Severe Acute Bronchial Asthma with Sepsis: Determining the Status of Biomarkers in the Diagnosis of the Disease
Abstract
1. Introduction
2. Objective of the Study
3. Materials and Methods
4. Diagnosis of Asthma
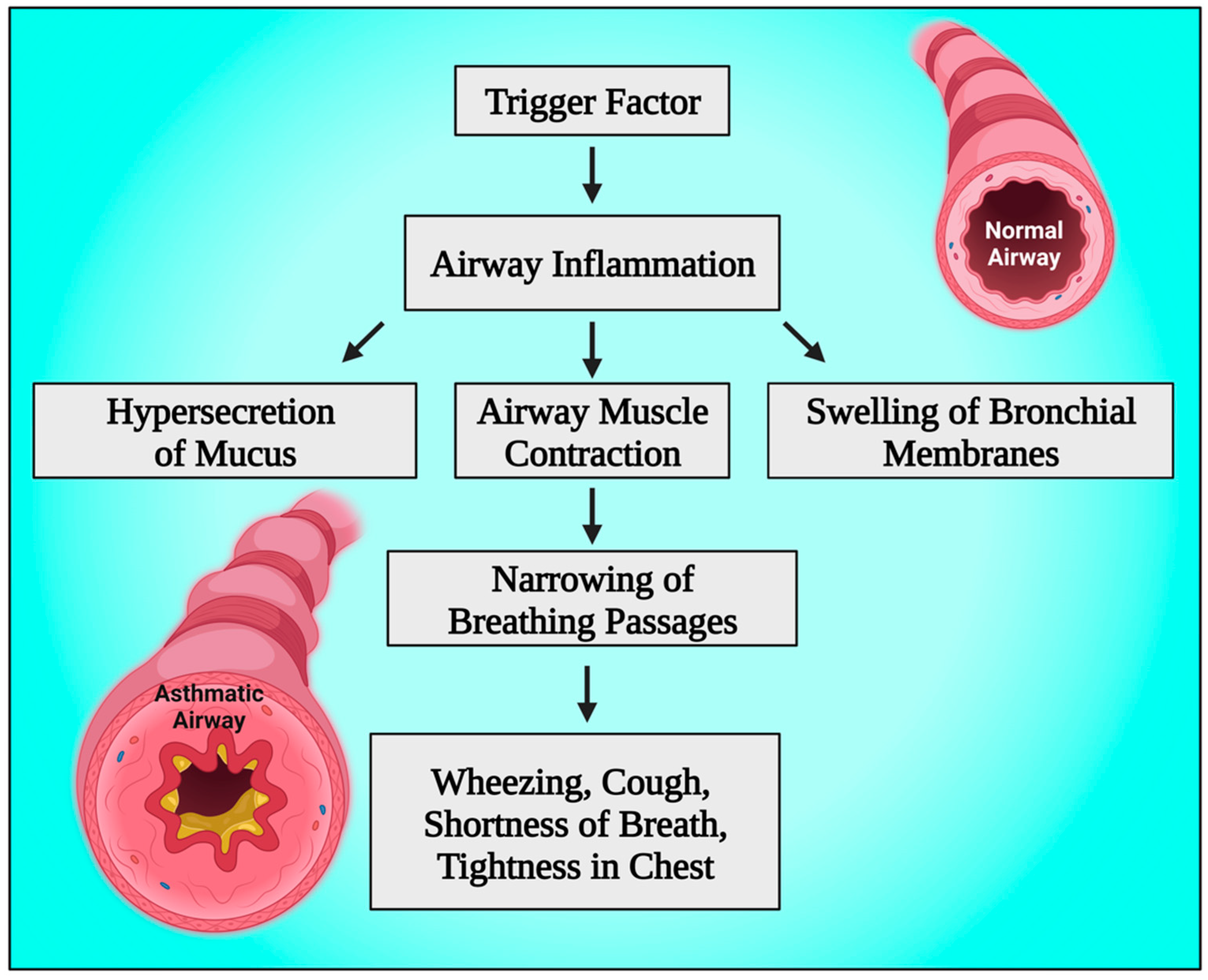
5. Pathophysiology of Bronchial Asthma
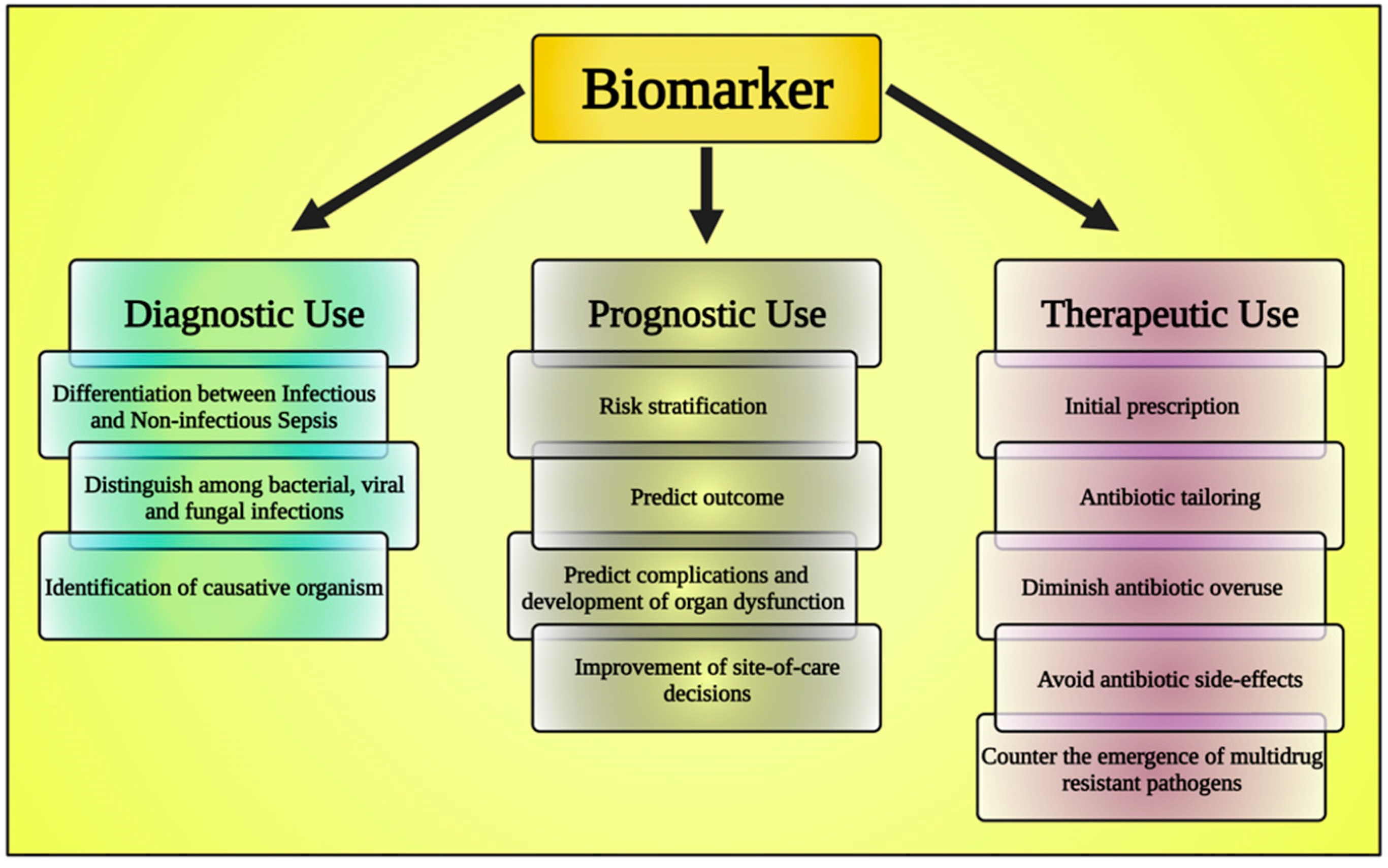
6. The Emerging Role of Biomarkers
6.1. Asthma Biomarkers
6.1.1. Fractional Exhaled Nitric Oxide (FeNO)
6.1.2. Sputum Inflammatory Cell Analysis
6.1.3. Blood Eosinophil (B-EOS)
6.1.4. Total Serum IgE Level
6.1.5. Soluble Form of the Triggering Receptor Expressed on Myeloid Cells-1 (sTREM-1)
6.1.6. Neutrophil to Lymphocyte Ratio (NLR)
6.2. Sepsis Biomarkers
6.2.1. Procalcitonin
6.2.2. Prognostic Role of Procalcitonin
6.2.3. C Reactive Protein
6.2.4. Type 2 Helper T-Cell (Th 2)
6.2.5. Omentin-1
6.2.6. H2S
7. Takeaway Message
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bond, K.R.; Horsley, C.A.; Williams, A.B. Non-invasive ventilation use in status asthmaticus: 16 years of experience in a tertiary intensive care. Emerg. Med. Australas. 2018, 30, 187–192. [Google Scholar] [CrossRef]
- Adams, J.Y.; Sutter, M.E.; Albertson, T.E. The patient with asthma in the emergency department. Clin. Rev. Allergy Immunol. 2012, 43, 14–29. [Google Scholar] [CrossRef]
- D’Amato, G.; Vitale, C.; Molino, A.; Stanziola, A.; Sanduzzi, A.; Vatrella, A.; Mormile, M.; Lanza, M.; Calabrese, G.; Antonicelli, L.; et al. Asthma-related deaths. Multidiscip. Respir. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- D’Amato, G.; Vitale, C.; Lanza, M.; Sanduzzi, A.; Molino, A.; Mormile, M.; Vatrella, A.; Bilò, M.B.; Antonicelli, L.; Bresciani, M.; et al. Near fatal asthma: Treatment and prevention. Eur. Ann. Allergy Clin. Immunol. 2016, 48, 116–122. [Google Scholar] [PubMed]
- Fernandes, A.G.; Souza-Machado, C.; Coelho, R.C.; Franco, P.A.; Esquivel, R.M.; Souza-Machado, A.; Cruz, A.A. Risk factors for death in patients with severe asthma. J. Bras. Pneumol. 2014, 40, 364–372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McFadden, E.R., Jr. Acute severe asthma. Am. J. Respir. Crit. Care Med. 2003, 168, 740–759. [Google Scholar] [CrossRef] [PubMed]
- Normansell, R.; Sayer, B.; Waterson, S.; Dennett, E.J.; Del Forno, M.; Dunleavy, A. Antibiotics for exacerbations of asthma. Cochrane Database Syst. Rev. 2018, 6, CD002741. [Google Scholar] [CrossRef]
- Iikura, M.; Hojo, M.; Koketsu, R.; Watanabe, S.; Sato, A.; Chino, H.; Ro, S.; Masaki, H.; Hirashima, J.; Ishii, S.; et al. The importance of bacterial and viral infections associated with adult asthma exacerbations in clinical practice. PLoS ONE 2015, 10, e0123584. [Google Scholar] [CrossRef] [PubMed]
- Majellano, E.C.; Clark, V.L.; Winter, N.A.; Gibson, P.G.; McDonald, V.M. Approaches to the assessment of severe asthma: Barriers and strategies. J. Asthma Allergy 2019, 12, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Narumoto, O. Adult bronchial asthma, definition, cause, and severity assessment. Nihon Rinsho. Jpn. J. Clin. Med. 2016, 74, 1617–1621. [Google Scholar]
- Cheong, A.T.; Lee, P.Y.; Shariff-Ghazali, S.; Salim, H.; Hussein, N.; Ramli, R.; Pinnock, H.; Liew, S.M.; Hanafi, N.S.; Abu Bakar, A.I.; et al. Implementing asthma management guidelines in public primary care clinics in Malaysia. NPJ Prim. Care Respir. Med. 2021, 31, 47. [Google Scholar] [CrossRef] [PubMed]
- Saglani, S.; Fleming, L.; Sonnappa, S.; Bush, A. Advances in the etiology, management, and prevention of acute asthma attacks in children. Lancet Child. Adolesc. Health 2019, 3, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Kaza, V.; Bandi, V.; Guntupalli, K.K. Acute severe asthma: Recent advances. Curr. Opin. Pulm. Med. 2007, 13, 1–7. [Google Scholar] [CrossRef]
- Klingenberg, C.; Kornelisse, R.F.; Buonocore, G.; Maier, R.F.; Stocker, M. Culture-Negative Early-Onset Neonatal Sepsis—At the Crossroad between Efficient Sepsis Care and Antimicrobial Stewardship. Front. Pediatr. 2018, 6, 285. [Google Scholar] [CrossRef] [PubMed]
- Lever, A.; Mackenzie, I. Sepsis: Definition, epidemiology, and diagnosis. BMJ 2007, 335, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Peters, M.J.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schlapbach, L.J.; Tasker, R.C.; Argent, A.C.; et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020, 46 (Suppl. S1), 10–67. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Lubell, Y.; Blacksell, S.D.; Dunachie, S.; Tanganuchitcharnchai, A.; Althaus, T.; Watthanaworawit, W.; Paris, D.H.; Mayxay, M.; Peto, T.J.; Dondorp, A.M.; et al. Performance of C-reactive protein and procalcitonin to distinguish viral from bacterial and malarial causes of fever in Southeast Asia. BMC Infect. Dis. 2015, 15, 511. [Google Scholar] [CrossRef]
