Hepatic Inflammatory Pseudotumor—Focusing on Its Heterogeneity
Abstract
1. Introduction
2. Pathological Findings and Clinical Features
3. Differential Diagnosis
3.1. Hepatic Inflammatory Myofibroblastic Tumor (IMT)
3.2. Primary Sclerosing Cholangitis (PSC)
3.3. Follicular Dendritic Cell Tumor (FDC)
3.4. Hepatic Pseudolymphoma
4. Pathogenesis
- (1)
- Although the pathogenesis of IPT is still unclear, several hypotheses have been proposed.
- (2)
- The relation between IPT and post-infection and inflammatory processes:
- (3)
- The relation between IPT and eosinophilia:
- (4)
- The relation between IPT and hepatitis B virus (HBV):
- (5)
- The close relation between IPT and IgG4-related immune reactions:
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Shek, T.W.; Ng, I.O.; Chan, K.W. Inflammatory pseudotumor of the liver. Report of four cases and re-view of the literature. Am. J. Surg. Pathol. 1993, 17, 231–238. [Google Scholar] [CrossRef]
- Horiuchi, R.; Uchida, T.; Kojima, T.; Shikata, T. Inflammatory pseudotumor of the liver. Clinicopathologic study and review of the literature. Cancer 1990, 65, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Coffin, C.M.; Humphrey, P.A.; Dehner, L.P. Extrapulmonary inflammatory myofibroblastic tumor: A clinical and pathological survey. Semin. Diagn. Pathol. 1998, 15, 85–101. [Google Scholar] [PubMed]
- Anthony, P.P. Inflammatory pseudotumour (plasma cell granuloma) of lung, liver and other organs. Diagnostics 1993, 23, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Tsuneyama, K.; Masuda, S.; Tomioka, T. Hepatic inflammatory pseudotumor associated with chronic cholangitis: Report of three cases. Hum. Pathol. 1994, 25, 86–91. [Google Scholar] [CrossRef]
- Zen, Y.; Fujii, T.; Sato, Y.; Masuda, S.; Nakanuma, Y. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod. Pathol. 2007, 20, 884–894. [Google Scholar] [CrossRef]
- Zhao, J.; Olino, K.; Low, L.E.; Qiu, S.; Stevenson, H.L. Hepatic Inflammatory Pseudotumor: An Important Differential Diagnosis in Patients With a History of Previous Biliary Procedures. ACG Case Rep. J. 2019, 6, e00015. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Biskup, E.; Cai, F.; Li, A. Inflammatory pseudotumors of the liver: Experience of 114 cases. Tumour Biol. 2015, 36, 5143–5148. [Google Scholar] [CrossRef]
- Milias, K.; Madhavan, K.K.; Bellamy, C.; Garden, O.J.; Parks, R.W. Inflammatory pseudotumors of the liver: Experience of a specialist surgical unit. J. Gastroenterol. Hepatol. 2009, 24, 1562–1566. [Google Scholar] [CrossRef]
- Pack, G.T.; Baker, H.W. Total right hepatic lobectomy; report of a case. Ann. Surg. 1953, 138, 253–258. [Google Scholar] [CrossRef]
- Mathiak, G.; Meyer-Pannwitt, U.; Mathiak, M.; Schroder, S.; Henne-Bruns, D.; Froschle, G. Inflammatory pseudotumor of the liver-rare differential diagnosis of undetermined hepatic space-occupying lesion. Case report and review of the literature. Langenbecks Arch. Chir. 1996, 381, 309–317. [Google Scholar] [PubMed]
