Applications and Prospects of Artificial Intelligence-Assisted Endoscopic Ultrasound in Digestive System Diseases
Abstract
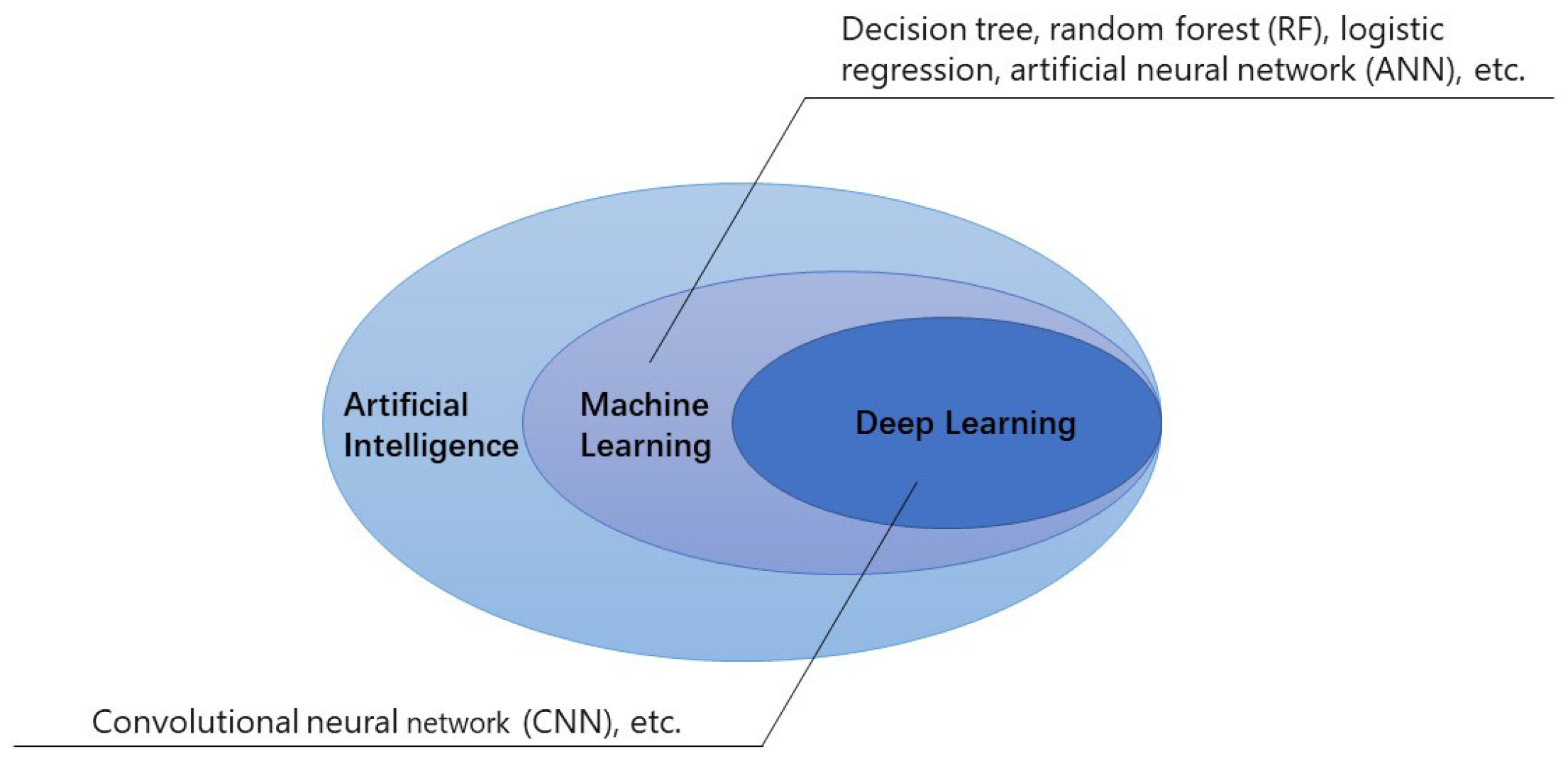
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
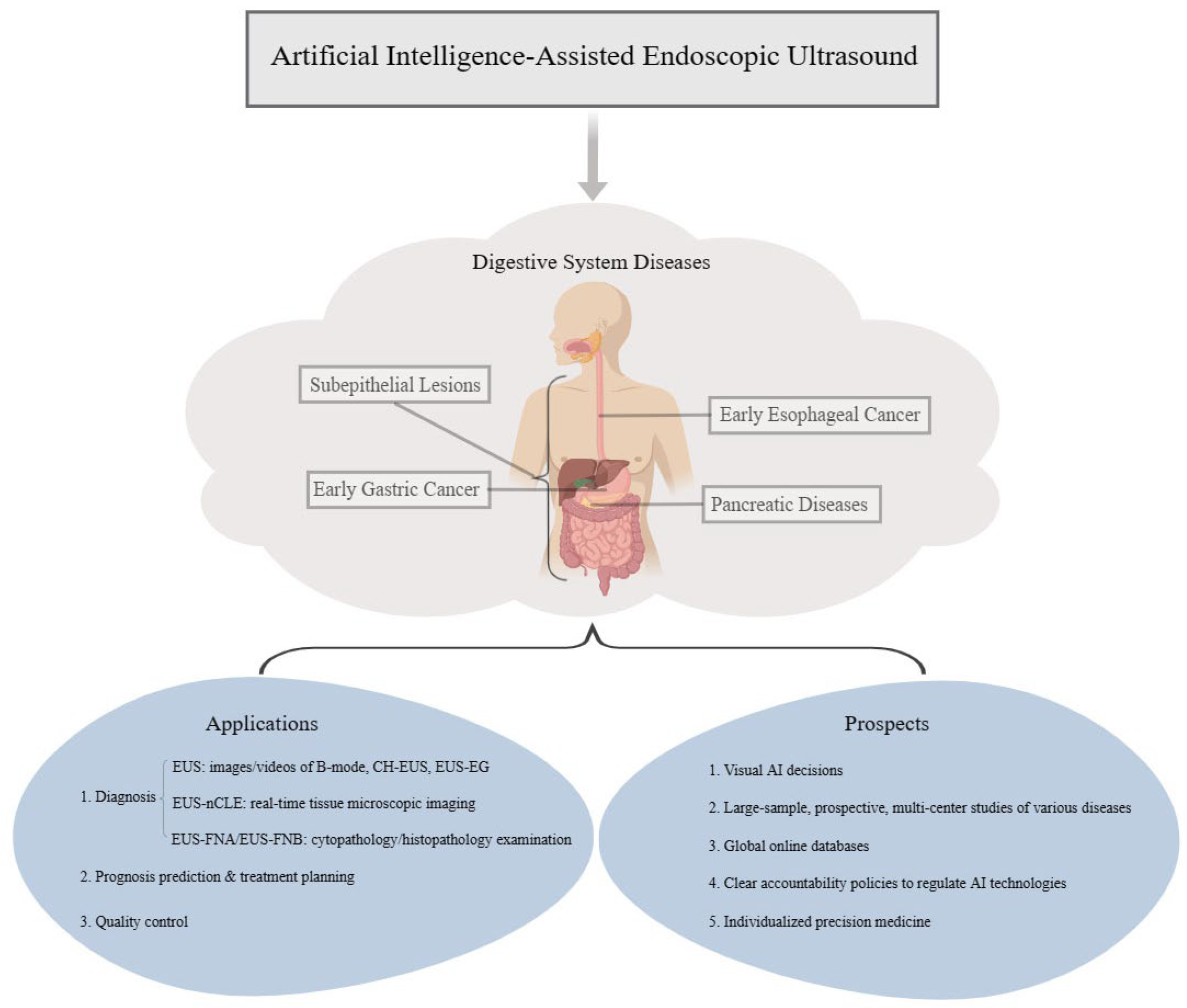
3. Types of Digestive System Diseases Diagnosed and Prognosed by EUS-AI
3.1. Subepithelial Lesions
| Study | Study Design | AI Model | Patient Population | Research Object | Outcomes for the AI Model |
|---|---|---|---|---|---|
| Minoda et al. [21] | Retrospective (Japan) | CNN | SELs < 20 mm: Total Patients = 30 GISTs = 23 Leiomyoma = 5 Schwannoma = 1 Ectopic Pancreas = 1 SELs ≥ 20 mm: Total Patients = 30 GISTs = 24 Leiomyoma = 4 Schwannoma = 1 Ectopic Pancreas = 1 | EUS Images | Recognition of GISTs in SELs < 20 mm: Sensitivity = 86.3% Specificity = 62.5% Accuracy = 86.3% AUC = 0.861 Recognition of GISTs in SELs ≥ 20 mm: Sensitivity = 83.3% Specificity = 91.7% Accuracy = 90.0% AUC = 0.965 |
| Minoda et al. [22] | Retrospective (Japan) | CNN | Total Patients = 52 GISTs = 36 Leiomyoma = 14 Ectopic Pancreas = 1 Appendiceal Mucocele = 1 | EUS Images | Recognition of GISTs: Sensitivity = 100% Specificity = 86.1% Accuracy = 94.4% AUC = 0.980 |
| Tanaka et al. [24] | Retrospective (Japan) | DL | Total Patients = 53 GISTs = 42 Leiomyoma = 11 | CH-EUS Images | Recognition of GISTs: Sensitivity = 90.5% Specificity = 90.9% Accuracy = 90.6% |
| Hirai et al. [25] | Retrospective (Japan) | CNN DCGAN Semi-supervised Learning | Total Patients = 631 GISTs = 435 non-GISTs = 196 (Leiomyoma = 97, Schwannoma = 33, NET = 47, Ectopic Pancreas = 19) | EUS Images | Recognition of GISTs: Sensitivity = 98.8% Specificity = 67.6% Accuracy = 89.3% |
3.2. Early Esophageal Cancer
3.3. Early Gastric Cancer
3.4. Pancreatic Diseases
3.4.1. Pancreatic Cystic Lesions
3.4.2. Autoimmune Pancreatitis
3.4.3. Pancreatic Cancer
| Study | Study Design | AI Model | Patient Population | Research Object | Outcomes for the AI Model |
|---|---|---|---|---|---|
| Kuwahara et al. [64] | Retrospective (Japan) | DL | Total Patients = 694 PC = 524 Non-Cancer Patients = 170 (PDAC = 518, PASC = 5, ACC = 1, MPT = 8, NEC = 6, NET = 57, SPN = 6, CP = 58, AIP = 35) | EUS Images | Recognition of PC: Sensitivity = 94% Specificity = 82% Accuracy = 91% AUC = 0.90 |
| Tonozuka et al. [11] | Retrospective (Japan) | CNN | Total Patients = 139 PDAC = 76 CP = 34 NP = 29 | EUS Images | Recognition of PC: Sensitivity = 92.4% Specificity = 84.1% AUC = 0.940 |
| Goyal et al. [65] | Systematic Review (United States) | ANN CNN SVM | Total Patients = 2292 PC = 1409 Non-Cancer Patients = 883 | EUS Images EUS Videos EUS-EG | Recognition of PC: Sensitivity = 83–100% Specificity = 50–99% Accuracy = 80–97.5% |
| Zhang et al. [67] | Retrospective (China) | DCNN | Total Patients = 194 PC = 110 Non-Cancer Patients = 84 | Staining EUS-FNA Specimens | Recognition of PC: Sensitivity = 92.8–94.4% Specificity = 87.5–97.1% Accuracy = 91.2–95.8% AUC = 0.948–0.976 |
| Ishikawa et al. [68] | Retrospective (Japan) | Contrastive Learning (Unsupervised Learning) | Total Patients = 97 PDAC = 66 MFP = 13 AIP = 11 Pancreatic Neuroendocrine Tumor = 3 MPT = 3 IPMC = 1 | Staining EUS-FNB Specimens | Recognition of Pancreatic Diseases: Sensitivity = 90.34% Specificity = 53.5% Accuracy = 84.39% |
