Abstract
Bronchial asthma is a widely prevalent illness that substantially impacts an individual’s health standard worldwide and has a significant financial impact on society. Global guidelines for managing asthma do not recommend the routine use of antimicrobial agents because most episodes of the condition are linked to viral respiratory tract infections (RTI), and bacterial infection appears to have an insignificant impact. However, antibiotics are recommended when there is a high-grade fever, a consolidation on the chest radiograph, and purulent sputum that contains polymorphs rather than eosinophils. Managing acute bronchial asthma with sepsis, specifically the choice of whether or not to initiate antimicrobial treatment, remains difficult since there are currently no practical clinical or radiological markers that allow for a simple distinction between viral and bacterial infections. Researchers found that serum procalcitonin (PCT) values can efficiently and safely minimize antibiotic usage in individuals with severe acute asthma. Again, the clinical manifestations of acute asthma and bacterial RTI are similar, as are frequently used test values, like C-reactive protein (CRP) and white blood cell (WBC) count, making it harder for doctors to differentiate between viral and bacterial infections in asthma patients. The role and scope of each biomarker have not been precisely defined yet, although they have all been established to aid healthcare professionals in their diagnostics and treatment strategies.
1. Introduction
More than 300 million people worldwide have asthma, which poses an alarming danger to public health. Bronchial asthma is a non-communicable disease that affects a person’s standard of living and psychological and physical wellness. The consequences of this illness can be severe and continue throughout the patient’s life, influencing caregivers, family members, societies, and the healthcare sector. A precise diagnosis of this condition is essential to track the health of those with severe asthma. The recently developed clinical biomarkers have been determined to be a practical tool for disease diagnosis in the clinical management of critical illness. Even though all biomarkers have been developed to assist healthcare providers in their diagnostic and therapeutic approaches, their specific roles and scope are still unclear. This review aimed to provide insight into the various biomarkers of severe acute asthma with sepsis and determine their prognostic implications.
The progression of an asthmatic episode in severe acute asthma typically occurs over days or weeks, though in a few individuals, it may happen over hours or even minutes. This condition is potentially fatal and is one of the “gateways” of access to asthmatic deaths [1]. The diagnosis of severe acute asthma must be made at the emergency room since it might rarely appear as an entirely novel problem in a patient unaware of asthma [2]. Therefore, morbidity and mortality are primarily caused by underestimating the severity of the exacerbation, delaying hospital referrals, and/or providing insufficient emergency care. Acute bronchial asthma is a paramount global healthcare concern regarding complications, death [3,4], and economic impact. Episodes of coughing (especially at night or in the earlier mornings), dyspnea, wheezing, or tightness in the chest that are linked to an extensive but varying airflow restriction inside the lung are referred to as severe episodes of asthma [5]. This condition is frequently recoverable either spontaneously or with medical treatment. In general care and emergencies, it is the source of a significant percentage of antibiotic prescriptions [6]. Until now, antibiotic treatment has not been advised for viral and bronchial infections. Antibiotics are recommended when there is a high-grade fever, a consolidation on the chest radiograph, and purulent sputum that contains polymorphs rather than eosinophils [7]. Bronchial asthma is caused by lower respiratory tract inflammation and bronchial smooth muscle spasms that are usually mediated by IgE.
Since there are currently no feasible clinical or radiological signs that can easily distinguish between viral and bacterial infections, managing acute bronchial asthma with sepsis is difficult. Again, the specific decision about whether or not to commence antimicrobial treatment remains challenging [8]. The role and scope of each biomarker have not been precisely defined yet, even though they have all been established to aid healthcare professionals in their diagnostics and treatment strategies [9,10,11]. Since viral infections are typically involved in asthma attacks brought on by a medical condition, antibiotics are not recommended as a standard treatment [12,13].