- Zamir, D.; Jarchowsky, J.; Singer, C.; Abumoch, S.; Groisman, G.; Ammar, M.; Weiner, P. Inflammatory pseudotumor of the liver—A rare entity and a diagnostic challenge. Am. J. Gastroenterol. 1998, 93, 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Shen, M.S.; Yin, T. Liver inflammatory pseudotumor or parasitic granuloma? World J. Gastroenterol. 2000, 6, 458–460. [Google Scholar] [CrossRef]
- Lupovitch, A.; Chen, R.; Mishra, S. Inflammatory pseudotumor of the liver. Report of the fine needle aspiration cytologic findings in a case initially misdiagnosed as malignant. Acta Cytol. 1989, 33, 259–262. [Google Scholar]
- Sasahira, N.; Kawabe, T.; Nakamura, A.; Shimura, K.; Shimura, H.; Itobayashi, E.; Asada, M.; Shiratori, Y.; Omata, M. Inflammatory pseudotumor of the liver and peripheral eosinophilia in autoimmune pancreatitis. World J. Gastroenterol. 2005, 11, 922–925. [Google Scholar] [CrossRef]
- Lin, M.; Cao, L.; Wang, J.; Zhou, J. Diagnosis of hepatic inflammatory pseudotumor by fine-needle biopsy. J. Interv. Med. 2022, 5, 166–170. [Google Scholar] [CrossRef]
- Kanagalingam, G.; Dulymamode, K.N.; Jafroodifar, A.; Huda, S.A.; May, A.; Masood, U.; John, S. En larging Liver Mass: Inflammatory Pseudotumor in a Patient With Polymyalgia Rheumatica. J. Investig. Med. High. Impact Case Rep. 2022, 10, 23247096211070387. [Google Scholar]
- Amankonah, T.D.; Strom, C.B.; Vierling, J.M.; Petrovic, L.M. Inflammatory pseudotumor of the liver as the first manifestation of Crohn’s disease. Am. J. Gastroenterol. 2001, 96, 2520–2522. [Google Scholar] [CrossRef] [PubMed]
- Mergan, F.; Jaubert, F.; Sauvat, F.; Hartmann, O.; Lortat-Jacob, S.; Révillon, Y.; Nihoul-Fékété, C.; Sarnacki, S. Inflammatory myofibroblastic tumor in children: Clinical review with anaplastic lymphoma kinase, Epstein–Barr virus, and human herpesvirus 8 detection analysis. J. Pediatr. Surg. 2005, 40, 1581–1586. [Google Scholar] [CrossRef]
- Priebe-Richter, C.; Ivanyi, P.; Buer, J.; Länger, F.; Lotz, J.; Hertenstein, B.; Ganser, A.; Franzke, A. In-flammatory pseudotumor of the lung following invasive aspergillosis in a patient with chronic graft-vs host disease. Eur. J. Haematol. 2005, 75, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Colby, T.V.; Koss, M.N.; Rosado-de-Christenson, M.L.; Muller, N.L.; King, T.E., Jr. Miscellaneous diseases of uncertain etiology. In Atlas of Nontumor Pathology. Non-Neoplastic Disorders of the Lower Respiratory Tract, 1st ed.; King, D.W., Ed.; American Registry of Pathology and Armed Forced Institute of Pathology: Washington, DC, USA, 2002; pp. 857–900. [Google Scholar]
- Arora, K.S.; Anderson, M.A.; Neyaz, A.; Yilmaz, O.; Pankaj, A.; Ferrone, C.R.; Zen, Y.; England, J.; Deshpande, V. Fibrohistiocytic Variant of Hepatic Pseudotumor: An Antibiotic Responsive Tumefactive Lesion. Am. J. Surg. Pathol. 2021, 45, 1314–1323. [Google Scholar] [CrossRef]
