Migraine Aura—Catch Me If You Can with EEG and MRI—A Narrative Review
Abstract
1. Introduction
2. Methods
Search Strategy
3. Narrative Summary of studies
3.1. EEG Studies during Migraine Aura
3.2. EEG Studies during Aura in Hemiplegic Migraine
3.3. EEG during Migraine with brainstem aura
3.4. MRI studies during Migraine Aura
3.4.1. Perfusion MRI Studies
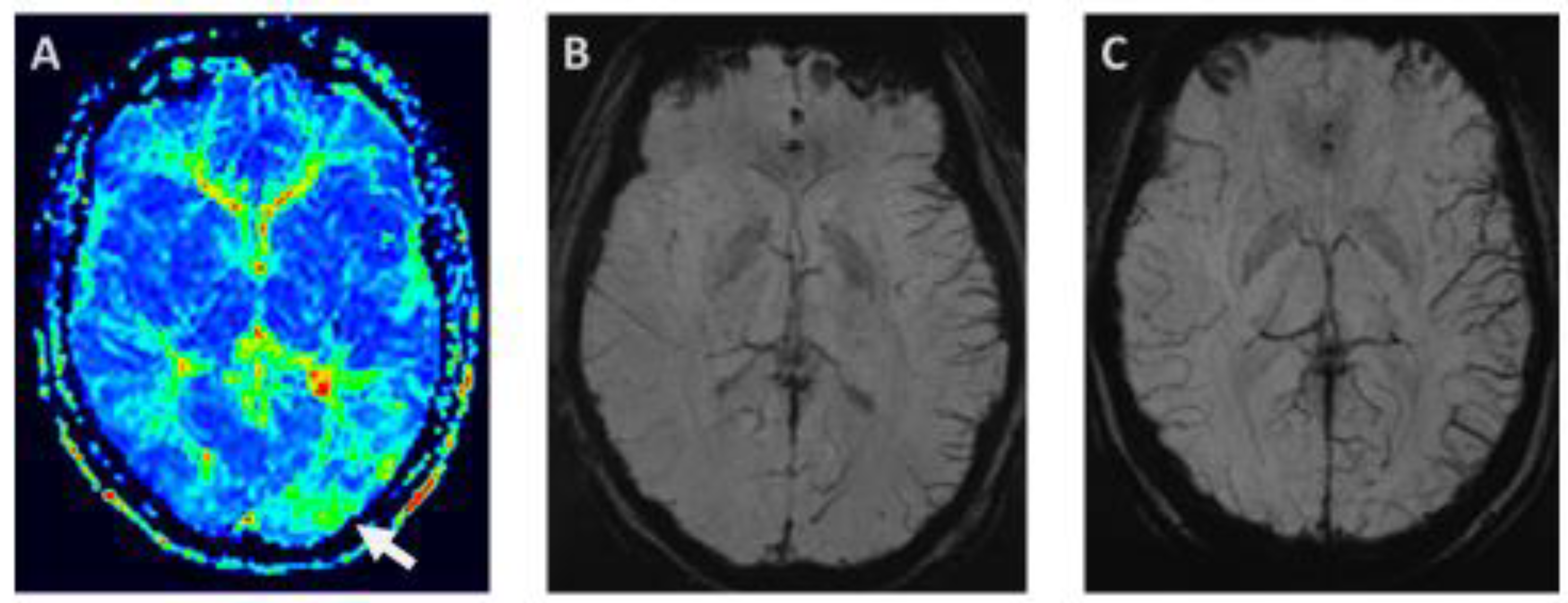
3.4.2. Susceptibility—Weighted Imaging—Prominent focal veins—Index Vein
3.4.3. Diffusion Abnormalities
4. Discussion
4.1. EEG Signatures of the Migraine Aura
Familial Hemiplegic Migraine, Epilepsy and EEG
4.2. MRI Signatures of Migraine Aura
5. Conclusions, Context, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lampl, C.; Buzath, A.; Baumhackl, U.; Klingler, D. One-year prevalence of migraine in Austria: A nation-wide survey. Cephalalgia 2003, 23, 280–286. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Ludvigsson, P.; Hesdorffer, D.; Olafsson, E.; Kjartansson, O.; Hauser, W.A. Migraine with aura is a risk factor for unprovoked seizures in children. Ann. Neurol. 2006, 59, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Tronvik, E.A.; Hagen, K. The bidirectional temporal relationship between headache and affective disorders: Longitudinal data from the HUNT studies. J. Headache Pain 2022, 23, 14. [Google Scholar] [CrossRef]
- Mahmoud, A.N.; Mentias, A.; Elgendy, A.Y.; Qazi, A.; Barakat, A.F.; Saad, M.; Mohsen, A.; Abuzaid, A.; Mansoor, H.; Mojadidi, M.K.; et al. Migraine and the risk of cardiovascular and cerebrovascular events: A meta-analysis of 16 cohort studies including 1,152,407 subjects. BMJ Open 2018, 8, e020498. [Google Scholar] [CrossRef]
- Bashir, A.; Lipton, R.B.; Ashina, S.; Ashina, M. Migraine and structural changes in the brain: A systematic review and meta-analysis. Neurology 2013, 81, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Schott, G.D. Exploring the visual hallucinations of migraine aura: The tacit contribution of illustration. Brain 2007, 130, 1690–1703. [Google Scholar] [CrossRef]
- Scutelnic, A.; Kreis, L.A.; Beyeler, M.; Heldner, M.R.; Meinel, T.R.; Kaesmacher, J.; Hakim, A.; Arnold, M.; Fischer, U.; Mattle, H.P.; et al. Migraine aura-like symptoms at onset of stroke and stroke-like symptoms in migraine with aura. Front. Neurol. 2022, 13, 1004058. [Google Scholar] [CrossRef]
- Lauritzen, M.; Trojaborg, W.; Olesen, J. EEG during attacks of common and classical migraine. Cephalalgia 1981, 1, 63–66. [Google Scholar] [CrossRef]
- Dow, D.J.; Whitty, C.W. Electroencephalographic changes in migraine; review of 51 cases. Lancet 1947, 2, 52–54. [Google Scholar] [CrossRef]
- Sand, T. Electroencephalography in migraine: A review with focus on quantitative electroencephalography and the migraine vs. epilepsy relationship. Cephalalgia 2003, 23 (Suppl. S1), 5–11. [Google Scholar] [CrossRef]
- Seri, S.; Cerquiglini, A.; Guidetti, V. Computerized EEG topography in childhood migraine between and during attacks. Cephalalgia 1993, 13, 53–56. [Google Scholar] [CrossRef]