Various inflammatory reactions with clinical suspicion or confirmation of a microbial etiology are called sepsis [14,15]. Again, sepsis continues to be a significant cause of morbidity and death and a global concern in various therapeutic contexts, despite rising acknowledgment of its significance [16,17]. A simple bedside or fast laboratory evaluation with highly accurate traits that could distinguish a bacterial cause that requires antimicrobial medication from a nonbacterial cause would be crucial for advising treatment involving the start and stoppage of antimicrobial agents [18,19].
Cellular and organ functioning has been evaluated using biological markers, often known as “biomarkers,” along with the spectrum of wellness and disease [20]. The goal of biomarkers is to guide medical professionals in identifying the bacterial or viral cause of acute respiratory infection to minimize or at least reduce the requirement for antimicrobial medication [21].
As markers of airway or systemic inflammation, fractional exhaled nitric oxide (FeNO), blood eosinophil counts (EOS), and neutrophil-to-lymphocyte ratio (NLR) have been used to increase the precision of asthma diagnosis, direct asthma interventions, track the effectiveness of inhaled corticosteroids (ICS) therapy, evaluate eosinophilic airway inflammation, and determine the likelihood of acute exacerbation. FeNO [22], EOS [23], and NLR [24] are efficient, feasible, consistent, and non-invasive inflammatory biomarkers. Even so, elements like smoking and prescribed medicines impact all of them [25,26,27,28].
2. Objective of the Study
Various difficulties can be classified as impediments that prevent the accurate diagnosis of sepsis, including patients, medical personnel, and others. Additionally, sepsis is a primary cause of fatalities in intensive care units (ICUs), and it can be hard to foresee the progression of the disease in patients. In the clinical management of critical illness, the recently designed clinical biomarkers have been acknowledged as a feasible tool for diagnosing diseases. The goal of this review was to illuminate the distinct biomarkers of severe acute asthma with sepsis and figure out their prognostic relevance.
3. Materials and Methods
This article explains common biomarkers for evaluating and managing severe acute bronchial asthma with septicemia. Google Scholar, Science Direct, PubMed, and ResearchGate were among the online archives reviewed for the scientific literature search (Figure 1). The reference list of relevant works was reviewed to retrieve additional materials. Keywords included acute severe bronchial asthma, sepsis, septicemia, biomarkers, bronchial asthma prognosis, procalcitonin, C reactive protein, blood eosinophil in bronchial asthma, neutrophil to lymphocyte ratio, and hydrogen sulfide. Additionally, predominant keywords included: severe bronchial asthma and sepsis, described in Table 1, as PICO format. Keywords also include insulin resistance, polyunsaturated fatty acids (PUFA), omega-fatty acids, and omega-3 fatty acids. Papers written in languages other than English and released prior to 2000 were not included. The papers’ eligibility was thoroughly considered before they were included in the study. Duplicate publications were removed. After the recommended works of literature were independently evaluated and included, an additional discussion was organized to discuss any doubts, issues, inaccuracies, or prejudices relevant to the individual article.
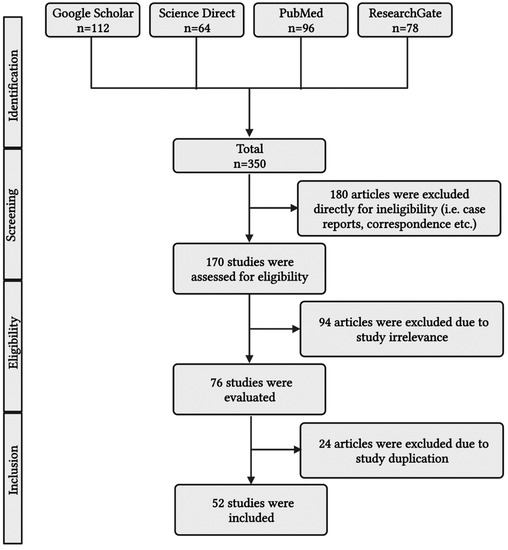
Figure 1.