- Lamrous, A.; Repetto, E.; Depp, T.; Jimenez, C.; Chua, A.C.; Kanapathipillai, R.; Jensen, T.O. C-reactive protein and procalcitonin use in adults in low- and middle-income countries: A narrative review. JAC Antimicrob. Resist. 2023, 5, dlad057. [Google Scholar] [CrossRef]
- Lassere, M.N.; Johnson, K.R.; Boers, M.; Tugwell, P.; Brooks, P.; Simon, L.; Strand, V.; Conaghan, P.G.; Ostergaard, M.; Maksymowych, W.P.; et al. Definitions and validation criteria for biomarkers and surrogate endpoints: Development and testing of a quantitative hierarchical levels of evidence schema. J. Rheumatol. 2007, 34, 607–615. [Google Scholar]
- Aabenhus, R.; Jensen, J.U.; Jørgensen, K.J.; Hróbjartsson, A.; Bjerrum, L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst. Rev. 2014, 11, CD010130. [Google Scholar] [CrossRef]
- Pijnenburg, M.W. The Role of FeNO in Predicting Asthma. Front. Pediatr. 2019, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S. Severe asthma: From characteristics to phenotypes to endotypes. Clin. Exp. Allergy 2012, 42, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Huang, G.T.; Zhan, Q.M.; Chen, J.L.; Luo, W.T.; Wu, L.H.; Wu, L.Y.; Wu, L.Y.; Lu, Z.N.; Sun, Y.F. The neutrophil to lymphocyte ratio as a novel predictor of asthma and its exacerbation: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11719–11728. [Google Scholar] [CrossRef]
- Pan, R.; Ren, Y.; Li, Q.; Zhu, X.; Zhang, J.; Cui, Y.; Yin, H. Neutrophil-lymphocyte ratios in blood to distinguish children with asthma exacerbation from healthy subjects. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320221149849. [Google Scholar] [CrossRef]
- Arwas, N.; Shvartzman, S.U.; Goldbart, A.; Bari, R.; Hazan, I.; Horev, A.; Golan Tripto, I. Elevated Neutrophil-to-Lymphocyte Ratio Is Associated with Severe Asthma Exacerbation in Children. J. Clin. Med. 2023, 12, 3312. [Google Scholar] [CrossRef]
- Landry, V.; Coburn, P.; Kost, K.; Liu, X.; Li-Jessen, N.Y.K. Diagnostic Accuracy of Liquid Biomarkers in Airway Diseases: Toward Point-of-Care Applications. Front. Med. 2022, 9, 855250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, M.; Wang, Y.; Su, X.; Lei, T.; Yu, H.; Liu, J. Diagnostic value of fractional exhaled nitric oxide in differentiating the asthma-COPD overlap from COPD: A systematic review and meta-analysis. Expert. Rev. Respir. Med. 2022, 16, 679–687. [Google Scholar] [CrossRef]
- Grover, H.L.; Higgins, B.G. GPs have a key role in improving outcomes in acute asthma. Practitioner 2016, 260, 15–19. [Google Scholar]
- Zelicof Paul, A.; Rutherford, K.A.; Abuso, S.M. Emergency department management of pediatric acute asthma: An evidence-based review. Pediatr. Emerg. Med. Pract. 2023, 20, 1–28. [Google Scholar] [PubMed]
- Aniapravan, R.; Pullattayil, A.; Al Ansari, K.; Powell, C.V.E. Question 5: Magnesium Sulphate for Acute Asthma in children. Paediatr. Respir. Rev. 2020, 36, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.; Sweeney, J.; Rumantir, M.; Coates, A.L.; Willan, A.R.; Stephens, D.; Atenafu, E.G.; Finkelstein, Y.; Thompson, G.; Zemek, R.; et al. Effect of Nebulized Magnesium vs Placebo Added to Albuterol on Hospitalization Among Children with Refractory Acute Asthma Treated in the Emergency Department: A Randomized Clinical Trial. JAMA 2020, 324, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Kwofie, K.; Wolfson, A.B. Intravenous Magnesium Sulfate for Acute Asthma Exacerbation in Children and Adults. Am. Fam. Physician 2021, 103, 245–246. [Google Scholar] [PubMed]
- Chavasse, R.; Scott, S. The Differences in Acute Management of Asthma in Adults and Children. Front. Pediatr. 2019, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Alangari, A.A. Corticosteroids in the treatment of acute asthma. Ann. Thorac. Med. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Kearns, N.; Maijers, I.; Harper, J.; Beasley, R.; Weatherall, M. Inhaled Corticosteroids in Acute Asthma: A Systemic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2020, 8, 605–617.e6. [Google Scholar] [CrossRef]