| Tang et al. [77] | Prospective (China) | Model 1: DCNN Model 2: RF Algorithm | Total Patients in Model 1 = 950 PC = 760 Benign Pancreatic Masses = 190 Total Patients in Model 2 = 295 PC = 167 Pancreatitis = 128 | Model 1: CH-EUS Images Model 2: CH-EUS Videos | Recognition of Pancreatic Diseases in Model 1: the Average Overlap Rate = 0.708; Accuracy = 87.8% Recognition of Pancreatic Diseases in Model 2: Sensitivity = 100% Specificity = 75% Accuracy = 88.9% |
| Săftoiu et al. [78] | Prospective (Europe) | ANN | Total Patients = 258 PC = 211 CP = 47 | Hue Histogram Data Extracted from Dynamic Sequences of EUS-EG | Recognition of Pancreatic Diseases: Sensitivity = 87.59% Specificity = 82.94% Accuracy = 84.27% |
4. EUS-AI in Quality Control
5. Discussion and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samuel, A.L. Some Studies in Machine Learning Using the Game of Checkers. IBM J. Res. Dev. 1959, 3, 210–229. [Google Scholar]
- Kuwahara, T.; Hara, K.; Mizuno, N.; Haba, S.; Okuno, N.; Koda, H.; Miyano, A.; Fumihara, D. Current status of artificial intelligence analysis for endoscopic ultrasonography. Dig. Endosc. 2021, 33, 298–305. [Google Scholar] [CrossRef]
- Tonozuka, R.; Mukai, S.; Itoi, T. The Role of Artificial Intelligence in Endoscopic Ultrasound for Pancreatic Disorders. Diagnostics 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, H.; Ramalakshmi, K.; Swaminathan, D.K.; Mazzara, M. GIT-Net: An Ensemble Deep Learning-Based GI Tract Classification of Endoscopic Images. Bioengineering 2023, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, K.; George, T.T.; Shah, Y.; Sasidhar, P. A Novel Multi-Feature Fusion Method for Classification of Gastrointestinal Diseases Using Endoscopy Images. Diagnostics 2022, 12, 2316. [Google Scholar] [CrossRef]
- Zhao, A.; Du, X.; Yuan, S.; Shen, W.; Zhu, X.; Wang, W. Automated Detection of Endometrial Polyps from Hysteroscopic Videos Using Deep Learning. Diagnostics 2023, 13, 1409. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamashita, Y.; Kitano, M. Endoscopic Ultrasound for Early Diagnosis of Pancreatic Cancer. Diagnostics 2019, 9, 81. [Google Scholar] [CrossRef]
- Sooklal, S.; Chahal, P. Endoscopic Ultrasound. Surg. Clin. N. Am. 2020, 100, 1133–1150. [Google Scholar] [CrossRef]
- Dye, C.E.; Waxman, I. Endoscopic ultrasound. Gastroenterol. Clin. N. Am. 2002, 31, 863–879. [Google Scholar] [CrossRef]
- Yin, H.; Yang, X.; Sun, L.; Pan, P.; Peng, L.; Li, K.; Zhang, D.; Cui, F.; Xia, C.; Huang, H.; et al. The value of artificial intelligence techniques in predicting pancreatic ductal adenocarcinoma with EUS images: A meta-analysis and systematic review. Endosc. Ultrasound 2023, 12, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tonozuka, R.; Itoi, T.; Nagata, N.; Kojima, H.; Sofuni, A.; Tsuchiya, T.; Ishii, K.; Tanaka, R.; Nagakawa, Y.; Mukai, S. Deep learning analysis for the detection of pancreatic cancer on endosonographic images: A pilot study. J. Hepatobiliary Pancreat. Sci. 2021, 28, 95–104. [Google Scholar] [CrossRef]