- Parain, D.; Hitzel, A.; Guegan-Massardier, E.; Lebas, A.; Blondeau, C.; Fedina, I.; Feray, D.; Vera, P.; Mihout, B. Migraine aura lasting 1-24 h in children: A sequence of EEG slow-wave abnormalities vs. vascular events. Cephalalgia 2007, 27, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, E.; Galimberti, C.A.; Soragna, D.; Ferrandi, D.; Maurelli, M.; Ratti, M.T.; Bo, P.; Montalbetti, L.; Albergati, A.; Savoldi, F. Familial hemiplegic migraine versus migraine with prolonged aura: An uncertain diagnosis in a family report. Neurology 1995, 45, 33–37. [Google Scholar] [CrossRef]
- Indelicato, E.; Nachbauer, W.; Eigentler, A.; Donnemiller, E.; Wagner, M.; Unterberger, I.; Boesch, S. Ten years of follow-up in a large family with familial hemiplegic migraine type 1: Clinical course and implications for treatment. Cephalalgia 2018, 38, 1167–1176. [Google Scholar] [CrossRef]
- Oberndorfer, S.; Wober, C.; Nasel, C.; Asenbaum, S.; Lahrmann, H.; Fueger, B.; Grisold, W. Familial hemiplegic migraine: Follow-up findings of diffusion-weighted magnetic resonance imaging (MRI), perfusion-MRI and [99mTc] HMPAO-SPECT in a patient with prolonged hemiplegic aura. Cephalalgia 2004, 24, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Murphy, O.C.; Merwick, A.; O’Mahony, O.; Ryan, A.M.; McNamara, B. Familial Hemiplegic Migraine with Asymmetric Encephalopathy Secondary to ATP1A2 Mutation: A Case Series. J. Clin. Neurophysiol. 2018, 35, e3–e7. [Google Scholar] [CrossRef]
- Ducros, A.; Denier, C.; Joutel, A.; Cecillon, M.; Lescoat, C.; Vahedi, K.; Darcel, F.; Vicaut, E.; Bousser, M.G.; Tournier-Lasserve, E. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N. Engl. J. Med. 2001, 345, 17–24. [Google Scholar] [CrossRef] [PubMed]
- De Fusco, M.; Marconi, R.; Silvestri, L.; Atorino, L.; Rampoldi, L.; Morgante, L.; Ballabio, A.; Aridon, P.; Casari, G. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 2003, 33, 192–196. [Google Scholar] [CrossRef]
- Dichgans, M.; Freilinger, T.; Eckstein, G.; Babini, E.; Lorenz-Depiereux, B.; Biskup, S.; Ferrari, M.D.; Herzog, J.; van den Maagdenberg, A.M.; Pusch, M.; et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 2005, 366, 371–377. [Google Scholar] [CrossRef]
- Chan, Y.C.; Burgunder, J.M.; Wilder-Smith, E.; Chew, S.E.; Lam-Mok-Sing, K.M.; Sharma, V.; Ong, B.K. Electroencephalographic changes and seizures in familial hemiplegic migraine patients with the CACNA1A gene S218L mutation. J. Clin. Neurosci. 2008, 15, 891–894. [Google Scholar] [CrossRef]
- Fitzsimons, R.B.; Wolfenden, W.H. Migraine coma. Meningitic migraine with cerebral oedema associated with a new form of autosomal dominant cerebellar ataxia. Brain 1985, 108 Pt 3, 555–577. [Google Scholar] [CrossRef]