- Vázquez, Y.; González, L.; Noguera, L.; González, P.A.; Riedel, C.A.; Bertrand, P.; Bueno, S.M. Cytokines in the Respiratory Airway as Biomarkers of Severity and Prognosis for Respiratory Syncytial Virus Infection: An Update. Front. Immunol. 2019, 10, 1154. [Google Scholar] [CrossRef]
- Rubin, B.K.; Pohanka, V. Beyond the guidelines: Fatal and near-fatal asthma. Paediatr. Respir. Rev. 2012, 13, 106–111. [Google Scholar] [CrossRef]
- Pardue Jones, B.; Fleming, G.M.; Otillio, J.K.; Asokan, I.; Arnold, D.H. Pediatric acute asthma exacerbations: Evaluation and management from emergency department to intensive care unit. J. Asthma 2016, 53, 607–617. [Google Scholar] [CrossRef]
- Uffen, J.W.; Oosterheert, J.J.; Schweitzer, V.A.; Thursky, K.; Kaasjager, H.A.H.; Ekkelenkamp, M.B. Interventions for rapid recognition and treatment of sepsis in the emergency department: A narrative review. Clin. Microbiol. Infect. 2021, 27, 192–203. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensiv. Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Zawistowski, C.A. The management of sepsis. Curr. Probl. Pediatr. Adolesc. Health Care 2013, 43, 285–291. [Google Scholar] [CrossRef]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Tan, J.C.; Harvey, S.E.; Bell, D.; et al. Protocolised Management In Sepsis (ProMISe): A multicentre randomised controlled trial of the clinical effectiveness and cost-effectiveness of early, goal-directed, protocolised resuscitation for emerging septic shock. Health Technol. Assess. 2015, 19, 1–150. [Google Scholar] [CrossRef] [PubMed]
- Mangioni, D.; Viaggi, B.; Giani, T.; Arena, F.; D’Arienzo, S.; Forni, S.; Tulli, G.; Rossolini, G.M. Diagnostic stewardship for sepsis: The need for risk stratification to triage patients for fast microbiology workflows. Future Microbiol. 2019, 14, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Haktanir Abul, M.; Phipatanakul, W. Severe asthma in children: Evaluation and management. Allergol. Int. 2019, 68, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.C.; Levy, M.L.; Moreiras, J.; Fleming, L. Managing problematic severe asthma: Beyond the guidelines. Arch. Dis. Child. 2018, 103, 392–397. [Google Scholar] [CrossRef]
- Castagnoli, R.; Marseglia, A.; Brambilla, I.; Marseglia, G.L.; Licari, A. Severe uncontrolled asthma in children: Practical approach on diagnosis and management. Minerva Pediatr. 2020, 72, 196–205. [Google Scholar] [CrossRef]
- Heffler, E.; Blasi, F.; Latorre, M.; Menzella, F.; Paggiaro, P.; Pelaia, G.; Senna, G.; Canonica, G.W.; SANI Network. The Severe Asthma Network in Italy: Findings and Perspectives. J. Allergy Clin. Immunol. Pract. 2019, 7, 1462–1468. [Google Scholar] [CrossRef]
- Agnihotri, N.T.; Saltoun, C. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2019, 40, 406–409. [Google Scholar] [CrossRef]
- Lofrese, J.J.; Tupper, C.; Denault, D.; Lappin, S.L. Physiology, Residual Volume. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Haddad, M.; Sharma, S. Physiology, Lung. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hammer, J. Acute respiratory failure in children. Paediatr. Respir. Rev. 2013, 14, 64–69. [Google Scholar] [CrossRef]
- Schneider, J.; Sweberg, T. Acute respiratory failure. Crit. Care Clin. 2013, 29, 167–183. [Google Scholar] [CrossRef]
- Trachsel, D.; Erb, T.O.; Hammer, J.; von Ungern-Sternberg, B.S. Developmental respiratory physiology. Paediatr. Anaesth. 2022, 32, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Grasso, S.; Karbing, D.S.; Fogagnolo, A.; Contoli, M.; Bollini, G.; Ragazzi, R.; Cinnella, G.; Verri, M.; Cavallesco, N.G.; et al. Physiologic Evaluation of Ventilation Perfusion Mismatch and Respiratory Mechanics at Different Positive End-expiratory Pressure in Patients Undergoing Protective One-lung Ventilation. Anesthesiology 2018, 128, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Powers, K.A.; Dhamoon, A.S. Physiology, Pulmonary Ventilation, and Perfusion. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rubin, B.K.; Priftis, K.N.; Schmidt, H.J.; Henke, M.O. Secretory hyperresponsiveness and pulmonary mucus hypersecretion. Chest 2014, 146, 496–507. [Google Scholar] [CrossRef]