- Wang, L.; Song, H.; Wang, M.; Wang, H.; Ge, R.; Shen, Y.; Yu, Y. Utilization of Ultrasonic Image Characteristics Combined with Endoscopic Detection on the Basis of Artificial Intelligence Algorithm in Diagnosis of Early Upper Gastrointestinal Cancer. J. Healthc. Eng. 2021, 2021, 2773022. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, K.; Oya, M.; Koga, T.; Shiratsuchi, Y. Current clinical management of gastrointestinal stromal tumor. World J. Gastroenterol. 2018, 24, 2806–2817. [Google Scholar] [CrossRef]
- Jaros, D.; Bozic, B.; Sebesta, C. [Gastrointestinal stromal tumors (GIST)]. Wien Med. Wochenschr. 2023, 173, 201–205. [Google Scholar] [CrossRef]
- Joensuu, H.; Hohenberger, P.; Corless, C.L. Gastrointestinal stromal tumour. Lancet 2013, 382, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Davila, R.E. A Gastroenterologist’s Approach to the Diagnosis and Management of Gastrointestinal Stromal Tumors. Gastroenterol. Clin. N. Am. 2022, 51, 609–624. [Google Scholar] [CrossRef]
- Joensuu, H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008, 39, 1411–1419. [Google Scholar] [CrossRef]
- Kalkmann, J.; Zeile, M.; Antoch, G.; Berger, F.; Diederich, S.; Dinter, D.; Fink, C.; Janka, R.; Stattaus, J. Consensus report on the radiological management of patients with gastrointestinal stromal tumours (GIST): Recommendations of the German GIST Imaging Working Group. Cancer Imaging 2012, 12, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Landi, B. Gastrointestinal stromal tumors: Clinical features and diagnosis. Bull. Acad. Natl. Med. 2012, 196, 845–852; discussion 852–853. [Google Scholar]
- Panbude, S.N.; Ankathi, S.K.; Ramaswamy, A.T.; Saklani, A.P. Gastrointestinal Stromal Tumor (GIST) from esophagus to anorectum—Diagnosis, response evaluation and surveillance on computed tomography (CT) scan. Indian J. Radiol. Imaging 2019, 29, 133–140. [Google Scholar] [CrossRef]
- Minoda, Y.; Ihara, E.; Komori, K.; Ogino, H.; Otsuka, Y.; Chinen, T.; Tsuda, Y.; Ando, K.; Yamamoto, H.; Ogawa, Y. Efficacy of endoscopic ultrasound with artificial intelligence for the diagnosis of gastrointestinal stromal tumors. J. Gastroenterol. 2020, 55, 1119–1126. [Google Scholar] [CrossRef]
- Minoda, Y.; Ihara, E.; Fujimori, N.; Nagatomo, S.; Esaki, M.; Hata, Y.; Bai, X.; Tanaka, Y.; Ogino, H.; Chinen, T.; et al. Efficacy of ultrasound endoscopy with artificial intelligence for the differential diagnosis of non-gastric gastrointestinal stromal tumors. Sci. Rep. 2022, 12, 16640. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, F.; Li, P.; Zhu, J. Artificial intelligence-assisted endoscopic ultrasound in the diagnosis of gastrointestinal stromal tumors: A meta-analysis. Surg. Endosc. 2023, 37, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kamata, K.; Ishihara, R.; Handa, H.; Otsuka, Y.; Yoshida, A.; Yoshikawa, T.; Ishikawa, R.; Okamoto, A.; Yamazaki, T.; et al. Value of artificial intelligence with novel tumor tracking technology in the diagnosis of gastric submucosal tumors by contrast-enhanced harmonic endoscopic ultrasonography. J. Gastroenterol. Hepatol. 