- Kors, E.E.; Terwindt, G.M.; Vermeulen, F.L.; Fitzsimons, R.B.; Jardine, P.E.; Heywood, P.; Love, S.; van den Maagdenberg, A.M.; Haan, J.; Frants, R.R.; et al. Delayed cerebral edema and fatal coma after minor head trauma: Role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann. Neurol. 2001, 49, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G.; Anzalone, N.; Baldoli, C.; Impellizzeri, M.; Minicucci, F.; Comi, G.; Colombo, B. Pediatric sporadic hemiplegic migraine (ATP1A2 gene): A case report and brief literature review. Neurol. Sci. 2018, 39, 69–71. [Google Scholar] [CrossRef]
- Hirsch, L.J.; Fong, M.W.K.; Leitinger, M.; LaRoche, S.M.; Beniczky, S.; Abend, N.S.; Lee, J.W.; Wusthoff, C.J.; Hahn, C.D.; Westover, M.B.; et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J. Clin. Neurophysiol. 2021, 38, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Chastan, N.; Lebas, A.; Legoff, F.; Parain, D.; Guyant-Marechal, L. Clinical and electroencephalographic abnormalities during the full duration of a sporadic hemiplegic migraine attack. Neurophysiol. Clin. 2016, 46, 307–311. [Google Scholar] [CrossRef]
- Ramelli, G.P.; Sturzenegger, M.; Donati, F.; Karbowski, K. EEG findings during basilar migraine attacks in children. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 374–378. [Google Scholar] [CrossRef]
- Cutrer, F.M.; Sorensen, A.G.; Weisskoff, R.M.; Ostergaard, L.; Sanchez del Rio, M.; Lee, E.J.; Rosen, B.R.; Moskowitz, M.A. Perfusion-weighted imaging defects during spontaneous migrainous aura. Ann. Neurol. 1998, 43, 25–31. [Google Scholar] [CrossRef]
- Sanchez del Rio, M.; Bakker, D.; Wu, O.; Agosti, R.; Mitsikostas, D.D.; Ostergaard, L.; Wells, W.A.; Rosen, B.R.; Sorensen, G.; Moskowitz, M.A.; et al. Perfusion weighted imaging during migraine: Spontaneous visual aura and headache. Cephalalgia 1999, 19, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Floery, D.; Vosko, M.R.; Fellner, F.A.; Fellner, C.; Ginthoer, C.; Gruber, F.; Ransmayr, G.; Doerfler, A.; Uder, M.; Bradley, W.G. Acute-onset migrainous aura mimicking acute stroke: MR perfusion imaging features. AJNR Am. J. Neuroradiol. 2012, 33, 1546–1552. [Google Scholar] [CrossRef]
- Forster, A.; Wenz, H.; Kerl, H.U.; Brockmann, M.A.; Groden, C. Perfusion patterns in migraine with aura. Cephalalgia 2014, 34, 870–876. [Google Scholar] [CrossRef]
- Fandler-Hofler, S.; Enzinger, C.; Gattringer, T. Speechless from pain: Migraine with prolonged aura. Lancet 2020, 396, e11. [Google Scholar] [CrossRef]
- Cadiot, D.; Longuet, R.; Bruneau, B.; Treguier, C.; Carsin-Vu, A.; Corouge, I.; Gomes, C.; Proisy, M. Magnetic resonance imaging in children presenting migraine with aura: Association of hypoperfusion detected by arterial spin labelling and vasospasm on MR angiography findings. Cephalalgia 2018, 38, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Cobb-Pitstick, K.M.; Munjal, N.; Safier, R.; Cummings, D.D.; Zuccoli, G. Time Course of Cerebral Perfusion Changes in Children with Migraine with Aura Mimicking Stroke. AJNR Am. J. Neuroradiol. 2018, 39, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- Hadjikhani, N.; Sanchez Del Rio, M.; Wu, O.; Schwartz, D.; Bakker, D.; Fischl, B.; Kwong, K.K.; Cutrer, F.M.; Rosen, B.R.; Tootell, R.B.; et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc. Natl. Acad. Sci. USA 2001, 98, 4687–4692. [Google Scholar] [CrossRef]