- Evans, C.M.; Kim, K.; Tuvim, M.J.; Dickey, B.F. Mucus hypersecretion in asthma: Causes and effects. Curr. Opin. Pulm. Med. 2009, 15, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Papiris, S.A.; Manali, E.D.; Kolilekas, L.; Triantafillidou, C.; Tsangaris, I. Acute severe asthma: New approaches to assessment and treatment. Drugs 2009, 69, 2363–2391. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.E.; Leung, A.K.C. Medications and Recent Patents for Status Asthmaticus in Children. Recent. Pat. Inflamm. Allergy Drug Discov. 2017, 11, 12–21. [Google Scholar] [CrossRef]
- Lammers, S.; Scott, D.; Hunter, K.; Tan, W.; Shandas, R.; Stenmark, K.R. Mechanics and Function of the Pulmonary Vasculature: Implications for Pulmonary Vascular Disease and Right Ventricular Function. Compr. Physiol. 2012, 2, 295–319. [Google Scholar] [CrossRef]
- Dhand, R. Ventilator graphics and respiratory mechanics in the patient with obstructive lung disease. Respir. Care 2005, 50, 246–261. [Google Scholar]
- Hamzaoui, O.; Monnet, X.; Teboul, J.L. Pulsus paradoxus. Eur. Respir. J. 2013, 42, 1696–1705. [Google Scholar] [CrossRef]
- Sarkar, M.; Bhardwaz, R.; Madabhavi, I.; Modi, M. Physical signs in patients with chronic obstructive pulmonary disease. Lung India 2019, 36, 38–47. [Google Scholar] [CrossRef]
- Giacomelli, R.; Afeltra, A.; Alunno, A.; Bartoloni-Bocci, E.; Berardicurti, O.; Bombardieri, M.; Bortoluzzi, A.; Caporali, R.; Caso, F.; Cervera, R.; et al. Guidelines for biomarkers in autoimmune rheumatic diseases—Evidence-based analysis. Autoimmun. Rev. 2019, 18, 93–106. [Google Scholar] [CrossRef]
- Burke, H.B. Predicting Clinical Outcomes Using Molecular Biomarkers. Biomark. Cancer 2016, 8, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.D.; McCabe, S.; White, N.; Clancy, R.L. Biomarkers: An important clinical assessment tool. Am. J. Nurs. 2012, 112, 52–58. [Google Scholar] [CrossRef] [PubMed]
- di Palmo, E.; Cantarelli, E.; Catelli, A.; Ricci, G.; Gallucci, M.; Miniaci, A.; Pession, A. The Predictive Role of Biomarkers and Genetics in Childhood Asthma Exacerbations. Int. J. Mol. Sci. 2021, 22, 4651. [Google Scholar] [CrossRef]
- Wu, A.C.; Kiley, J.P.; Noel, P.J.; Amur, S.; Burchard, E.G.; Clancy, J.P.; Galanter, J.; Inada, M.; Jones, T.K.; Kropski, J.A.; et al. Current Status and Future Opportunities in Lung Precision Medicine Research with a Focus on Biomarkers. An American Thoracic Society/National Heart, Lung, and Blood Institute Research Statement. Am. J. Respir. Crit. Care Med. 2018, 198, e116–e136. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Baines, K.J.; Pavord, I.D.; Gibson, P.G. The role of biomarkers in the management of airways disease. Int. J. Tuberc. Lung Dis. 2014, 18, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.F.; Ko, F.W.; Wong, G.W. Recent advances in asthma biomarker research. Ther. Adv. Respir. Dis. 2013, 7, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Koczulla, A.R.; Vogelmeier, C.F.; Garn, H.; Renz, H. New concepts in asthma: Clinical phenotypes and pathophysiological mechanisms. Drug Discov. Today 2017, 22, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Poon, A.H.; Hamid, Q. Asthma phenotypes and endotypes. Curr. Opin. Pulm. Med. 2013, 19, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, K.; Van Den Heuvel, R.; Govarts, E.; Hooyberghs, J.; Nelen, V.; Witters, E.; Desager, K.; Schoeters, G. A new approach to study exhaled proteins as potential biomarkers for asthma. Clin. Exp. Allergy 2011, 41, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Bartminski, G.; Crossley, M.; Turcanu, V. Novel biomarkers for asthma stratification and personalized therapy. Expert. Rev. Mol. Diagn. 2015, 15, 415–430. [Google Scholar] [CrossRef]
- Richards, L.B.; Neerincx, A.H.; van Bragt, J.J.M.H.; Sterk, P.J.; Bel, E.H.D.; Maitland-van der Zee, A.H. Biomarkers and asthma management: Analysis and potential applications. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 96–108. [Google Scholar] [CrossRef]
- Arnold, R.J.; Massanari, M.; Lee, T.A.; Brooks, E. A Review of the Utility and Cost Effectiveness of Monitoring Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management. Manag. Care 2018, 27, 34–41. [Google Scholar]
- Rao, D.R.; Phipatanakul, W. An Overview of Fractional Exhaled Nitric Oxide and Children with Asthma. Expert. Rev. Clin. Immunol. 2016, 12, 521–530. [Google Scholar] [CrossRef]
- Zhou, A.; Zhou, Z.; Deng, D.; Zhao, Y.; Duan, J.; Cheng, W.; Liu, C.; Chen, P. The Value of FENO Measurement for Predicting Treatment Response in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct Pulmon Dis. 2020, 15, 2257–2266. [Google Scholar] [CrossRef]