2022, 37, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Kuwahara, T.; Furukawa, K.; Kakushima, N.; Furune, S.; Yamamoto, H.; Marukawa, T.; Asai, H.; Matsui, K.; Sasaki, Y.; et al. Artificial intelligence-based diagnosis of upper gastrointestinal subepithelial lesions on endoscopic ultrasonography images. Gastric Cancer 2022, 25, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.W.; Lee, H.; Song, B.G.; Min, B.H.; Kim, H.K.; Choi, Y.S.; Lee, J.H.; Hwang, N.Y.; Carriere, K.C.; Rhee, P.L.; et al. Comparison of endoscopic submucosal dissection and surgery for superficial esophageal squamous cell carcinoma: A propensity score-matched analysis. Gastrointest. Endosc. 2018, 88, 624–633. [Google Scholar] [CrossRef]
- Van de Ven, S.E.M.; Spaander, M.C.W.; Pouw, R.E.; Tang, T.J.; Houben, M.; Schoon, E.J.; de Jonge, P.J.F.; Bruno, M.J.; Koch, A.D. Favorable effect of endoscopic reassessment of clinically staged T2 esophageal adenocarcinoma: A multicenter prospective cohort study. Endoscopy 2022, 54, 163–169. [Google Scholar] [CrossRef]
- Schmidlin, E.J.; Gill, R.R. New frontiers in esophageal radiology. Ann. Transl. Med. 2021, 9, 904. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Souza, R.F.; Yadlapati, R.H.; Sauer, B.G.; Wani, S. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Am. J. Gastroenterol. 2022, 117, 559–587. [Google Scholar] [CrossRef]
- Meining, A.; Rösch, T.; Wolf, A.; Lorenz, R.; Allescher, H.D.; Kauer, W.; Dittler, H.J. High interobserver variability in endosonographic staging of upper gastrointestinal cancers. Z. Gastroenterol. 2003, 41, 391–394. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Y.; Li, Z.; Su, P.; Gao, W.; Huang, C.; Tian, Z. Impact of endoscopic ultrasonography on the accuracy of T staging in esophageal cancer and factors associated with its accuracy: A retrospective study. Medicine 2022, 101, e28603. [Google Scholar] [CrossRef]
- Knabe, M.; Welsch, L.; Blasberg, T.; Müller, E.; Heilani, M.; Bergen, C.; Herrmann, E.; May, A. Artificial intelligence-assisted staging in Barrett’s carcinoma. Endoscopy 2022, 54, 1191–1197. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, Y.; Hayashi, Y.; Ishihara, R.; Yamaguchi, S.; Yamamoto, M.; Inoue, T.; Nagai, K.; Ogiyama, H.; Yamada, T.; Nakahara, M.; et al. Diagnostic value of endoscopic ultrasonography for the depth of gastric cancer suspected of submucosal invasion: A multicenter prospective study. Surg. Endosc. 2023, 37, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Uedo, N.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig. Endosc. 2021, 33, 4–20. [Google Scholar] [CrossRef]
- Min, Y.W.; Lee, J.H. Endoscopic Resection for Early Gastric Cancer beyond Absolute Indication with Emphasis on Controversial Issues. J. Gastric. Cancer. 2014, 14, 7–14. [Google Scholar] [CrossRef]
- Giandola, T.; Maino, C.; Marrapodi, G.; Ratti, M.; Ragusi, M.; Bigiogera, V.; Talei Franzesi, C.; Corso, R.; Ippolito, D. Imaging in Gastric Cancer: Current Practice and Future Perspectives. Diagnostics 2023, 13, 1276. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.Y.; Chung, H.J.; Choi, K.S.; Lee, H.; Kim, T.J.; Soh, H.; Kang, E.A.; Cho, S.J.; Ye, J.C.; Im, J.P.; et al. Deep learning model for diagnosing gastric mucosal lesions using endoscopic images: Development, validation, and method comparison. Gastrointest. Endosc. 2022, 95, 258–268.e210. [Google Scholar] [CrossRef]