A simplified PRISMA diagram showing the methodology. This figure has been drawn using the premium version of BioRender with the license number LK25NTV1VO. Image Credit: Susmita Sinha.

Table 1.
Depicted the Principal Keywords Severe Bronchial Asthma and Sepsis in the PICO format [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].
4. Diagnosis of Asthma
Incorrect diagnosis could lead to adverse effects from asthma treatments. Therefore, it is crucial to perform a thorough examination to determine whether the patient has severe asthma. The initial step in the diagnostic process is comprehensive history-taking and careful general physical examination [45]. Symptoms, how frequently they occur, and their seriousness must be pointed out in the patient’s medical record. It is also essential to assess the degree of exacerbations and related comorbidities and clarify the onset of symptoms. Diagnosis of severe asthma exacerbations is of the utmost importance since they are associated with adverse effects and involve regular surveillance and intensive management. The history of medications could point to insufficient care or poor adherence to a recommended course of therapy. Socioeconomic factors and the absence of a written asthma action plan are also linked to an increased risk for severe exacerbation [46,47].
5. Pathophysiology of Bronchial Asthma
Severe acute asthma requires the most excellent attention, monitoring, and management skill to keep a patient’s asthma from becoming uncontrolled with fatal clinical outcomes. Patients with harsh asthma experience notable troubles with everyday life, such as decreased activity levels, less efficiency at work, and isolation from society. In addition, patients with severe asthma must cope with a higher incidence of complications [48]. Increased resistance to airflow, decreased expiratory flow, the accumulation of air with each breath, and lung hyperinflation are all effects of small airway obstructions within the lungs and make the expiration process an active process [49,50]. Additional mechanical difficulties result from a flat diaphragm resulting from hyperinflation [51]. Forced vital capacity and expiratory volume are reduced while total lung volumes are raised. Total lung capacity (TLC) continues to rise in acute severe asthma exacerbations, aiding in keeping narrowed airways open. At the same time, in physiological conditions, the quiet expiration process occurs passively through the elastic recoil tendency of the lungs. The time required for the inspired tidal volume to expire fully will increase with declined elastic forces, and inspiration starts at a volume when the respiratory system displays a positive recoil pressure due to incomplete exhalation of the given tidal volume. Moreover, positive alveolar pressure at the end of expiration causes the flow, known as dynamic hyperinflation of the lungs [52,53].
In addition, increased intrapulmonary shunt, increased dead space, and mismatched ventilation-perfusion ratio (V/Q) are the causes of abnormal gas exchange [54,55]. Airway inflammation and smooth muscle constriction, which may sometimes be severe enough to cause a potentially fatal airway obstruction even without mucus plugging, are the primary contributors to decreased airflow [56,57]. Asthma-related inflammation includes airway edema, eosinophilic cellular infiltration, activated CD4+ T lymphocytes and mast cells, and intraluminal mucous plugs made of plasma proteins, mucin glycoproteins, epithelial and inflammatory cells, as well as cellular debris [58,59]. A severe asthma attack that results in considerable dynamic hyperinflation also has another crucial component called hemodynamic impairment. In people with severe asthma, dynamic hyperinflation can stretch the pulmonary vasculature, raising right ventricular afterload and vascular resistance [60,61]. By reducing the proper heart preload and increasing the right heart afterload, the development of positive intrathoracic pressures causes a decrease in the right heart outputs. A significant reduction in systolic arterial pressure during inspiration and the presence of the pulses paradoxes is acknowledged by the decreased right heart output in conjunction with the left heart’s diastolic dysfunction and its incomplete filling [62,63] (Figure 2).