- Masuzaki, M.; Utsunomiya, H.; Yasumoto, S.; Mitsudome, A. A case of hemiplegic migraine in childhood: Transient unilateral hyperperfusion revealed by perfusion MR imaging and MR angiography. AJNR Am. J. Neuroradiol. 2001, 22, 1795–1797. [Google Scholar]
- Lindahl, A.J.; Allder, S.; Jefferson, D.; Allder, S.; Moody, A.; Martel, A. Prolonged hemiplegic migraine associated with unilateral hyperperfusion on perfusion weighted magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry 2002, 73, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.R.; Friberg, L.; Olsen, T.S.; Olesen, J. Delayed hyperemia following hypoperfusion in classic migraine. Single photon emission computed tomographic demonstration. Arch. Neurol. 1988, 45, 154–159. [Google Scholar] [CrossRef]
- Hougaard, A.; Amin, F.M.; Christensen, C.E.; Younis, S.; Wolfram, F.; Cramer, S.P.; Larsson, H.B.W.; Ashina, M. Increased brainstem perfusion, but no blood-brain barrier disruption, during attacks of migraine with aura. Brain 2017, 140, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Michels, L.; Villanueva, J.; O’Gorman, R.; Muthuraman, M.; Koirala, N.; Buchler, R.; Gantenbein, A.R.; Sandor, P.S.; Luechinger, R.; Kollias, S.; et al. Interictal Hyperperfusion in the Higher Visual Cortex in Patients with Episodic Migraine. Headache 2019, 59, 1808–1820. [Google Scholar] [CrossRef]
- Kellner-Weldon, F.; Lehmann, V.F.; Breiding, P.S.; Grunder, L.; Muri, R.; Pastore-Wapp, M.; Bigi, S.; Wiest, R.; El-Koussy, M.; Slavova, N. Findings in susceptibility weighted imaging in pediatric patients with migraine with aura. Eur. J. Paediatr. Neurol. 2020, 28, 221–227. [Google Scholar] [CrossRef]
- Shimoda, Y.; Kudo, K.; Kuroda, S.; Zaitsu, Y.; Fujima, N.; Terae, S.; Sasaki, M.; Houkin, K. Susceptibility-weighted imaging and magnetic resonance angiography during migraine attack: A case report. Magn. Reson. Med. Sci. 2011, 10, 49–52. [Google Scholar] [CrossRef][Green Version]
- Kellner-Weldon, F.; Jossen, M.; Breiding, P.S.; Grunder, L.; Schankin, C.; Scutelnic, A.; Fischer, U.; Muri, R.; Pastore-Wapp, M.; Wiest, R.; et al. Imaging Neurovascular Uncoupling in Acute Migraine with Aura with Susceptibility Weighted Imaging. Clin. Neuroradiol. 2021, 31, 581–588. [Google Scholar] [CrossRef]
- Bosemani, T.; Burton, V.J.; Felling, R.J.; Leigh, R.; Oakley, C.; Poretti, A.; Huisman, T.A. Pediatric hemiplegic migraine: Role of multiple MRI techniques in evaluation of reversible hypoperfusion. Cephalalgia 2014, 34, 311–315. [Google Scholar] [CrossRef]