- Kim, J.; Chung, H.; Kim, J.L.; Lee, E.; Kim, S.G. Hierarchical Analysis of Factors Associated with T Staging of Gastric Cancer by Endoscopic Ultrasound. Dig. Dis. Sci. 2021, 66, 612–618. [Google Scholar] [CrossRef]
- Garcea, G.; Ong, S.L.; Rajesh, A.; Neal, C.P.; Pollard, C.A.; Berry, D.P.; Dennison, A.R. Cystic lesions of the pancreas. A diagnostic and management dilemma. Pancreatology 2008, 8, 236–251. [Google Scholar] [CrossRef]
- Moris, M.; Bridges, M.D.; Pooley, R.A.; Raimondo, M.; Woodward, T.A.; Stauffer, J.A.; Asbun, H.J.; Wallace, M.B. Association Between Advances in High-Resolution Cross-Section Imaging Technologies and Increase in Prevalence of Pancreatic Cysts From 2005 to 2014. Clin. Gastroenterol. Hepatol. 2016, 14, 585–593.e583. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, Y.J.; Park, Y.T.; Kim, K.G. Automatic Pancreatic Cyst Lesion Segmentation on EUS Images Using a Deep-Learning Approach. Sensors 2021, 22, 245. [Google Scholar] [CrossRef]
- Nguon, L.S.; Seo, K.; Lim, J.H.; Song, T.J.; Cho, S.H.; Park, J.S.; Park, S. Deep Learning-Based Differentiation between Mucinous Cystic Neoplasm and Serous Cystic Neoplasm in the Pancreas Using Endoscopic Ultrasonography. Diagnostics 2021, 11, 1052. [Google Scholar] [CrossRef]
- Vilas-Boas, F.; Ribeiro, T.; Afonso, J.; Cardoso, H.; Lopes, S.; Moutinho-Ribeiro, P.; Ferreira, J.; Mascarenhas-Saraiva, M.; Macedo, G. Deep Learning for Automatic Differentiation of Mucinous versus Non-Mucinous Pancreatic Cystic Lesions: A Pilot Study. Diagnostics 2022, 12, 2041. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I. Invited Editorial: Comprehensive Analysis of Molecular Biological Characteristics of Pancreatic Ductal Adenocarcinoma Concomitant with Intraductal Papillary Mucinous Neoplasm. Ann. Surg. Oncol. 2022, 29, 4683–4685. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Hara, K.; Mizuno, N.; Okuno, N.; Matsumoto, S.; Obata, M.; Kurita, Y.; Koda, H.; Toriyama, K.; Onishi, S.; et al. Usefulness of Deep Learning Analysis for the Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Clin. Transl. Gastroenterol. 2019, 10, e00045. [Google Scholar] [CrossRef]
- Nakai, Y.; Iwashita, T.; Park, D.H.; Samarasena, J.B.; Lee, J.G.; Chang, K.J. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest. Endosc. 2015, 81, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.K.; Modi, R.M.; Swanson, B.; Conwell, D.L.; Krishna, S.G. A comprehensive examination of the novel techniques used for in vivo and ex vivo confocal laser endomicroscopy of pancreatic cystic lesions. VideoGIE 2016, 1, 6–7. [Google Scholar] [CrossRef][Green Version]
- Chin, Y.K.; Wu, C.C.H.; Tan, D.M.Y. The Role of Needle-Based Confocal Laser Endomicroscopy in the Evaluation of Pancreatic Cystic Lesions: A Systematic Review. Clin. Endosc. 2021, 54, 38–47. [Google Scholar] [CrossRef]