- Hamano, H.; Kawa, S.; Horiuchi, A.; Unno, H.; Furuya, N.; Akamatsu, T.; Fukushima, M.; Nikaido, T.; Nakayama, K.; Usuda, N.; et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N. Engl. J. Med. 2001, 344, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Neild, G.H.; Rodriguez-Justo, M.; Wall, C.; Connolly, J.O. Hyper-IgG4 disease: Report and characterisation of a new disease. BMC Med. 2006, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Funata, N.; Hayashi, Y.; Tsuruta, K.; Okamoto, A.; Amemiya, K.; Egawa, N.; Nakajima, H. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut 2003, 52, 683–687. [Google Scholar] [CrossRef]
- Kitagawa, S.; Zen, Y.; Harada, K.; Sasaki, M.; Sato, Y.; Minato, H.; Watanabe, K.; Kurumaya, H.; Katayanagi, K.; Masuda, S.; et al. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Küttner’s tumor). Am. J. Surg. Pathol. 2005, 29, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Kitagawa, S.; Minato, H.; Kurumaya, H.; Katayanagi, K.; Masuda, S.; Niwa, H.; Fujimura, M.; Nakanuma, Y. IgG4-positive plasma cells in inflammatory pseudotumor (plasma cell granuloma) of the lung. Hum. Pathol. 2005, 36, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Ko, M.; Seko, S.; Nishida, O.; Inoue, F.; Kobayashi, H.; Saiga, T.; Okamoto, M.; Fukuse, T. Interstitial pneumonia associated with autoimmune pancreatitis. Gut 2004, 53, 770. [Google Scholar]
- Zen, Y.; Sawazaki, A.; Miyayama, S.; Notsumata, K.; Tanaka, N.; Nakanuma, Y. A case of retroperitoneal and mediastinal fibrosis exhibiting elevated levels of IgG4 in the absence of sclerosing pancreatitis (au-toimmune pancreatitis). Hum. Pathol. 2006, 37, 239–243. [Google Scholar] [CrossRef]
- Hamano, H.; Kawa, S.; Ochi, Y.; Unno, H.; Shiba, N.; Wajiki, M.; Nakazawa, K.; Shimojo, H.; Kiyosawa, K. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet 2002, 359, 1403–1404. [Google Scholar] [CrossRef]
- Solomon, G.J.; Kinkhabwala, M.M.; Akhtar, M. Inflammatory myofibroblastic tumor of the liver. Arch. Pathol. Lab. Med. 2006, 130, 1548–1551. [Google Scholar] [CrossRef]
- Fritchie, K.J.; Hornick, J.L.; Rossi, S. Inflammatory myofibroblastic tumour. In WHO Classification of Tumours, Digestive System Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 444–445. [Google Scholar]
- Makhlouf, H.R.; Sobin, L.H. Inflammatory myofibroblastic tumors (inflammatory pseudotumors) of the gastrointestinal tract: How closely are they related to inflammatory fibroid polyps? Hum. Pathol. 2002, 33, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Mariño-Enríquez, A.; Wang, W.L.; Roy, A.; Lopez-Terrada, D. Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am. J. Surg. Pathol. 2011, 35, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Strainiene, S.; Sedleckaite, K.; Jarasunas, J.; Savlan, I.; Stanaitis, J.; Stundiene, I.; Strainys, T.; Liakina, V.; Valantinas, J. Complicated course of biliary inflammatory myofibroblastic tumor mimicking hilar cholangiocarcinoma: A case report and literature review. World J. Clin. Cases. 2021, 9, 6155–6169. [Google Scholar] [CrossRef] [PubMed]