- Tamura, H.; Hatazawa, J.; Toyoshima, H.; Shimosegawa, E.; Okudera, T. Detection of deoxygenation-related signal change in acute ischemic stroke patients by T2*-weighted magnetic resonance imaging. Stroke 2002, 33, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Aellen, J.; Abela, E.; Buerki, S.E.; Kottke, R.; Springer, E.; Schindler, K.; Weisstanner, C.; El-Koussy, M.; Schroth, G.; Wiest, R.; et al. Focal hemodynamic patterns of status epilepticus detected by susceptibility weighted imaging (SWI). Eur. Radiol. 2014, 24, 2980–2988. [Google Scholar] [CrossRef]
- Slavova, N.; Denier, N.; El-Koussy, M.; Wiest, R.; Kellner-Weldon, F.; Fischer, U.; Schankin, C.J. The index vein pointing to the origin of the migraine aura symptom: A case series. Neurology 2020, 94, e2577–e2580. [Google Scholar] [CrossRef] [PubMed]
- Scutelnic, A.; Petroulia, V.; Schraml, L.; Jung, S.; Branca, M.; Beyeler, M.; Fischer, U.; Wiest, R.; Slavova, N.; Schankin, C.J. The “index vein” as a sign for migraine aura in the emergency setting. Cephalalgia 2023, 43, 3331024221132010. [Google Scholar] [CrossRef]
- Lewis, R.; Ruiz, A.; Monteith, T. Reversible Lesion of the Corpus Callosum in a Patient with Migraine with Aura: A Case Study. Headache 2020, 60, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D. Transient lesion in the splenium of the corpus callosum in status migrainosus. Acta Neurol. Belg. 2015, 115, 397–398. [Google Scholar] [CrossRef] [PubMed]
- Nozari, A.; Dilekoz, E.; Sukhotinsky, I.; Stein, T.; Eikermann-Haerter, K.; Liu, C.; Wang, Y.; Frosch, M.P.; Waeber, C.; Ayata, C.; et al. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann. Neurol. 2010, 67, 221–229. [Google Scholar] [CrossRef]
- Resnick, S.; Reyes-Iglesias, Y.; Carreras, R.; Villalobos, E. Migraine with aura associated with reversible MRI abnormalities. Neurology 2006, 66, 946–947. [Google Scholar] [CrossRef]
- Kumar, G.; Topper, L.; Maytal, J. Familial hemiplegic migraine with prolonged aura and multimodality imaging: A case report. Headache 2009, 49, 139–142. [Google Scholar] [CrossRef]
- Butteriss, D.J.; Ramesh, V.; Birchall, D. Serial MRI in a case of familial hemiplegic migraine. Neuroradiology 2003, 45, 300–303. [Google Scholar] [CrossRef]
- Bhatia, R.; Desai, S.; Tripathi, M.; Garg, A.; Padma, M.V.; Prasad, K.; Singh, M.B. Sporadic hemiplegic migraine: Report of a case with clinical and radiological features. J. Headache Pain 2008, 9, 385–388. [Google Scholar] [CrossRef]
- Arca, K.N.; VanderPluym, J.H.; Halker Singh, R.B. Narrative review of neuroimaging in migraine with aura. Headache 2021, 61, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Belvis, R.; Ramos, R.; Villa, C.; Segura, C.; Pagonabarraga, J.; Ormazabal, I.; Kulisevsky, J. Brain apparent water diffusion coefficient magnetic resonance image during a prolonged visual aura. Headache 2010, 50, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Bereczki, D.; Kollar, J.; Kozak, N.; Viszokay, K.; Barta, Z.; Sikula, J.; Magyar, M.T. Cortical spreading edema in persistent visual migraine aura. Headache 2008, 48, 1226–1229. [Google Scholar] [CrossRef]