- Licari, A.; Manti, S.; Castagnoli, R.; Leonardi, S.; Marseglia, G.L. Measuring inflammation in pediatric severe asthma: Biomarkers in clinical practice. Breathe 2020, 16, 190301. [Google Scholar] [CrossRef]
- Guida, G.; Bagnasco, D.; Carriero, V.; Bertolini, F.; Ricciardolo, F.L.M.; Nicola, S.; Brussino, L.; Nappi, E.; Paoletti, G.; Canonica, G.W.; et al. Critical evaluation of asthma biomarkers in clinical practice. Front. Med. 2022, 9, 969243. [Google Scholar] [CrossRef]
- Kim, H.; Ellis, A.K.; Fischer, D.; Noseworthy, M.; Olivenstein, R.; Chapman, K.R.; Lee, J. Asthma biomarkers in the age of biologics. Allergy Asthma Clin. Immunol. 2017, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qiu, R.; Yang, Z.; Li, J.; Chung, K.F.; Zhong, N.; Zhang, Q. Sputum microbiota in severe asthma patients: Relationship to eosinophilic inflammation. Respir. Med. 2017, 131, 192–198. [Google Scholar] [CrossRef]
- Li, W.; Gao, P.; Zhi, Y.; Xu, W.; Wu, Y.; Yin, J.; Zhang, J. Periostin: Its role in asthma and its potential as a diagnostic or therapeutic target. Respir. Res. 2015, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Refaat, M.M.; El Sayed, E.; Abd El-Fattah, W.; Elbanna, A.H.; Sayed, H.M.E. Relationship between sputum periostin level and inflammatory asthma phenotypes in Egyptian patients. J. Asthma 2021, 58, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Bobolea, I.; Barranco, P.; Del Pozo, V.; Romero, D.; Sanz, V.; López-Carrasco, V.; Canabal, J.; Villasante, C.; Quirce, S. Sputum periostin in patients with different severe asthma phenotypes. Allergy 2015, 70, 540–546. [Google Scholar] [CrossRef]
- Bruijnzeel, P.L.; Uddin, M.; Koenderman, L. Targeting neutrophilic inflammation in severe neutrophilic asthma: Can we target the disease-relevant neutrophil phenotype? J. Leukoc. Biol. 2015, 98, 549–556. [Google Scholar] [CrossRef]
- Kostakou, E.; Kaniaris, E.; Filiou, E.; Vasileiadis, I.; Katsaounou, P.; Tzortzaki, E.; Koulouris, N.; Koutsoukou, A.; Rovina, N. Acute Severe Asthma in Adolescent and Adult Patients: Current Perspectives on Assessment and Management. J. Clin. Med. 2019, 8, 1283. [Google Scholar] [CrossRef]
- Kostikas, K.; Brindicci, C.; Patalano, F. Blood Eosinophils as Biomarkers to Drive Treatment Choices in Asthma and COPD. Curr. Drug Targets 2018, 19, 1882–1896. [Google Scholar] [CrossRef]
- Mogensen, I.; James, A.; Malinovschi, A. Systemic and breath biomarkers for asthma: An update. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 71–79. [Google Scholar] [CrossRef]
- Lv, M.Y.; Qiang, L.X.; Li, Z.H.; Jin, S.D. The lower the eosinophils, the stronger the inflammatory response? The relationship of different levels of eosinophils with the degree of inflammation in acute exacerbation chronic obstructive pulmonary disease (AECOPD). J. Thorac. Dis. 2021, 13, 232–243. [Google Scholar] [CrossRef]
- Naumova, V.; Beltyukov, E.; Niespodziana, K.; Errhalt, P.; Valenta, R.; Karaulov, A.; Kiseleva, D. Cumulative IgE-levels specific for respiratory allergens as biomarker to predict efficacy of anti-IgE-based treatment of severe asthma. Front. Immunol. 2022, 13, 941492. [Google Scholar] [CrossRef]
- Pelham, C.J.; Agrawal, D.K. Emerging roles for triggering receptor expressed on myeloid cells receptor family signaling in inflammatory diseases. Expert. Rev. Clin. Immunol. 2014, 10, 243–256. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, H.; Fu, X.; Han, L.; Zhang, H.; Zhang, L.; Zhao, J.; Xiao, D.; Li, H.; Li, P. Autophagy-driven neutrophil extracellular traps: The dawn of sepsis. Pathol. Res. Pract. 2022, 234, 153896. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.F.; Cao, K.; Jiang, J.P.; Guan, W.X.; Du, J.F. Neutrophil dysregulation during sepsis: An overview and update. J. Cell Mol. Med. 2017, 21, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Zhou, M.; Chen, G.B.; Du, J.; Wang, Y.; Ye, C. The value of lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio in differentiating pneumonia from upper respiratory tract infection (URTI) in children: A cross-sectional study. BMC Pediatr. 2021, 21, 545. [Google Scholar] [CrossRef]
- Tsang, M.S.; Hou, T.; Chan, B.C.; Wong, C.K. Immunological Roles of NLR in Allergic Diseases and Its Underlying Mechanisms. Int. J. Mol. Sci. 2021, 22, 1507. [Google Scholar] [CrossRef]