- Haith, E.E.; Kepes, J.J.; Holder, T.M. Inflammatory pseudotumor involving the common bile duct of a six-year-old boy: Successful pancreaticoduodenectomy. Surgery 1964, 56, 436–441. [Google Scholar]
- Stamatakis, J.D.; Howard, E.R.; Williams, R. Benign inflammatory tumour of the common bile duct. Br. J. Surg. 1979, 66, 257–258. [Google Scholar] [CrossRef]
- Ikeda, H.; Oka, T.; Imafuku, I.; Yamada, S.; Yamada, H.; Fujiwara, K.; Hirata, M.; Idezuki, Y.; Oka, H. A case of inflammatory pseudotumor of the gallbladder and bile duct. Am. J. Gastroenterol. 1990, 85, 203–206. [Google Scholar]
- Fukushima, N.; Suzuki, M.; Abe, T.; Fukayama, M. A case of inflammatory pseudotumour of the common bile duct. Virchows Arch. 1997, 431, 219–224. [Google Scholar] [CrossRef]
- Walsh, S.V.; Evangelista, F.; Khettry, U. Inflammatory myofibroblastic tumor of the pancreaticobiliary region: Morphologic and immunocytochemical study of three cases. Am. J. Surg. Pathol. 1998, 22, 412–418. [Google Scholar] [CrossRef]
- Sobesky, R.; Chollet, J.M.; Prat, F.; Karkouche, B.; Pelletier, G.; Fritsch, J.; Choury, A.D.; Allonier, C.; Bedossa, P.; Buffet, C. Inflammatory pseudotumor of the common bile duct. Endoscopy 2003, 35, 698–700. [Google Scholar]
- Venkataraman, S.; Semelka, R.C.; Braga, L.; Danet, I.M.; Woosley, J.T. Inflammatory myofibroblastic tumor of the hepatobiliary system: Report of MR imaging appearance in four patients. Radiology 2003, 227, 758–763. [Google Scholar] [CrossRef]
- Büyükyavuz, I.; Karnak, I.; Haliloglu, M.; Senocak, M.E. Inflammatory myofibroblastic tumour of the extrahepatic bile ducts: An unusual cause of obstructive jaundice in children. Eur. J. Pediatr. Surg. 2003, 13, 421–424. [Google Scholar] [PubMed]
- Lopez-Tomassetti Fernandez, E.M.; Luis, H.D.; Malagon, A.M.; Gonzalez, I.A.; Pallares, A.C. Recur-rence of inflammatory pseudotumor in the distal bile duct: Lessons learned from a single case and re-ported cases. World J. Gastroenterol. 2006, 12, 3938–3943. [Google Scholar] [CrossRef]
- Martín Malagón, A.; López-Tomassetti Fernández, E.M.; Arteaga González, I.; Carrillo Pallarés, A.; Díaz Luis, H. Inflammatory myofibroblastic tumor of the distal bile duct associated with lymphoplasmacytic sclerosing pancreatitis. Case report and review of the literature. Pancreatology 2006, 6, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Joo, S.H.; Kim, G.Y.; Joo, K.R. Aggressive hilar inflammatory myofibroblastic tumor with hilar bile duct carcinoma in situ. J. Korean Surg. Soc. 2011, 81, S59–S63. [Google Scholar] [CrossRef]