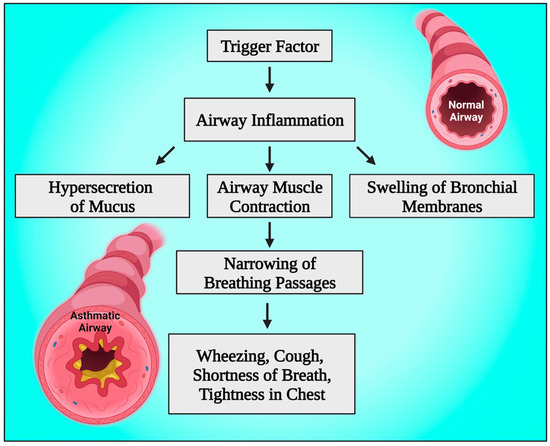
Figure 2.
Schematic diagram showing the pathophysiology of bronchial asthma. This figure has been drawn using the premium version of BioRender with the license number GD25JNBBY1. Image Credit: Susmita Sinha.
6. The Emerging Role of Biomarkers
Biomarkers are observable traits that can be quantitatively assessed to determine if a biological process is typical or pathological. There are four primary functions that biomarkers play in therapeutic settings: (1) diagnostic; (2) disease grading; (3) continual monitoring of the disease’s advancement; and (4) evaluation of the efficacy of treatment. In addition to serving as a clinical reference, the technique of biomarker analysis enables the identification of potential opportunities for innovative therapeutics and an in-depth knowledge of the primary molecular pathways that contribute to disease progression [64].
In every aspect of medical management, the value of biomarkers is increasing. Whether used to anticipate, identify, or track health conditions, biomarkers are helpful at every stage of the course of treatment [65] (Figure 3). Biomarkers can be assessed by examining blood, sputum, and urine samples [66,67]. All medical practitioners should understand biomarkers, their uses, and their potential consequences on patient outcomes when biomarker research is implemented into clinical practice [68]. The employment of biomarkers has evolved into an integral component of the standard of care for several illnesses because of considerable research and clinical evidence, which has increased the significance of biomarkers in detecting and managing many diseases [69]. Advancement in the implementation of biomarkers for disease classification, monitoring, and assessment should lead to more effective disease management and enhanced tailoring of therapy [70,71].
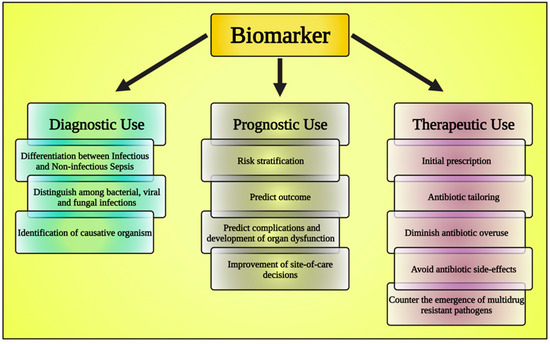
Figure 3.
The chart shows the classification of biomarkers. This figure has been drawn utilizing the premium version of BioRender with the license number DA25JLV4IV. Image Credit: Susmita Sinha.
6.1. Asthma Biomarkers
Asthma is a persistent breathing disorder caused by respiratory tract inflammation [72]. Regarding asthma, endotyping and phenotyping are intimately related to biomarkers. The purpose is to anticipate a response to a specific treatment using a variety of signals, either systemic, local, or clinical [73,74].
6.1.1. Fractional Exhaled Nitric Oxide (FeNO)
FeNO is the most frequently examined potential biomarker for asthma [75,76]. Nitric Oxide (NO) is produced in the lungs in response to inflammation by the enzyme Nitric Oxide synthase from the amino acid L-arginine [77,78]. Assessing FeNO is simple, rapid, and noninvasive. FeNO values are influenced by height, age, weight, race, gender, and exhaling flow rates [79,80].
6.1.2. Sputum Inflammatory Cell Analysis
The most reliable, precise, and non-invasive tool for determining airway inflammation is sputum inflammatory cell examination, which identifies the many inflammatory phenotypes of asthma [81,82]. Again, its dependability, sensitivity, and authenticity are established, and processing and evaluation are controlled [83].