- Zhang, X.; Levy, D.; Noseda, R.; Kainz, V.; Jakubowski, M.; Burstein, R. Activation of meningeal nociceptors by cortical spreading depression: Implications for migraine with aura. J. Neurosci. 2010, 30, 8807–8814. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.A.; Nozaki, K.; Kraig, R.P. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J. Neurosci. 1993, 13, 1167–1177. [Google Scholar] [CrossRef]
- Bolay, H.; Reuter, U.; Dunn, A.K.; Huang, Z.; Boas, D.A.; Moskowitz, M.A. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat. Med. 2002, 8, 136–142. [Google Scholar] [CrossRef]
- Zhang, X.; Levy, D.; Kainz, V.; Noseda, R.; Jakubowski, M.; Burstein, R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann. Neurol. 2011, 69, 855–865. [Google Scholar] [CrossRef]
- Charles, A.C.; Baca, S.M. Cortical spreading depression and migraine. Nat. Rev. Neurol. 2013, 9, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Kors, E.E.; Melberg, A.; Vanmolkot, K.R.; Kumlien, E.; Haan, J.; Raininko, R.; Flink, R.; Ginjaar, H.B.; Frants, R.R.; Ferrari, M.D.; et al. Childhood epilepsy, familial hemiplegic migraine, cerebellar ataxia, and a new CACNA1A mutation. Neurology 2004, 63, 1136–1137. [Google Scholar] [CrossRef] [PubMed]
- Vanmolkot, K.R.; Kors, E.E.; Hottenga, J.J.; Terwindt, G.M.; Haan, J.; Hoefnagels, W.A.; Black, D.F.; Sandkuijl, L.A.; Frants, R.R.; Ferrari, M.D.; et al. Novel mutations in the Na+, K+-ATPase pump gene ATP1A2 associated with familial hemiplegic migraine and benign familial infantile convulsions. Ann. Neurol. 2003, 54, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, M.; Jorgensen, M.B.; Diemer, N.H.; Gjedde, A.; Hansen, A.J. Persistent oligemia of rat cerebral cortex in the wake of spreading depression. Ann. Neurol. 1982, 12, 469–474. [Google Scholar] [CrossRef]
- Olesen, J.; Larsen, B.; Lauritzen, M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann. Neurol. 1981, 9, 344–352. [Google Scholar] [CrossRef]
- Olesen, J.; Friberg, L.; Olsen, T.S.; Iversen, H.K.; Lassen, N.A.; Andersen, A.R.; Karle, A. Timing and topography of cerebral blood flow, aura, and headache during migraine attacks. Ann. Neurol. 1990, 28, 791–798. [Google Scholar] [CrossRef]
- Bowyer, S.M.; Aurora, K.S.; Moran, J.E.; Tepley, N.; Welch, K.M. Magnetoencephalographic fields from patients with spontaneous and induced migraine aura. Ann. Neurol. 2001, 50, 582–587. [Google Scholar] [CrossRef]
- Coppola, G.; Di Lorenzo, C.; Parisi, V.; Lisicki, M.; Serrao, M.; Pierelli, F. Clinical neurophysiology of migraine with aura. J. Headache Pain 2019, 20, 42. [Google Scholar] [CrossRef]
- Umesh Rudrapatna, S.; Hamming, A.M.; Wermer, M.J.; van der Toorn, A.; Dijkhuizen, R.M. Measurement of distinctive features of cortical spreading depolarizations with different MRI contrasts. NMR Biomed. 2015, 28, 591–600. [Google Scholar] [CrossRef]