- Sekaran, A.; Lakhtakia, S.; Pradeep, R.; Santosh, D.; Gupta, R.; Tandan, M.; Reddy, D.B.; Rao, G.V.; Reddy, D.N. Inflammatory myofibroblastic tumor of biliary tract presenting as recurrent GI bleed (with video). Gastrointest. Endosc. 2006, 63, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Abu-Wasel, B.; Eltawil, K.M.; Molinari, M. Benign inflammatory pseudotumour mimicking extrahepatic bile duct cholangiocarcinoma in an adult man presenting with painless obstructive jaundice. BMJ Case Rep. 2012, 2012, 006514. [Google Scholar] [CrossRef]
- Vasiliadis, K.; Fortounis, K.; Papavasiliou, C.; Kokarhidas, A.; Al Nimer, A.; Fachiridis, D.; Pervana, S.; Makridis, C. Mid common bile duct inflammatory pseudotumor mimicking cholangiocarcinoma. A case report and literature review. Int. J. Surg. Case Rep. 2014, 5, 12–15. [Google Scholar] [CrossRef]
- D’Cunha, A.; Jehangir, S.; Thomas, R. Inflammatory Myofibroblastic Tumor of Common Bile Duct in a Girl. APSP J. Case Rep. 2016, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Saha, A.; Saha, K. Inflammatory Myofibroblastic Tumor of the Mid Common Bile Duct Masquerading as Cholangiocarcinoma. J. Gastrointest. Cancer 2019, 50, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Tizmaghz, A.; Shabestanipour, G. An interesting case of inflammatory myofibroblastic tumor presenting as cholangiocarcinoma. Int. J. Surg. Case Rep. 2018, 47, 38–40. [Google Scholar] [CrossRef]
- Dehner, L. Inflammatory myofibroblastic tumor: The continued definition of one type of so-called in-flammatory pseudotumor. Am. J. Surg. Pathol. 2004, 28, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Chougule, A.; Taylor, M.S.; MacLeay, A.R.; Kurzawa, P.; Chebib, I.; Le, L.; Deshpande, V. Morpho-logic Overlap Between Inflammatory Myofibroblastic Tumor and IgG4-related Disease: Lessons from Next-generation Sequencing. Am. J. Surg. Pathol. 2019, 43, 314–324. [Google Scholar]
- Li, Y.-Y.; Zang, J.-F.; Zhang, C. Laparoscopic treatment of inflammatory myofibroblastic tumor in liver: A case report. World J. Clin. Cases. 2022, 10, 11853–11860. [Google Scholar] [CrossRef]
- Arber, D.A.; Kamel, O.W.; van de Rijn, M.; Davis, R.E.; Medeiros, L.J.; Jaffe, E.S.; Weiss, L.M. Frequent presence of the Epstein-Barr virus in inflammatory pseudotumor. Hum. Pathol. 1995, 26, 1093–1098. [Google Scholar] [CrossRef]
- Lewis, J.T.; Gaffney, R.L.; Casey, M.B.; Farrell, M.A.; Morice, W.G.; Macon, W.R. Inflammatory pseudotumor of the spleen associated with a clonal Epstein-Barr virus genome. Case report and review of the literature. Am. J. Clin. Pathol. 2003, 120, 56–61. [Google Scholar] [CrossRef]
- Neuhauser, T.S.; Derringer, G.A.; Thompson, L.D.; Fanburg-Smith, J.C.; Aguilera, N.S.; Andriko, J.; Chu, W.S.; Abbondanzo, S.L. Splenic inflammatory myofibroblastic tumor (inflammatory pseudotumor): A clinicopathologic and immunophenotypic study of 12 cases. Arch. Pathol. Lab. Med. 2001, 125, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Ideda, H.; Suzuki, N.; Takahashi, A.; Kuroiwa, M.; Hirato, J.; Hatakeyama, S.; Tsuchida, Y. Inflammatory pseudotumor of the liver: Case report and review of the literature. J. Pediatr. Surg. 2001, 36, 663–666. [Google Scholar] [CrossRef]