Asthma characteristics have been reported to be related to higher sputum neutrophil levels. In individuals with chronic asthma, prolonged narrowing of the airways and a gradual decline in lung function have been linked to airway neutrophilia. It has also been related to increased bronchial responsiveness unrelated to elevated eosinophil count [84].
Patients with asthma had considerably greater sputum periostin levels than non-asthmatics. Periostin is a potential biomarker capable of identifying intensity and outcome and serving as a possible treatment focus [85]. Sputum periostin has an inverse relationship with forced expiratory volume in the first second (FEV1) and is highly associated with sputum TLC and age. It is linked to both the sputum eosinophil percentage and neutrophil count [86]. Again, periostin is a matricellular protein expressed by lung fibroblast and respiratory epithelium. In addition to this, periostin is a secondary product of type-2 immunological reactions’ distinguishing cytokines, interleukin-13 and interleukin-4 [87].
Th-1 and Th-17 actions are shifted due to neutrophil infiltration and engagement into the respiratory passages, initiating toll-like receptor (TLR) activity and triggering innate immunity. Again, this process produces elevated levels of neutrophil elastase, interleukin-8, matrix metalloproteinase 9, and interleukin-17. IL-8 causes neutrophils to release enzymes and other impacts on immunological monitoring and bacterial death. Neutrophil subgroups may exert diverse effects on immunological monitoring and bacterial death. In addition, bacteria, ozone, and viruses cause the release of cytokines and chemokines that ultimately encourage neutrophil migration [88,89].
6.1.3. Blood Eosinophil (B-EOS)
Eosinophils have been playing a more important part as a biomarker in determining the response to treatment in clinical practice for several years [90]. High B-EOS findings were observed to be a significant indicator of treatment response. Contradictory findings have been found regarding the proper B-EOS cut-off for estimating airway eosinophilia in severe asthma [91,92].
6.1.4. Total Serum IgE Level
In severe asthma caused by allergies, this biomarker guides anti-IgE antibody treatments. Blood eosinophil count (more than 260 per liter) and fractional exhaled nitric oxide (FeNO) values above 19.5 parts per billion strongly indicate whether individuals with severe allergic asthma are responsive to anti-IgE antibodies that will lower exacerbation incidence in asthma patients [22,93,94].
6.1.5. Soluble Form of the Triggering Receptor Expressed on Myeloid Cells-1 (sTREM-1)
The immunoglobulin superfamily member sTREM-1 is represented on the outer membranes of neutrophils, monocytes, and macrophages [95]. Its production is boosted by extracellular bacteria, which also causes the release of cytokines that promote inflammation. Thus, it amplifies the inflammatory response in contact with bacteria [95,96].
6.1.6. Neutrophil to Lymphocyte Ratio (NLR)
NLR could be utilized as a biomarker to differentiate and diagnose various forms of obstructive illnesses since neutrophils and NLR, as markers for circulatory immune complexes, can be significantly more prevalent among individuals with respiratory insufficiency than in the healthy population [97,98].
Healthcare research on early diagnostic markers in asthma patients is an area of interest. The study of readily available biomarkers for the diagnosis of asthma has drawn more attention in the past few years. As an indicator of persistent infection, the blood neutrophil-to-lymphocyte ratio (NLR) is a simple, easily accessible, and reasonably priced index obtained from complete blood counts. The NLR has been considered to be a potential indicator of episodes of inflammation in persistent illnesses and several other conditions in a number of recent studies. In addition, a rise in neutrophils is a result of cytokines in the pathophysiology of asthma. Additionally, patients with asthma exacerbations had greater blood NLR values than those with stable asthma [24,99].