- Zuo, H.; Xie, X.; Peng, J.; Wang, L.; Zhu, R. Predictive Value of Novel Inflammation-Based Biomarkers for Pulmonary Hypertension in the Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Anal. Cell. Pathol. 2019, 2019, 5189165. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Teng, S.; Li, K. Prediction of sepsis mortality using metabolite biomarkers in the blood: A meta-analysis of death-related pathways and prospective validation. BMC Med. 2020, 18, 83. [Google Scholar] [CrossRef]
- Pregernig, A.; Müller, M.; Held, U.; Beck-Schimmer, B. Prediction of mortality in adult patients with sepsis using six biomarkers: A systematic review and meta-analysis. Ann. Intensive Care 2019, 9, 125. [Google Scholar] [CrossRef]
- Vincent, J.L. The Clinical Challenge of Sepsis Identification and Monitoring. PLoS Med. 2016, 13, e1002022. [Google Scholar] [CrossRef] [PubMed]
- van Engelen, T.S.R.; Wiersinga, W.J.; Scicluna, B.P.; van der Poll, T. Biomarkers in Sepsis. Crit. Care Clin. 2018, 34, 139–152. [Google Scholar] [CrossRef]
- Li, H.X.; Liu, Z.M.; Zhao, S.J.; Zhang, D.; Wang, S.J.; Wang, Y.S. Measuring both procalcitonin and C-reactive protein for a diagnosis of sepsis in critically ill patients. J. Int. Med. Res. 2014, 42, 1050–1059. [Google Scholar] [CrossRef]
- Tan, M.; Lu, Y.; Jiang, H.; Zhang, L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J. Cell Biochem. 2019, 120, 5852–5859. [Google Scholar] [CrossRef] [PubMed]
- Hamade, B.; Huang, D.T. Procalcitonin: Where Are We Now? Crit. Care Clin. 2020, 36, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Naot, D.; Musson, D.S.; Cornish, J. The Activity of Peptides of the Calcitonin Family in Bone. Physiol. Rev. 2019, 99, 781–805. [Google Scholar] [CrossRef]
- Xie, J.; Guo, J.; Kanwal, Z.; Wu, M.; Lv, X.; Ibrahim, N.A.; Li, P.; Buabeid, M.A.; Arafa, E.A.; Sun, Q. Calcitonin and Bone Physiology: In Vitro, In Vivo, and Clinical Investigations. Int. J. Endocrinol. 2020, 2020, 3236828. [Google Scholar] [CrossRef]
- Angeletti, S.; Spoto, S.; Fogolari, M.; Cortigiani, M.; Fioravanti, M.; De Florio, L.; Curcio, B.; Cavalieri, D.; Costantino, S.; Dicuonzo, G. Diagnostic and prognostic role of procalcitonin (PCT) and MR-pro-Adrenomedullin (MR-proADM) in bacterial infections. APMIS 2015, 123, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Kip, M.M.; Kusters, R.; IJzerman, M.J.; Steuten, L.M. A PCT algorithm for discontinuation of antibiotic therapy is a cost-effective way to reduce antibiotic exposure in adult intensive care patients with sepsis. J. Med. Econ. 2015, 18, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Mustafić, S.; Brkić, S.; Prnjavorac, B.; Sinanović, A.; Porobić Jahić, H.; Salkić, S. Diagnostic and prognostic value of procalcitonin in patients with sepsis. Med. Glas. 2018, 15, 93–100. [Google Scholar] [CrossRef]
- Ozger, H.S.; Senol, E. Use of infection biomarkers in the emergency department. Turk. J. Emerg. Med. 2022, 22, 169–176. [Google Scholar] [CrossRef]
- Hausfater, P. Biomarkers and infection in the emergency unit. Med. Mal. Infect. 2014, 44, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.; Bigham, M.T.; Giuliano, J.S., Jr. Use of procalcitonin for the prediction and treatment of acute bacterial infection in children. Curr. Opin. Pediatr. 2014, 26, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Long, W.; Yan, L.; Zhang, Y.; Xie, J.; Lu, G.; Yang, C. Procalcitonin guided antibiotic therapy of acute exacerbations of asthma: A randomized controlled trial. BMC Infect. Dis. 2013, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.T.; Sun, L.C.; Jia, H.B.; Gao, W.; Yang, J.P.; Zhang, G.Q. Procalcitonin levels in bloodstream infections caused by different sources and species of bacteria. Am. J. Emerg. Med. 2017, 35, 579–583. [Google Scholar] [CrossRef]
- Carbonell, R.; Moreno, G.; Martín-Loeches, I.; Gomez-Bertomeu, F.; Sarvisé, C.; Gómez, J.; Bodí, M.; Díaz, E.; Papiol, E.; Trefler, S.; et al. Prognostic Value of Procalcitonin and C-Reactive Protein in 1608 Critically Ill Patients with Severe Influenza Pneumonia. Antibiotics 2021, 10, 350. [Google Scholar] [CrossRef]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Pal, M.; Febbraio, M.A.; Whitham, M. From cytokine to myokine: The emerging role of interleukin-6 in metabolic regulation. Immunol. Cell Biol. 2014, 92, 331–339. [Google Scholar] [CrossRef]
- von Witting, E.; Lindbo, S.; Lundqvist, M.; Möller, M.; Wisniewski, A.; Kanje, S.; Rockberg, J.; Tegel, H.; Åstrand, M.; Uhlén, M.; et al. Small Bispecific Affinity Proteins for Simultaneous Target Binding and Albumin-Associated Half-Life Extension. Mol. Pharm. 2021, 18, 328–337. [Google Scholar] [CrossRef]