- Coffin, C.M.; Fletcher, J.A. Inflammatory myofibroblastic tumor. In World Health Organization Classification of Tumours; Pathology and Genetics of Tumours of Soft Tissue and Bone; Fletcher, C.D.M., Unni, K.K., Mertens, F., Eds.; IARC Press: Lyon, France, 2002; Volume 5, pp. 91–93. [Google Scholar]
- Zen, Y.; Hubscher, S.G.; Nakanuma, Y. Bile duct Diseases. In MacSween’s Pathology of the Liver, 7th ed.; Burt, A.D., Ferrell, L.D., Hubscher, S.G., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 515–593. [Google Scholar]
- Schiff, E.R.; Sorrell, M.F.; Maddrey, W.C. Schiff’s Diseases of the Liver; Lippincott Williams &Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Cheuk, W.; Chan, J.K.; Shek, T.W.; Chang, J.H.; Tsou, M.H.; Yuen, N.W.; Ng, W.F.; Chan, A.C.; Prat, J. Inflammatory pseudotumor-like follicular dendritic cell tumor: A distinctive low-grade malignant in-tra-abdominal neoplasm with consistent Epstein-Barr virus association. Am. J. Surg. Pathol. 2001, 25, 721–731. [Google Scholar] [CrossRef]
- Kim, S.R.; Hayashi, Y.; Kudo, M.; Matsuoka, T.; Imoto, S.; Sasaki, K.; Shintani, S.; Song, K.B.; Park, S.Y.; Kim, J.H.; et al. Inflammatory pseudotumor of the liver in a patient with chronic hepatitis C: Difficulty in differentiating it from hepatocellular carcinoma. Pathol. Int. 1999, 49, 726–730. [Google Scholar] [CrossRef]
- Kim, S.R.; Hayashi, Y.; Kang, K.B.; Soe, C.G.; Kim, J.H.; Yang, M.K.; Itoh, H. A case of pseudolym-phoma of the liver with chronic hepatitis C. J. Hepatol. 1997, 26, 209–214. [Google Scholar] [CrossRef]
- Katayanagi, K.; Terada, T.; Nakanuma, Y.; Ueno, T. A case of pseudolymphoma of the liver. Pathol. Int. 1994, 44, 704–711. [Google Scholar] [CrossRef]
- Ishak, K.G.; Goodman, Z.D.; Stocked, J.T. Tumors of the Liver and Intrahepatic Bile Ducts; Armed Forces Institute of Pathology: Washington, DC, USA, 1999; pp. 128–133. [Google Scholar]
- Malatjalian, D.A.; Morris, J.; Bodurtha, A. Isolation of Klebsiella Pneumoniae from an Hepatic Inflammatory Pseudotumour. Can. J. Gastroenterol. 1992, 6, 84–86. [Google Scholar] [CrossRef]
- Fauci, A.S.; Harley, J.B.; Roberts, W.C.; Ferrans, V.J.; Gralnick, H.R.; Bjornson, B.H. NIH conference. The idiopathic hypereosinophilic syndrome. Clinical, pathophysiologic, and therapeutic considerations. Ann. Intern. Med. 1982, 97, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Adsay, N.V.; Basturk, O.; Klimstra, D.S.; Klöppel, G. Pancreatic pseudotumors: Non-neoplastic solid lesions of the pancreas that clinically mimic pancreas cancer. Semin. Diagn. Pathol. 2004, 21, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Koike, M.; Tsuruta, K.; Okamoto, A.; Tabata, I.; Fujita, N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: A variant of primary sclerosing cholangitis extensively involving pancreas. Hum. Pathol. 1991, 22, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Notohara, K.; Burgart, L.J.; Yadav, D.; Chari, S.; Smyrk, T.C. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: Clinicopathologic features of 35 cases. Am. J. Surg. Pathol. 2003, 27, 1119–1127. [Google Scholar] [CrossRef]