- Drenckhahn, C.; Winkler, M.K.; Major, S.; Scheel, M.; Kang, E.J.; Pinczolits, A.; Grozea, C.; Hartings, J.A.; Woitzik, J.; Dreier, J.P.; et al. Correlates of spreading depolarization in human scalp electroencephalography. Brain 2012, 135, 853–868. [Google Scholar] [CrossRef]
- Bastany, Z.J.R.; Askari, S.; Dumont, G.A.; Kellinghaus, C.; Kazemi, A.; Gorji, A. Association of cortical spreading depression and seizures in patients with medically intractable epilepsy. Clin. Neurophysiol. 2020, 131, 2861–2874. [Google Scholar] [CrossRef]
- Zhu, B.; Coppola, G.; Shoaran, M. Migraine classification using somatosensory evoked potentials. Cephalalgia 2019, 39, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, K.; Petrusic, I.; Radojicic, A.; Dakovic, M.; Savic, A. Migraine with aura detection and subtype classification using machine learning algorithms and morphometric magnetic resonance imaging data. Front. Neurol. 2023, 14, 1106612. [Google Scholar] [CrossRef]
- Hsiao, F.J.; Chen, W.T.; Wang, Y.F.; Chen, S.P.; Lai, K.L.; Coppola, G.; Wang, S.J. Identification of patients with chronic migraine by using sensory-evoked oscillations from the electroencephalogram classifier. Cephalalgia 2023, 43, 3331024231176074. [Google Scholar] [CrossRef] [PubMed]
- Petrusic, I.; Jovanovic, V.; Kovic, V.; Savic, A.M. P3 latency as a biomarker for the complexity of migraine with aura: Event-related potential study. Cephalalgia 2022, 42, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Abagnale, C.; Di Renzo, A.; Sebastianelli, G.; Casillo, F.; Tinelli, E.; Giuliani, G.; Tullo, M.G.; Serrao, M.; Parisi, V.; Fiorelli, M.; et al. Whole brain surface-based morphometry and tract-based spatial statistics in migraine with aura patients: Difference between pure visual and complex auras. Front. Hum. Neurosci. 2023, 17, 1146302. [Google Scholar] [CrossRef]
- Petrusic, I.; Jovanovic, V.; Kovic, V.; Savic, A. Characteristics of N400 component elicited in patients who have migraine with aura. J. Headache Pain 2021, 22, 157. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.; Ghanizada, H.; Nielsen, C.A.W.; Hougaard, A.; Ashina, M. Opening of ATP sensitive potassium channels causes migraine attacks with aura. Brain 2021, 144, 2322–2332. [Google Scholar] [CrossRef] [PubMed]

| Ref. | Diagnosis | Relevant Findings | Patients Studied during Aura | Age Range |
|---|---|---|---|---|
| Dow et al., Lancet 1947 [11] | Migraine, subgroup with visual aura | No focal changes during aura | 6 | 14–56 Not specified in subgroup with MwA |
| Lauritzen et al., Cephalalgia 1981 [10] | Classical migraine | No changes or unspecific changes during aura as well as interictally | 3 | 16, 32 |
| Seri et al., Cephalalgia 1993 [13] | Classical migraine | Contralateral decrease in α-power, followed increase in bilateral frontal δ-power; in the headache phase, increase in δ-power in posterior temporal and occipital regions | 10 | 8–14 |