- Facciorusso, A.; Buccino, V.R.; Sacco, R. Needle-based confocal laser endomicroscopy in pancreatic cysts: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1084–1090. [Google Scholar] [CrossRef]
- Napoleon, B.; Krishna, S.G.; Marco, B.; Carr-Locke, D.; Chang, K.J.; Ginès, À.; Gress, F.G.; Larghi, A.; Oppong, K.W.; Palazzo, L.; et al. Confocal endomicroscopy for evaluation of pancreatic cystic lesions: A systematic review and international Delphi consensus report. Endosc. Int. Open 2020, 8, E1566–E1581. [Google Scholar] [CrossRef]
- Machicado, J.D.; Chao, W.L.; Carlyn, D.E.; Pan, T.Y.; Poland, S.; Alexander, V.L.; Maloof, T.G.; Dubay, K.; Ueltschi, O.; Middendorf, D.M.; et al. High performance in risk stratification of intraductal papillary mucinous neoplasms by confocal laser endomicroscopy image analysis with convolutional neural networks (with video). Gastrointest. Endosc. 2021, 94, 78–87.e72. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.S.; Al-Haddad, M.; Chandan, S.; Gangwani, M.K.; Aziz, M.; Mohan, B.P.; Ramai, D.; Canakis, A.; Bapaye, J.; Sharma, N. Artificial Intelligence in Endoscopic Ultrasound for Pancreatic Cancer: Where Are We Now and What Does the Future Entail? J. Clin. Med. 2022, 11, 7476. [Google Scholar] [CrossRef]
- Mack, S.; Flattet, Y.; Bichard, P.; Frossard, J.L. Recent advances in the management of autoimmune pancreatitis in the era of artificial intelligence. World J. Gastroenterol. 2022, 28, 6867–6874. [Google Scholar] [CrossRef]
- Tacelli, M.; Zaccari, P.; Petrone, M.C.; Della Torre, E.; Lanzillotta, M.; Falconi, M.; Doglioni, C.; Capurso, G.; Arcidiacono, P.G. Differential EUS findings in focal type 1 autoimmune pancreatitis and pancreatic cancer: A proof-of-concept study. Endosc. Ultrasound 2022, 11, 216–222. [Google Scholar] [CrossRef]
- Yousaf, M.N.; Chaudhary, F.S.; Ehsan, A.; Suarez, A.L.; Muniraj, T.; Jamidar, P.; Aslanian, H.R.; Farrell, J.J. Endoscopic ultrasound (EUS) and the management of pancreatic cancer. BMJ Open Gastroenterol. 2020, 7, e000408. [Google Scholar] [CrossRef]
- Thomsen, M.M.; Larsen, M.H.; Di Caterino, T.; Hedegaard Jensen, G.; Mortensen, M.B.; Detlefsen, S. Accuracy and clinical outcomes of pancreatic EUS-guided fine-needle biopsy in a consecutive series of 852 specimens. Endosc. Ultrasound 2022, 11, 306–318. [Google Scholar] [CrossRef]
- Guo, T.; Xu, T.; Zhang, S.; Lai, Y.; Wu, X.; Wu, D.; Feng, Y.; Jiang, Q.; Wang, Q.; Qian, J.; et al. The role of EUS in diagnosing focal autoimmune pancreatitis and differentiating it from pancreatic cancer. Endosc. Ultrasound 2021, 10, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Marya, N.B.; Powers, P.D.; Chari, S.T.; Gleeson, F.C.; Leggett, C.L.; Abu Dayyeh, B.K.; Chandrasekhara, V.; Iyer, P.G.; Majumder, S.; Pearson, R.K.; et al. Utilisation of artificial intelligence for the development of an EUS-convolutional neural network model trained to enhance the diagnosis of autoimmune pancreatitis. Gut 2021, 70, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Spadaccini, M.; Koleth, G.; Emmanuel, J.; Khalaf, K.; Facciorusso, A.; Grizzi, F.; Hassan, C.; Colombo, M.; Mangiavillano, B.; Fugazza, A.; et al. Enhanced endoscopic ultrasound imaging for pancreatic lesions: The road to artificial intelligence. World J. Gastroenterol. 2022, 28, 3814–3824. [Google Scholar] [CrossRef] [PubMed]