- Deshpande, V.; Chicano, S.; Finkelberg, D.; Selig, M.K.; Mino-Kenudson, M.; Brugge, W.R.; Colvin, R.B.; Lauwers, G.Y. Autoimmune pancreatitis: A systemic immune complex mediated disease. Am. J. Surg. Pathol. 2006, 30, 1537–1545. [Google Scholar] [CrossRef]
- Zen, Y.; Harada, K.; Sasaki, M.; Sato, Y.; Tsuneyama, K.; Haratake, J.; Kurumaya, H.; Katayanagi, K.; Masuda, S.; Niwa, H.; et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: Do they belong to a spectrum of sclerosing pancreatitis? Am. J. Surg. Pathol. 2004, 28, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Kasahara, Y.; Horita, K.; Miyayama, S.; Miura, S.; Kitagawa, S.; Nakanuma, Y. Inflammatory pseudotumor of the breast in a patient with a high serum IgG4 level: Histologic similarity to sclerosing pancreatitis. Am. J. Surg. Pathol. 2005, 29, 275–278. [Google Scholar] [CrossRef]
- Jais, P.; Berger, J.F.; Vissuzaine, C.; Paramelle, O.; Clays-Schouman, E.; Potet, F.; Mignon, M. Regres-sion of inflammatory pseudotumor of the liver under conservative therapy. Dig. Dis. Sci. 1995, 40, 752–756. [Google Scholar] [CrossRef]
- Hakozaki, Y.; Katou, M.; Nakagawa, K.; Shirahama, T.; Matsumoto, T. Improvement of inflammatory pseudotumor of the liver after nonsteroidal anti-inflammatory agent therapy. Am. J. Gastroenterol. 1993, 88, 1121–1122. [Google Scholar] [PubMed]
- Gollapudi, P.; Chejfec, G.; Zarling, E.J. Spontaneous regression of hepatic pseudotumor. Am. J. Gastroenterol. 1992, 87, 214–217. [Google Scholar] [PubMed]
- Yamaguchi, J.; Sakamoto, Y.; Sano, T.; Shimada, K.; Kosuge, T. Spontaneous regression of inflammato-ry pseudotumor of the liver: Report of three cases. Surg. Today. 2007, 37, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Maller, E.; Redd, D.; Hebra, A.; Davidoff, A.; Buzby, M.; Hoffman, M.A. Orthotopic liver transplantation for inflammatory myofibroblastic tumor of the liver hilum. J. Pediatr. Surg. 1996, 31, 840–842. [Google Scholar] [CrossRef]
- Heneghan, M.A.; Kaplan, C.G.; Priebe, C.J.; Partin, J.S. Inflammatory pseudotumor of the liver: A rare cause of obstructive jaundice and portal hypertension in a child. Pediatr. Radiol. 1984, 14, 433–435. [Google Scholar] [CrossRef]
- Anthony, P.P.; Telesinghe, P.U. Inflammatory pseudotumour of the liver. J. Clin. Pathol. 1986, 39, 761–768. [Google Scholar] [CrossRef]
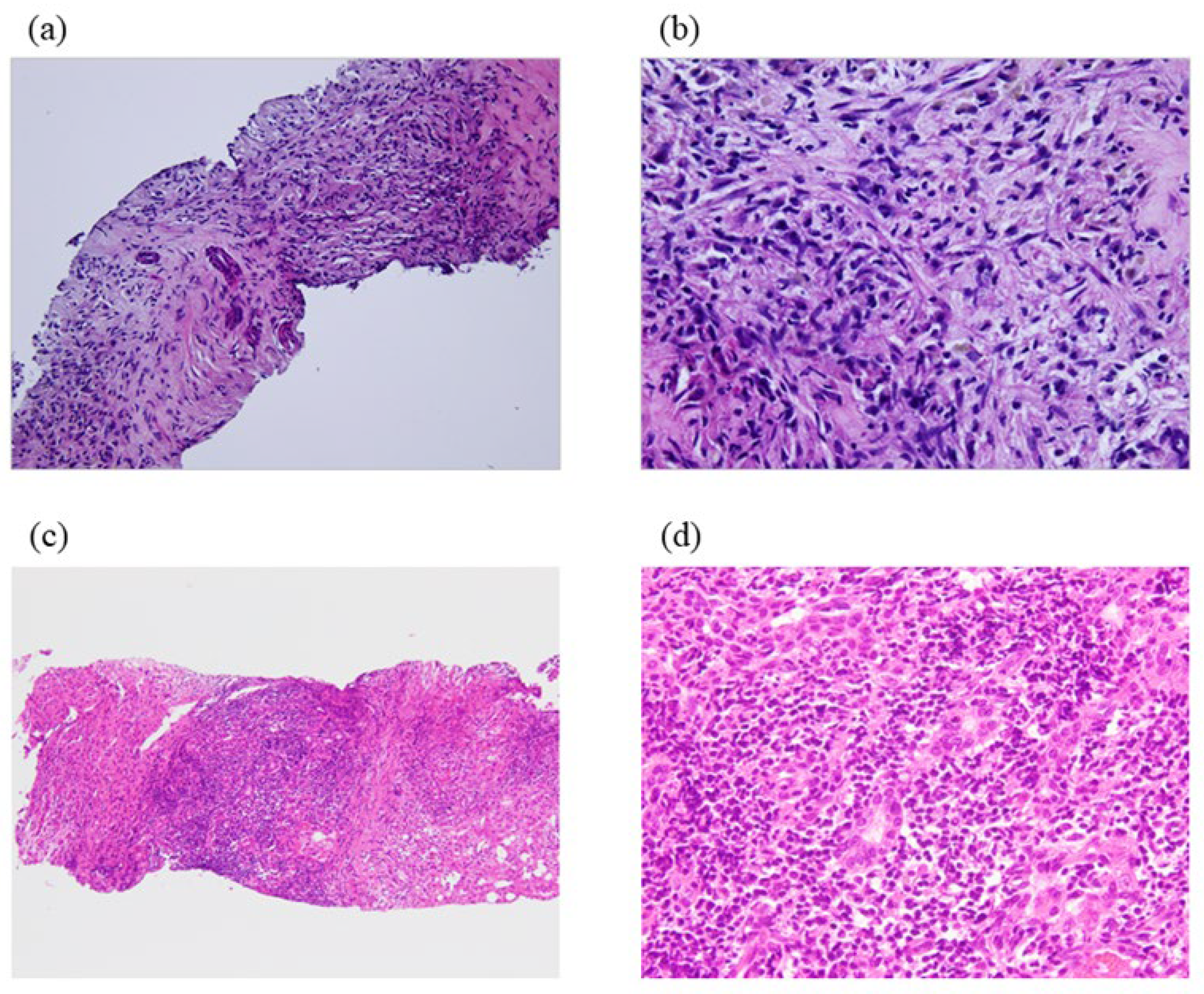
| Pathological Findings | Clinical Features | |
|---|---|---|
| Fibrohistiocytic |