6.2. Sepsis Biomarkers
The prognosis of sepsis remains detrimental regardless of the increased use of modern technologies for its management. Again, sepsis is one of the most common causes of death across the world, and its fatality rates are particularly significant because there is no accurate approach for predicting the course of the condition. Since sepsis has a mortality rate between 10% and 50%, managing this medical condition remains complicated. One of the main reasons for fatalities in the ICU is sepsis; hence, sepsis biomarker development and research are of utmost importance [100,101]. Since sepsis shares many clinical signs with other disorders that develop in ICU patients, recognizing the condition can be challenging for medical professionals [102,103]. The two most often utilized indicators for sepsis and other bacterial illnesses are C-reactive protein (CRP) and procalcitonin (PCT) [104,105].
6.2.1. Procalcitonin
Procalcitonin (PCT) is a precursor of calcitonin, consisting of 116 amino acid peptides synthesized in healthy people’s thyroid and adipose tissue [106]. To maintain calcium homeostasis, it is cleaved to produce calcitonin, which is then stored and produced in a controlled manner [107,108]. PCT has a serum value of 0.1 ng/mL in a healthy individual [109]. In addition to this, it was found to be more prevalent in patients with systemic illnesses [110]. Other illnesses, like surgery and trauma, as well as systemic viral infections to a much lesser amount, have also been linked to increased focus. The highest levels of serum PCT are observed in multiorgan dysfunction brought on by trauma and bacterial infection [111,112].
During trauma and surgery, PCT synthesis is induced throughout all parenchymal tissues by the systemic inflammatory response, especially by inflammatory mediators such as tumor necrosis factor-alpha (TNF alpha) [113]. Moreover, in reaction to an illness or damage, procalcitonin rises within 4 h, peaks at 6 h with an 8–24 h plateau, and then falls back to normal within 2–3 days. In contrast, CRP has an onset of 12–24 h, a plateau of 20–72 h, and a return to baseline of 3–7 days or more [114,115].
6.2.2. Prognostic Role of Procalcitonin
Even though PCT is more expensive, researchers have discovered that it helps separate bacterial from noninfectious inflammation causes [116]. Randomized controlled research revealed that serum procalcitonin (PCT) values could efficiently and safely minimize antibiotic usage in individuals with severe acute asthma [117]. Patients with systemic bacterial infections have elevated amounts of PCT in their blood. In contrast, patients with viral infections or inflammatory disorders still have relatively low levels of PCT in their blood. PCT levels may help clinical decision-making regarding the start and end of antibiotic therapy [118,119].
6.2.3. C Reactive Protein
CRP was regarded as a generalized but sensitive indicator of the beginning of inflammation [120]. The liver primarily synthesizes CRP in response to the cytokine interleukin-6, which is secreted during infections and several inflammatory conditions [121]. By attaching to the polysaccharides on pathogens, it begins a complement activation. Although sepsis is under control, its extended half-life indicates that it stays positive for a long time [122].
It is difficult for clinicians in feasible healthcare environments to determine which asthma patients with bacterial respiratory tract infection (RTI) will be effectively treated with antimicrobial therapy [123,124]. Despite current clinical guideline warnings against empiric antibiotic administration in severe asthma exacerbations, patients frequently receive antibiotics [125]. The clinical manifestations of acute asthma and bacterial RTI are similar, as are commonly used test values, like C-reactive protein (CRP) and white blood cell (WBC) count, making it harder for doctors to differentiate between viral and bacterial infections in asthma patients [126]. Due to the increased morbidity and mortality associated with severe exacerbations, patients are more typically managed with antibiotics.
Standard tests performed in laboratories, such as CRP level and WBC count, are frequently used; however, this application appears to be influenced more by traditional practices than by the diagnostic efficacy of these assays [127]. Furthermore, delayed peak values and poor specificity of CRP level and/or WBC count, particularly in individuals with systemic inflammation, limit their usefulness for directing antibiotic treatment [128].