- Machicado, J.D.; Obuch, J.C.; Goodman, K.A.; Schefter, T.E.; Frakes, J.; Hoffe, S.; Latifi, K.; Simon, V.C.; Santangelo, T.; Ezekwe, E.; et al. Endoscopic Ultrasound Placement of Preloaded Fiducial Markers Shortens Procedure Time Compared to Back-Loaded Markers. Clin. Gastroenterol. Hepatol. 2019, 17, 2749–2758.e2742. [Google Scholar] [CrossRef] [PubMed]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Hara, K.; Mizuno, N.; Haba, S.; Okuno, N.; Kuraishi, Y.; Fumihara, D.; Yanaidani, T.; Ishikawa, S.; Yasuda, T.; et al. Artificial intelligence using deep learning analysis of endoscopic ultrasonography images for the differential diagnosis of pancreatic masses. Endoscopy 2023, 55, 140–149. [Google Scholar] [CrossRef]
- Goyal, H.; Sherazi, S.A.A.; Gupta, S.; Perisetti, A.; Achebe, I.; Ali, A.; Tharian, B.; Thosani, N.; Sharma, N.R. Application of artificial intelligence in diagnosis of pancreatic malignancies by endoscopic ultrasound: A systemic review. Therap. Adv. Gastroenterol. 2022, 15, 17562848221093873. [Google Scholar] [CrossRef]
- Khalaf, K.; Terrin, M.; Jovani, M.; Rizkala, T.; Spadaccini, M.; Pawlak, K.M.; Colombo, M.; Andreozzi, M.; Fugazza, A.; Facciorusso, A.; et al. A Comprehensive Guide to Artificial Intelligence in Endoscopic Ultrasound. J. Clin. Med. 2023, 12, 3757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, Y.; Tang, D.; Ni, M.; Zheng, J.; Xu, G.; Peng, C.; Shen, S.; Zhan, Q.; Wang, X.; et al. A deep learning-based segmentation system for rapid onsite cytologic pathology evaluation of pancreatic masses: A retrospective, multicenter, diagnostic study. EBioMedicine 2022, 80, 104022. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Hayakawa, M.; Suzuki, H.; Ohno, E.; Mizutani, Y.; Iida, T.; Fujishiro, M.; Kawashima, H.; Hotta, K. Development of a Novel Evaluation Method for Endoscopic Ultrasound-Guided Fine-Needle Biopsy in Pancreatic Diseases Using Artificial Intelligence. Diagnostics 2022, 12, 434. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Mansueto, G.; Falconi, M.; Procacci, C. Neuroendocrine pancreatic tumor: Value of contrast enhanced ultrasonography. Abdom. Imaging 2004, 29, 246–258. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Zamboni, G.; Faccioli, N.; Capelli, P.; Pozzi Mucelli, R. Ultrasonography of the pancreas. 4. Contrast-enhanced imaging. Abdom. Imaging 2007, 32, 171–181. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Ignee, A.; Braden, B.; Barreiros, A.P.; Ott, M.; Hocke, M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin. Gastroenterol. Hepatol. 2008, 6, 590–597.e591. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Braden, B.; Hocke, M.; Ott, M.; Ignee, A. Improved characterisation of solitary solid pancreatic tumours using contrast enhanced transabdominal ultrasound. J. Cancer Res. Clin. Oncol. 