- Wildes, D.M.; Chisale, M.; Drew, R.J.; Harrington, P.; Watson, C.J.; Ledwidge, M.T.; Gallagher, J. A Systematic Review of Clinical Prediction Rules to Predict Hospitalisation in Children with Lower Respiratory Infection in Primary Care and their Validation in a New Cohort. EClinicalMedicine 2021, 41, 101164. [Google Scholar] [CrossRef]
- Williams, S.J.; Halls, A.V.; Tonkin-Crine, S.; Moore, M.V.; Latter, S.E.; Little, P.; Eyles, C.; Postle, K.; Leydon, G.M. General practitioner and nurse prescriber experiences of prescribing antibiotics for respiratory tract infections in UK primary care out-of-hours services (the UNITE study). J. Antimicrob. Chemother. 2018, 73, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.S.; Shieh, M.S.; Spitzer, K.A.; Pekow, P.S.; Krishnan, J.A.; Au, D.H.; Lindenauer, P.K. Association of Antibiotic Treatment with Outcomes in Patients Hospitalized for an Asthma Exacerbation Treated with Systemic Corticosteroids. JAMA Intern. Med. 2019, 179, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Koatz, A.M.; Coe, N.A.; Cicerán, A.; Alter, A.J. Clinical and Immunological Benefits of OM-85 Bacterial Lysate in Patients with Allergic Rhinitis, Asthma, and COPD and Recurrent Respiratory Infections. Lung 2016, 194, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.X.; Zhang, J.R.; Cao, Z.G.; Li, Y.; Wang, R.T. A decreased mean platelet volume is associated with stable and exacerbated asthma. Respiration 2014, 88, 31–37. [Google Scholar] [CrossRef]
- Couto, R.C.; Barbosa, J.A.; Pedrosa, T.M.; Biscione, F.M. C-reactive protein-guided approach may shorten length of antimicrobial treatment of culture-proven late-onset sepsis: An intervention study. Braz. J. Infect. Dis. 2007, 11, 240–245. [Google Scholar] [CrossRef]
- Krishack, P.A.; Louviere, T.J.; Decker, T.S.; Kuzel, T.G.; Greenberg, J.A.; Camacho, D.F.; Hrusch, C.L.; Sperling, A.I.; Verhoef, P.A. Protection against Staphylococcus aureus bacteremia-induced mortality depends on ILC2s and eosinophils. JCI Insight 2019, 4, e124168. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.; Wang, F.; Liang, J.; Chen, Q.; Lin, Z. Association between comorbid asthma and prognosis of critically ill patients with severe sepsis: A cohort study. Sci. Rep. 2021, 11, 15395. [Google Scholar] [CrossRef]
- Karampela, I.; Vallianou, N.G.; Tsilingiris, D.; Christodoulatos, G.S.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Armaganidis, A.; Dalamaga, M. Diagnostic and Prognostic Value of Serum Omentin-1 in Sepsis: A Prospective Study in Critically Ill Patients. Medicina 2023, 59, 833. [Google Scholar] [CrossRef]
- Suzuki, Y.; Saito, J.; Munakata, M.; Shibata, Y. Hydrogen sulfide as a novel biomarker of asthma and chronic obstructive pulmonary disease. Allergol. Int. 2021, 70, 181–189. [Google Scholar] [CrossRef]
- Li, H.; Hou, X.; Ding, Y.; Nie, L.; Zhou, H.; Nie, Z.; Tang, Y.; Chen, L.; Zheng, Y. Effects of H2S on the central regulation of respiration in adult rats. Neuroreport 2014, 25, 358–366. [Google Scholar] [CrossRef]
- Bazhanov, N.; Ansar, M.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Hydrogen Sulfide: A Novel Player in Airway Development, Pathophysiology of Respiratory Diseases, and Antiviral Defenses. Am. J. Respir. Cell Mol. Biol. 2017, 57, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Liu, S.J.; Tang, X.L.; Duan, G.L.; Ni, X.; Zhu, X.Y.; Liu, Y.J.; Wang, C.N. H2S Attenuates LPS-Induced Acute Lung Injury by Reducing Oxidative/Nitrative Stress and Inflammation. Cell Physiol. Biochem. 2016, 40, 1603–1612. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, G.; Wondimu, T.; Ross, B.; Wang, R. Hydrogen sulfide and asthma. Exp. Physiol. 2011, 96, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jaswani, P.; Sharma, R.K.; Agrawal, S.; Prasad, N.; Sahu, C.; Gupta, A.; Prasad, K.N. Procalcitonin as a diagnostic biomarker of sepsis: A tertiary care centre experience. J. Infect. Public Health 2019, 12, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Yu, F. Value of CRP, PCT, and NLR in Prediction of Severity and Prognosis of Patients with Bloodstream Infections and Sepsis. Front. Surg. 2022, 9, 857218. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wei, B.; Zhang, X.; Hu, L.; Ye, X. Value of Neutrophil:Lymphocyte Ratio Combined with Sequential Organ Failure Assessment Score in Assessing the Prognosis of Sepsis Patients. Int. J. Gen. Med. 2022, 15, 1901–1908. [Google Scholar] [CrossRef]