| Parain et al., Cephalalgia 2007 [14] | Prolonged aura 4–24 h with or without weakness | Slowing in recordings after 3 h in temporo-occipital regions or diffuse, occiptal slowing on the second day | 11 | 8–15 |
| Ref. | Diagnosis | Main Findings | Patients Studied during Aura | Age Range |
|---|---|---|---|---|
| Chan et al. J Clin Neurosci 2008 [22] | FHM Type 1 CANA1A mutation, 218 | Depressed EEG activity with low amplitudes over the hemisphere contralateral to hemiparesis, followed by high amplitude δ-activity; 1 patient who also suffered from childhood epilepsy showed in addition paroxysmal activity in the ϑ–δ range over the hemisphere ipsilateral to the hemiparesis | 3 | 12–19 |
| Fitzsimons et al. Brain 1985 [23] Kors et al. Ann Neurol 2001 [24] | FHM Type 1 CACNA1A, S218L | Slow waves over hemisphere related to the paresis and “paroxysms” over the contralateral hemisphere | 4 | 20–41 |
| Murphy J Clin Neurophysiol 2018 [18] | FHM Type 2, mutations in the ATP1A2 gene | Excess of slow-wave-activity in the ϑ–δ range; 1 case had encephalopathic features in the EEG with periods of suppression, which was still asymmetric. This case had cerebral edema of the affected hemisphere with signal increase in FLAIR sequences, diffusion restriction in grey matter and contrast enhancement of ipsilateral mesial temporal lobe | 5 | 12–32 |
| Schwarz et al. J Neurol Sci 2018 [25] | Sporadic hemiplegic migraine, mutations in the SCN1A-gene | Periodic interictal epileptic discharges over the hemisphere related to paresis in addition to continuous slow-wave abnormalities. This EEG pattern probably refers to lateralized periodic discharges (LPD) [26]. This patient was treated with levetiracetam and phenytoin and had cortical signal alterations on T2-weighted MRI but no diffusion restriction | 1 | 14 |
| Chastan et al. Neurophysiol Clin 2016 [27] | Sporadic hemiplegic migraine, mutations in the SCN1A-gene | Posterior slow waves were observed as soon as 15 min after onset of scintillating scotoma with spreading to anterior regions in parallel to symptom evolution to hemiparesis | 1 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riederer, F.; Beiersdorf, J.; Scutelnic, A.; Schankin, C.J. Migraine Aura—Catch Me If You Can with EEG and MRI—A Narrative Review. Diagnostics 2023, 13, 2844. https://doi.org/10.3390/diagnostics13172844
Riederer F, Beiersdorf J, Scutelnic A, Schankin CJ. Migraine Aura—Catch Me If You Can with EEG and MRI—A Narrative Review. Diagnostics. 2023; 13(17):2844. https://doi.org/10.3390/diagnostics13172844
Chicago/Turabian StyleRiederer, Franz, Johannes Beiersdorf, Adrian Scutelnic, and Christoph J. Schankin. 2023. "Migraine Aura—Catch Me If You Can with EEG and MRI—A Narrative Review" Diagnostics 13, no. 17: 2844. https://doi.org/10.3390/diagnostics13172844
APA StyleRiederer, F., Beiersdorf, J., Scutelnic, A., & Schankin, C. J. (2023). Migraine Aura—Catch Me If You Can with EEG and MRI—A Narrative Review. Diagnostics, 13(17), 2844. https://doi.org/10.3390/diagnostics13172844