2008, 134, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.R.; Jeong, S.H.; Kang, H.; Kim, E.J.; Kim, Y.S.; Jeon, S.; Cho, J.H. Diagnostic performance of endoscopic ultrasound elastography for differential diagnosis of solid pancreatic lesions: A propensity score-matched analysis. Pancreatology 2023, 23, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, H.; Liao, D.; Qian, B.; Zhang, Y.; Xu, M.; Han, S. Contrast-enhanced harmonic endoscopic ultrasonography for the differential diagnosis of pancreatic masses: A systematic review and meta-analysis. Mol. Clin. Oncol. 2019, 11, 425–433. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Yao, L.; Ding, X.; Chen, D.; Wu, H.; Lu, Z.; Zhou, W.; Zhang, L.; An, P.; et al. Deep learning-based pancreas segmentation and station recognition system in EUS: Development and validation of a useful training tool (with video). Gastrointest. Endosc. 2020, 92, 874–885.e873. [Google Scholar] [CrossRef]
- Takada, S.; Kato, H.; Saragai, Y.; Muro, S.; Uchida, D.; Tomoda, T.; Matsumoto, K.; Horiguchi, S.; Tanaka, N.; Okada, H. Contrast-enhanced harmonic endoscopic ultrasound using time-intensity curve analysis predicts pathological grade of pancreatic neuroendocrine neoplasm. J. Med. Ultrason. 2019, 46, 449–458. [Google Scholar] [CrossRef]
- Tang, A.; Tian, L.; Gao, K.; Liu, R.; Hu, S.; Liu, J.; Xu, J.; Fu, T.; Zhang, Z.; Wang, W.; et al. Contrast-enhanced harmonic endoscopic ultrasound (CH-EUS) MASTER: A novel deep learning-based system in pancreatic mass diagnosis. Cancer Med. 2023, 12, 7962–7973. [Google Scholar] [CrossRef]
- Săftoiu, A.; Vilmann, P.; Gorunescu, F.; Janssen, J.; Hocke, M.; Larsen, M.; Iglesias-Garcia, J.; Arcidiacono, P.; Will, U.; Giovannini, M.; et al. Efficacy of an artificial neural network-based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clin. Gastroenterol. Hepatol. 2012, 10, 84–90.e81. [Google Scholar] [CrossRef]
- Wani, S.; Keswani, R.N.; Petersen, B.; Edmundowicz, S.A.; Walsh, C.M.; Huang, C.; Cohen, J.; Cote, G. Training in EUS and ERCP: Standardizing methods to assess competence. Gastrointest. Endosc. 2018, 87, 1371–1382. [Google Scholar] [CrossRef]
- Irisawa, A.; Yamao, K. Curved linear array EUS technique in the pancreas and biliary tree: Focusing on the stations. Gastrointest. Endosc. 2009, 69, S84–S89. [Google Scholar] [CrossRef]
- Price, W.N. Big data and black-box medical algorithms. Sci. Transl. Med. 2018, 10, eaao5333. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Fan, X.; Liu, W. Applications and Prospects of Artificial Intelligence-Assisted Endoscopic Ultrasound in Digestive System Diseases. Diagnostics 2023, 13, 2815. https://doi.org/10.3390/diagnostics13172815
Huang J, Fan X, Liu W. Applications and Prospects of Artificial Intelligence-Assisted Endoscopic Ultrasound in Digestive System Diseases. Diagnostics. 2023; 13(17):2815. https://doi.org/10.3390/diagnostics13172815
Chicago/Turabian StyleHuang, Jia, Xiaofei Fan, and Wentian Liu. 2023. "Applications and Prospects of Artificial Intelligence-Assisted Endoscopic Ultrasound in Digestive System Diseases" Diagnostics 13, no. 17: 2815. https://doi.org/10.3390/diagnostics13172815
APA StyleHuang, J., Fan, X., & Liu, W. (2023). Applications and Prospects of Artificial Intelligence-Assisted Endoscopic Ultrasound in Digestive System Diseases. Diagnostics, 13(17), 2815. https://doi.org/10.3390/diagnostics13172815