- Drăgoescu, A.N.; Pădureanu, V.; Stănculescu, A.D.; Chiuțu, L.C.; Tomescu, P.; Geormăneanu, C.; Pădureanu, R.; Iovănescu, V.F.; Ungureanu, B.S.; Pănuș, A.; et al. Neutrophil to Lymphocyte Ratio (NLR)—A Useful Tool for the Prognosis of Sepsis in the ICU. Biomedicines 2021, 10, 75. [Google Scholar] [CrossRef]
- Rehman, F.U.; Khan, A.; Aziz, A.; Iqbal, M.; Mahmood, S.B.Z.; Ali, N. Neutrophils to Lymphocyte Ratio: Earliest and Efficacious Markers of Sepsis. Cureus 2020, 12, e10851. [Google Scholar] [CrossRef]
- Omran, A.; Maaroof, A.; Mohammad, M.H.S.; Abdelwahab, A. Salivary C-reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. J. Pediatr. 2018, 94, 82–87. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef]
- Imtiaz, F.; Shafique, K.; Mirza, S.S.; Ayoob, Z.; Vart, P.; Rao, S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int. Arch. Med. 2012, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Lee, K.O.; Choi, J.W.; Kim, N.K.; Kim, O.J.; Kim, S.H.; Oh, S.H.; Kim, W.C. Blood Neutrophil/Lymphocyte Ratio Is Associated with Cerebral Large-Artery Atherosclerosis but not with Cerebral Small-Vessel Disease. Front. Neurol. 2020, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, L.; Li, Q.; Pan, R.; Zhang, J.; Cui, Y. Combined score of C-reactive protein level and neutrophil-to-lymphocyte ratio: A novel marker in distinguishing children with exacerbated asthma. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211040641. [Google Scholar] [CrossRef] [PubMed]
- Yufei, Y.; Mingli, L.; Xuejiao, L.; Xuemei, D.; Yiming, J.; Qin, Q.; Hui, S.; Jie, G. Utility of the neutrophil-to-lymphocyte ratio and C-reactive protein level for coronavirus disease 2019 (COVID-19). Scand. J. Clin. Lab. Investig. 2020, 80, 536–540. [Google Scholar] [CrossRef]
- Cag, Y.; Pacal, Y.; Gunduz, M.; Isik, S.; Kertmen, B.A.; Toprak, N.; Ozaydin, S.E.; Ozcetin, M.; Kut, A. The effect of peripheral blood eosinophilia on inflammatory markers in asthmatic patients with lower respiratory tract infections. J. Int. Med. Res. 2019, 47, 2452–2460. [Google Scholar] [CrossRef]
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R.; Global Initiative for Asthma (GINA) Program. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]


| Patient/Problem | Intervention | Comparison | Outcome |
|---|---|---|---|
| Severe Bronchial Asthma | Biomarkers determine the severity of disease and either include or exclude sepsis [29,30,31,32,33]. | Clinical identification, evaluation, and therapeutic intervention of severe acute asthma [34,35,36]. | Unnecessary use of antibiotics is avoided [37,38,39]. |
| Sepsis | The mortality rate of sepsis decreases when the commencement of focused therapy and therapeutic interventions is delayed. Biomarker assessment may improve discrimination of inflammation from sepsis [40,41,42]. | The early goal-directed therapy aims to give early antibiotics to those with infection due to bacteria. Compared biomarker sensitivity to routine care [43] | Rapid detection and implementation of relevant measures may be achieved with biomarkers. In the patient group with bronchial asthma and sepsis, biomarkers improve identification of sepsis [44]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha, S.; Kumar, S.; Narwaria, M.; Singh, A.; Haque, M. Severe Acute Bronchial Asthma with Sepsis: Determining the Status of Biomarkers in the Diagnosis of the Disease. Diagnostics 2023, 13, 2691. https://doi.org/10.3390/diagnostics13162691
Sinha S, Kumar S, Narwaria M, Singh A, Haque M. Severe Acute Bronchial Asthma with Sepsis: Determining the Status of Biomarkers in the Diagnosis of the Disease. Diagnostics. 2023; 13(16):2691. https://doi.org/10.3390/diagnostics13162691
Chicago/Turabian StyleSinha, Susmita, Santosh Kumar, Mahendra Narwaria, Arya Singh, and Mainul Haque. 2023. "Severe Acute Bronchial Asthma with Sepsis: Determining the Status of Biomarkers in the Diagnosis of the Disease" Diagnostics 13, no. 16: 2691. https://doi.org/10.3390/diagnostics13162691
APA StyleSinha, S., Kumar, S., Narwaria, M., Singh, A., & Haque, M. (2023). Severe Acute Bronchial Asthma with Sepsis: Determining the Status of Biomarkers in the Diagnosis of the Disease. Diagnostics, 13(16), 2691. https://doi.org/10.3390/diagnostics13162691