| Gender: both genders involvedLocation and gross findings: mostly occurred in the peripheral hepatic parenchyma as nodule-forming lesions similar to intrahepatic cholangiocellular carcinoma Symptoms and examination: fever, abdominal pain, and general malaise |
| Lymphoplasmacytic |
| Gender: many cases involved menLocation and gross findings: more commonly observed in the left lobeSymptoms and examination: dysfunction by routine laboratory testing |
| Diagnostics | Characteristics |
|---|---|
| Hepatic inflammatory myofibroblastic tumor | The three histologic patterns are listed below.The first is the myxoid-vascular pattern.Characteristics:
|
| Primary sclerosing cholangitis | The fibrous-obliterative pattern is characterized by an “onionskin” pattern of periductal fibrosis near medium-sized or bigger bile ducts, associated with degeneration and atrophy of the epithelial interior and accidental replacement of the bile duct by fibrous cords.Lymphoid follicles or aggregates may be present, but granulomas are rarely detected. Small bile ducts may show degenerative epithelial changes or may be surrounded by a ring of edematous or hyaline fibrosis. |
| Follicular dendritic cell tumor | Tumors are dispersed in a background of abundant lymphocytes and plasma, spindle or ovoid cells with vesicular nuclei and distinct nucleoli. The extent of nuclear atypia varies, and some nuclei appear deformed or simulate Reed-Sternberg cells. The atypical cells are immunoreactive to FDC signs such as CD21/CD35, CD23, and CNA.42. The Epstein–Barr virus (EBV)-encoded RNA is positive in every case of in situ hybridization. |
| Pseudolymphoma | The hyperplastic lymphoid follicles with polymorphic and polyclonal cell populations consisted of small mature plasmacytes, histiocytes and stromal fibrosis.Lymphocytes consisting of many germinal centers are chiefly composed of L-26-positive B cell lymphocytes. The lymphocytes encircling the germinal centers are chiefly UCHL-1-positive T lymphocytes. The B cells in the lymphoid follicles are positive for both κ and λ light chains at sequential frequencies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.R.; Kim, S.K.; Koma, Y.-i.; Sasaki, M.; Asai, A.; Nishikawa, H. Hepatic Inflammatory Pseudotumor—Focusing on Its Heterogeneity. Diagnostics 2023, 13, 2857. https://doi.org/10.3390/diagnostics13172857
Kim SR, Kim SK, Koma Y-i, Sasaki M, Asai A, Nishikawa H. Hepatic Inflammatory Pseudotumor—Focusing on Its Heterogeneity. Diagnostics. 2023; 13(17):2857. https://doi.org/10.3390/diagnostics13172857
Chicago/Turabian StyleKim, Soo Ryang, Soo Ki Kim, Yu-ichiro Koma, Motoko Sasaki, Akira Asai, and Hiroki Nishikawa. 2023. "Hepatic Inflammatory Pseudotumor—Focusing on Its Heterogeneity" Diagnostics 13, no. 17: 2857. https://doi.org/10.3390/diagnostics13172857
APA StyleKim, S. R., Kim, S. K., Koma, Y.-i., Sasaki, M., Asai, A., & Nishikawa, H. (2023). Hepatic Inflammatory Pseudotumor—Focusing on Its Heterogeneity. Diagnostics, 13(17), 2857. https://doi.org/10.3390/diagnostics13172857







