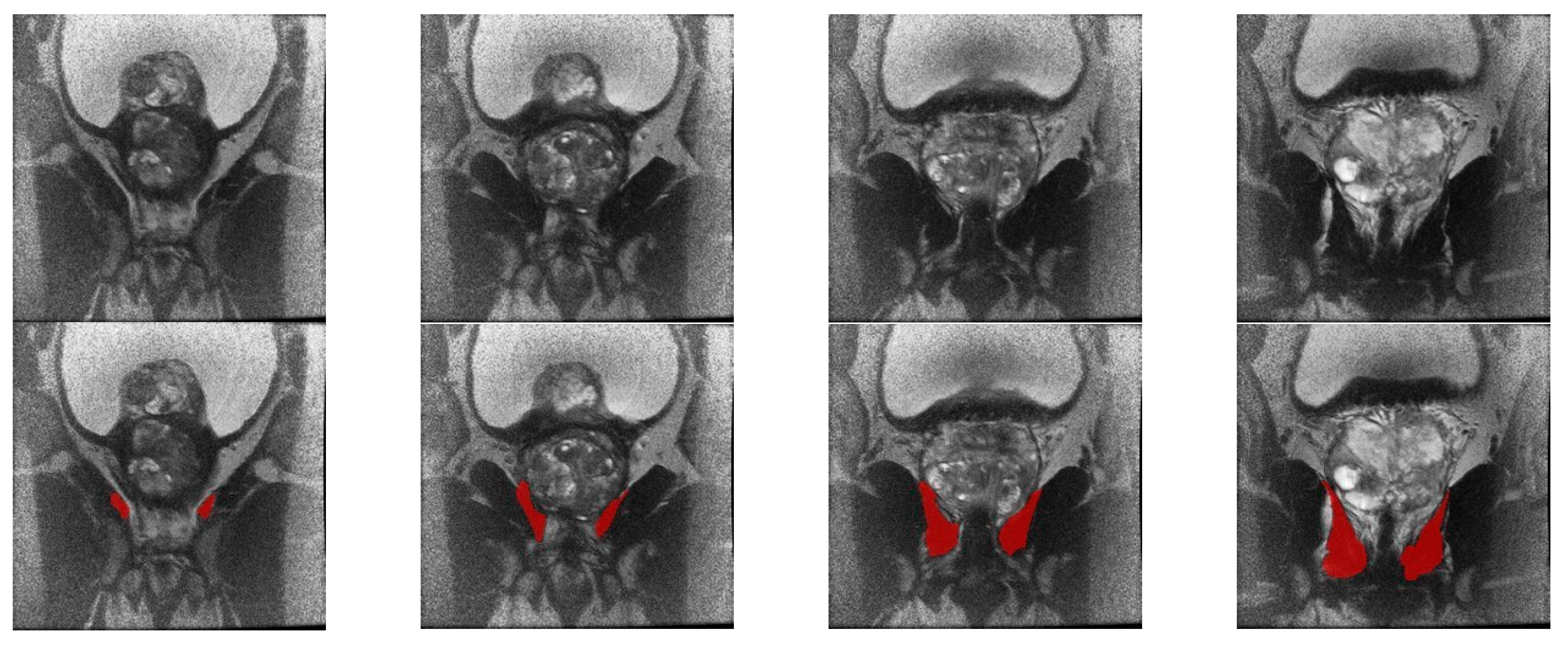
2.2. Image Acquisition and Preprocessing
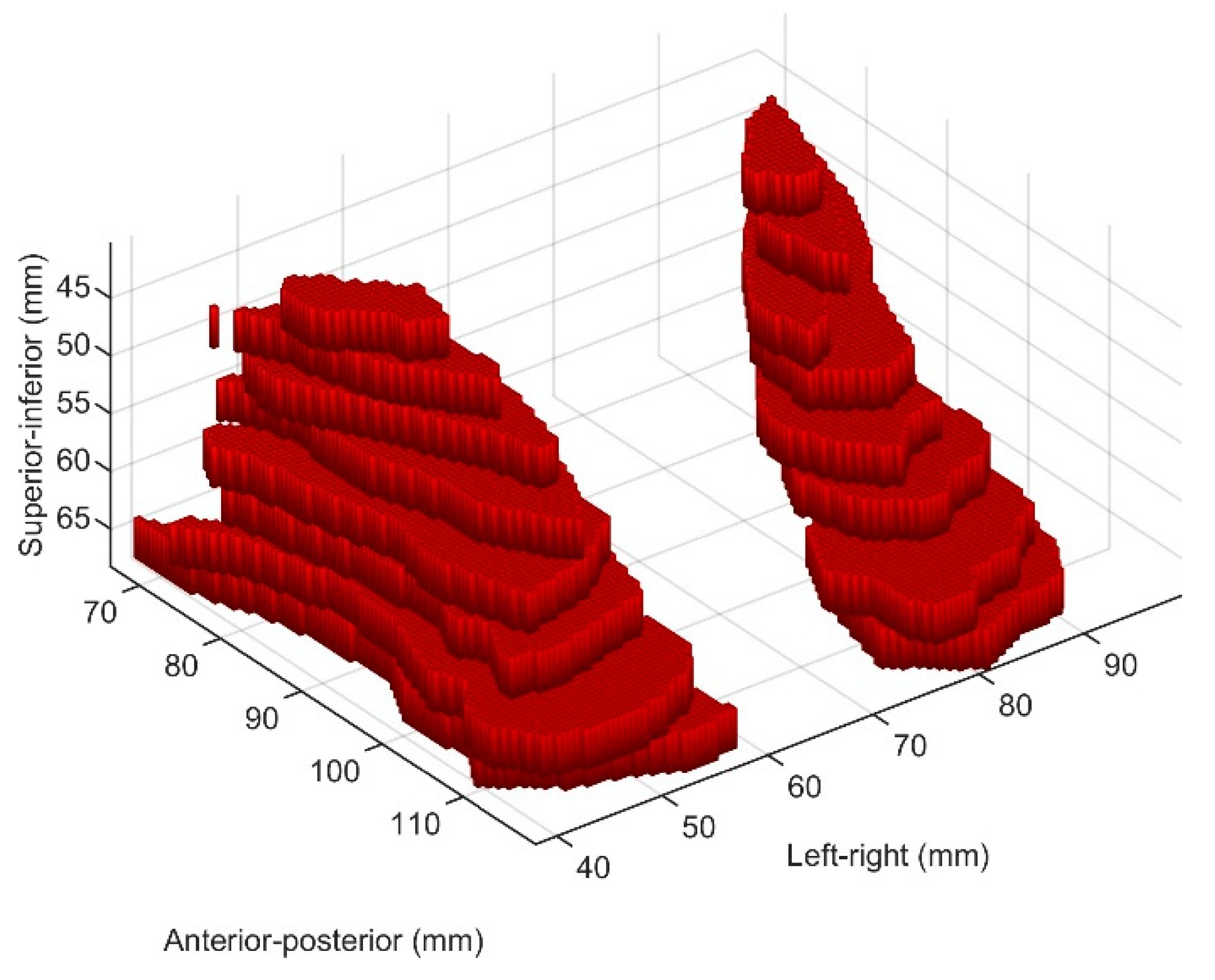
2.3. Biomarker Discovery Approach
2.4. Feature Extraction
2.5. Optimal Feature Selection
3. Results
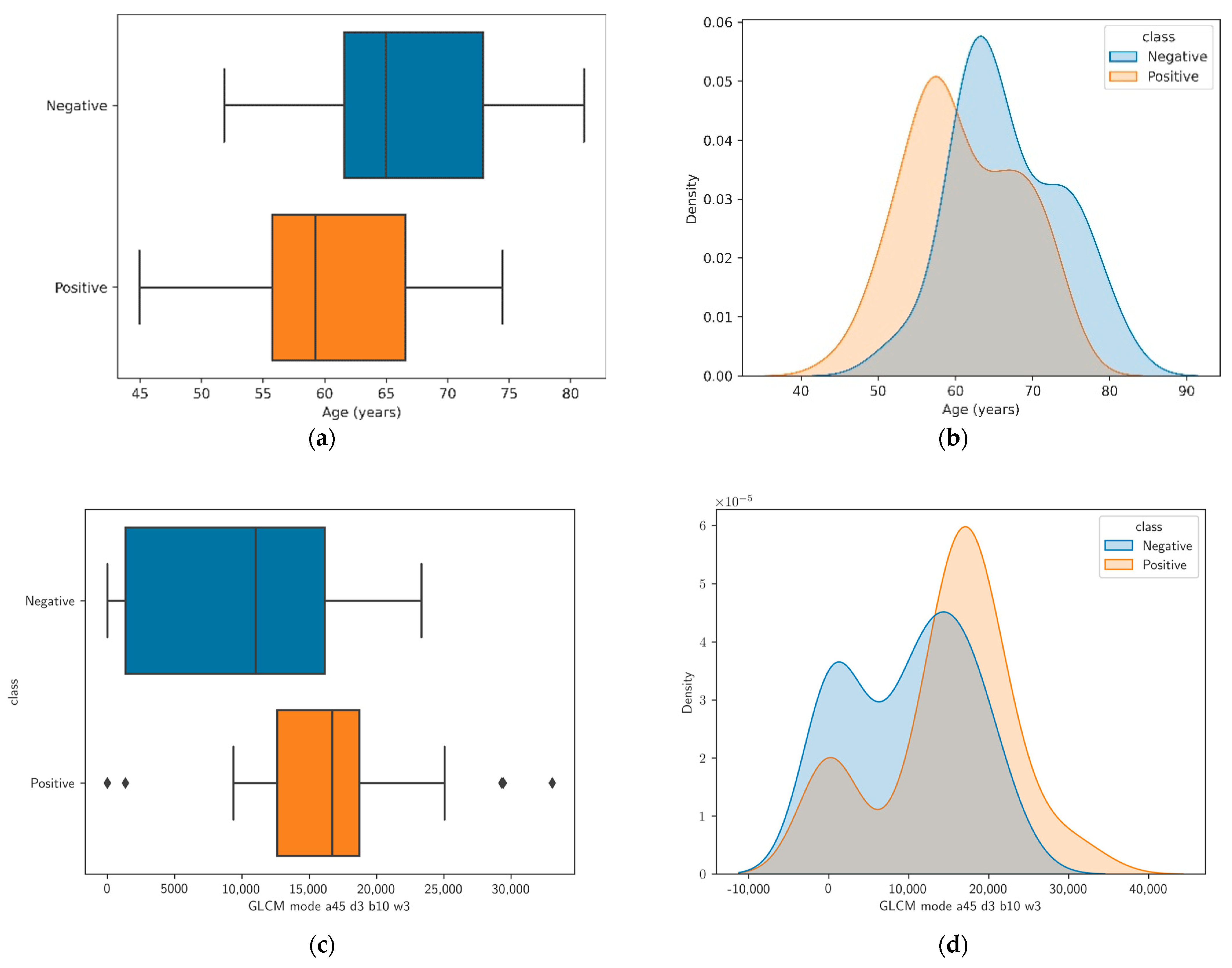
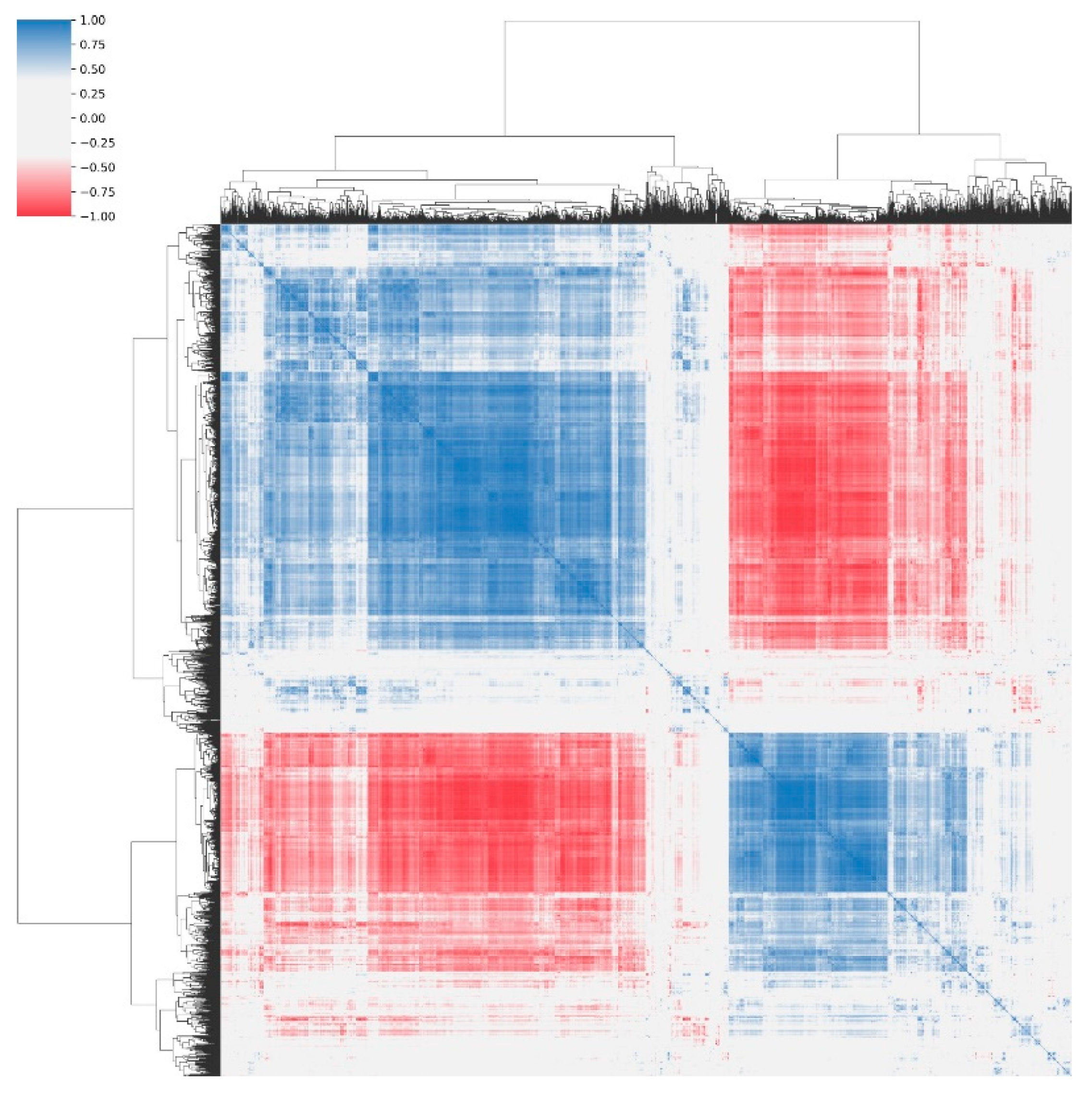
3.1. Univariate Analysis and Feature Correlation
3.2. Classification Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Feature Extraction, Selection, and Classification Algorithms
- Let λ1, λ2, and λ3 be the eigenvalues obtained from PCA.
- Let a, b, and c be the lengths of the equivalent ellipsoid’s major, intermediate, and minor axes, respectively.
- The relationship is: λ1 ∝ a2 λ2 ∝ b2 λ3 ∝ c2.
References
- Holze, S.; Mende, M.; Healy, K.V.; Koehler, N.; Gansera, L.; Truss, M.C.; Rebmann, U.; Degener, S.; Stolzenburg, J.-U. Comparison of various continence definitions in a large group of patients undergoing radical prostatectomy: A multicentre, prospective study. BMC Urol. 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Novara, G.; Rosen, R.C.; Artibani, W.; Carroll, P.R.; Costello, A.; Menon, M.; Montorsi, F.; Patel, V.R.; Stolzenburg, J.U.; et al. Systematic review and meta- analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Shafik, A. Levator ani muscle: New physioanatomical aspects and role in the micturition mechanism. World J. Urol. 1999, 17, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Hinata, N.; Murakami, G. The urethral rhabdosphincter, levator ani muscle, and perineal membrane: A review. BioMed Res. Int. 2014, 2014, 906921. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Muraki, O.; Yoshimura, Y. Evaluation of the effect of levator ani muscle contraction on post-prostatectomy urinary incontinence using cine MRI. Neurourol. Urodyn. 2022, 41, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Tutolo, M.; Rosiello, G.; Stabile, G.; Tasso, G.; Oreggia, D.; De Wever, L.; De Ridder, D.; Pellegrino, A.; Esposito, A.; De Cobelli, F.; et al. The key role of levator ani thickness for early urinary continence recovery in patients undergoing robot-assisted radical prostatectomy: A multi-institutional study. Eur. Urol. 2021, 79, S1640. [Google Scholar] [CrossRef]
- Lim, T.J.; Lee, J.H.; Lim, J.W.; Moon, S.K.; Jeon, S.H.; Chang, S.-G. Preoperative factors predictive of continence recovery after radical retropubic prostatectomy. Korean J. Urol. 2012, 53, 524–530. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nyu, L.G.; Udupa, J.K.; Zhang, X. New variants of a method of MRI scale standardization. IEEE Trans. Med. Imaging 2000, 19, 143–150. [Google Scholar]
- Grevera, G.; Udupa, J.; Odhner, D.; Zhuge, Y.; Souza, A.; Iwanaga, T.; Mishra, S. Cavass: A computer-assisted visualization and analysis software system. J. Digit. Imaging 2007, 20, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Udupa, J.K.; Sin, S.; Liu, Z.; Wileyto, E.P.; Torigian, D.A.; Arens, R. MR image analytics to characterize the upper airway structure in obese children with obstructive sleep apnea syndrome. PLoS ONE 2016, 11, e0159327. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Udupa, J.K.; Torigian, D.A.; Matsumoto, M.M.; Sin, S.; Arens, R. MR image analytics to characterize upper airway architecture in children with OSAS. In Medical Imaging 2015: Biomedical Applications in Molecular, Structural, and Functional Imaging; SPIE: Bellingham, WA, USA, 2015; Volume 9417, pp. 679–684. [Google Scholar]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural features for image classification. IEEE Trans. Syst. Man Cybern. 1973, 6, 610–621. [Google Scholar] [CrossRef]
- Scalco, E.; Rizzo, G. Texture analysis of medical images for radiotherapy applications. Br. J. Radiol. 2017, 90, 20160642. [Google Scholar] [CrossRef] [PubMed]
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Walz, J.; Burnett, A.L.; Costello, A.J.; Eastham, J.A.; Graefen, M.; Guillonneau, B.; Menon, M.; Montorsi, F.; Myers, R.P.; Rocco, B.; et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur. Urol. 2010, 57, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Frazier, P.I. Bayesian optimization. In Recent Advances in Optimization and Modeling of Contemporary Problems; Informs: Catonsville, MD, USA, 2018; pp. 255–278. [Google Scholar] [CrossRef]
- Davnall, F.; Yip, C.S.; Ljungqvist, G.; Selmi, M.; Ng, F.; Sanghera, B.; Ganeshan, B.; Miles, K.A.; Cook, G.J.; Goh, V. Assessment of tumor heterogeneity: An emerging imaging tool for clinical practice? Insights Imaging 2012, 3, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Muehlematter, U.J.; Mannil, M.; Becker, A.S.; Vokinger, K.N.; Finkenstaedt, T.; Osterhoff, G.; Fischer, M.A.; Guggenberger, R. Vertebral body insufficiency fractures: Detection of vertebrae at risk on standard CT images using texture analysis and machine learning. Eur. Radiol. 2019, 29, 2207–2217. [Google Scholar] [CrossRef]
- Mombiela, R.M.; Borr, C. The usefulness of radiomics methodology for developing descriptive and prognostic image-based phenotyping in the aging population: Results from a small feasibility study. Front. Aging 2022, 3, 853671. [Google Scholar] [CrossRef] [PubMed]
- Mammucari, C.; Rizzuto, R. Signaling pathways in mitochondrial dysfunction and aging. Mech. Ageing Dev. 2010, 131, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, G.; Sahin, F. A survey on feature selection methods. Comput. Electr. Eng. 2014, 40, 16–28. [Google Scholar] [CrossRef]
- Demircio, A. Benchmarking feature selection methods in radiomics. Investig. Radiol. 2022, 57, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.; Eriksson, D.; McCourt, M.; Kiili, J.; Laaksonen, E.; Xu, Z.; Guyon, I. Bayesian optimization is superior to random search for machine learning hyperparameter tuning: Analysis of the black-box optimization challenge 2020. In NeurIPS 2020 Competition and Demonstration Track; PMLR: Cambridge, MA, USA, 2021; pp. 3–26. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahait, M.; Usamentiaga, R.; Tong, Y.; Sandberg, A.; Lee, D.I.; Udupa, J.K.; Torigian, D.A. MRI-Based Radiomics Analysis of Levator Ani Muscle for Predicting Urine Incontinence after Robot-Assisted Radical Prostatectomy. Diagnostics 2023, 13, 2913. https://doi.org/10.3390/diagnostics13182913
Shahait M, Usamentiaga R, Tong Y, Sandberg A, Lee DI, Udupa JK, Torigian DA. MRI-Based Radiomics Analysis of Levator Ani Muscle for Predicting Urine Incontinence after Robot-Assisted Radical Prostatectomy. Diagnostics. 2023; 13(18):2913. https://doi.org/10.3390/diagnostics13182913
Chicago/Turabian StyleShahait, Mohammed, Ruben Usamentiaga, Yubing Tong, Alex Sandberg, David I. Lee, Jayaram K. Udupa, and Drew A. Torigian. 2023. "MRI-Based Radiomics Analysis of Levator Ani Muscle for Predicting Urine Incontinence after Robot-Assisted Radical Prostatectomy" Diagnostics 13, no. 18: 2913. https://doi.org/10.3390/diagnostics13182913
APA StyleShahait, M., Usamentiaga, R., Tong, Y., Sandberg, A., Lee, D. I., Udupa, J. K., & Torigian, D. A. (2023). MRI-Based Radiomics Analysis of Levator Ani Muscle for Predicting Urine Incontinence after Robot-Assisted Radical Prostatectomy. Diagnostics, 13(18), 2913. https://doi.org/10.3390/diagnostics13182913