6.2.4. Type 2 Helper T-Cell (Th 2)
Th-2 immune responses, an essential causative process, primarily cause asthma. Increased circulating Th-2 concentrations have been reported in patients who survived than in those who had passed away from Staphylococcus aureus infection [129]. In addition to this Th-2 path, research on nonTh-2 pathways further points towards possible positive effects of asthma for predicting the outcome of infection. Again, toll-like receptors (TLR) are crucial in the allergic response of the respiratory passageways because they are the primary detectors of intruding microorganisms. Furthermore, the pathogenesis of asthma involves the stimulation of interleukin 17 (IL-17), which is possibly significant in causing the attraction of neutrophils to the infection site and, thus, minimizing disease progression [130].
6.2.5. Omentin-1
A newly discovered adipokine with anti-inflammatory characteristics linked to sepsis and inflammatory disorders is omentin 1, also known as intelectin 1. Omentin-1 primarily appears in fat tissues of viscera, although it can be detected in the ovaries, endothelium, the bloodstream, mesothelial cells, and respiratory goblet cells. Again, the concentration of serum omentin-1 rises in sepsis, and the severity and 28-day mortality of sepsis correlate with more significant levels and slower kinetics during the first week of the condition [131].
6.2.6. H2S
It has long been established that hydrogen sulfide (H2S), a toxic gas with a strong, putrid egg odor that is linked to industrial and water pollution, counts as a harmful gas [132]. In addition to this, the respiratory and central nervous systems are significantly affected by H2S. Recent research, however, implies that H2S may belong to a unique class of endogenous gaseous transmitters and, along with carbon monoxide and nitric oxide, may constitute a third endogenous signaling transmitter that functions both as a vasodilator and a neurotransmitter [133,134]. Furthermore, it has been found that increased H2S production in endotoxemia contributes to the pathogenesis of organ damage [135]. Acting at the junction of leucocytes and endothelium, endogenous H2S is a significant facilitator of acute inflammatory processes [136]. In the etiology of sepsis, shock, cardiovascular injury, and pancreatitis, endogenous H2S may have anti-inflammatory or pro-inflammatory impacts, indicating that H2S may be linked to the development of systemic inflammation [137]. However, the diagnostic usefulness of serum H2S in bacterial infection in patients who are not critically ill has not been examined.
7. Takeaway Message
Acute severe asthma with sepsis can be treated more rapidly, with better results, and with less needless antibiotic therapy with an early diagnosis. Diagnostic biomolecular markers have the potential to considerably optimize, speed up, and accurately represent the entire recovery process, from diagnosis and management to confirmation and prompt therapeutic adjustment. Procalcitonin (PCT) has variable cut-off limits in various clinical circumstances, although it is still effective for the identification of sepsis in medical settings. Moreover, procalcitonin is an intriguing biomarker for the diagnosis of bacteria-related sepsis since it is capable of distinguishing culture-positive and culture-negative sepsis from non-infectious illnesses [138].
Blood neutrophils may be helpful as sepsis biomarkers. The blood neutrophil-to-lymphocyte ratio (NLR), which is derived from total blood counts, is a simple, easily available, and relatively affordable index used as a marker of chronic infection. In a number of recent studies, the NLR has been proposed as a potential marker of bouts of inflammation in chronic diseases and various other illnesses. Furthermore, patients with asthma exacerbations had greater blood NLR values than those with stable asthma [24,99].
Early sepsis causes a significant increase in the concentration of CRP, and because of this, it has been employed to diagnose sepsis and its prognosis. Furthermore, the importance of circulating HS-CRP (high sensitivity C-reactive protein) in the diagnosis of asthma is increased when paired with fractional exhaled nitric oxide (FeNO). In contrast, procalcitonin (PCT) is beneficial in the diagnosis of bacterial infections in patients and has an impact on decisions about antibiotic therapy. Most importantly, PCT is capable of distinguishing severe acute asthma from sepsis. Additionally, Pearson correlation analysis revealed that NLR, CRP, and PCT levels were conclusively correlated with graveness of septicemic patients, especially those with bloodstream infections (r-values were 0.468, 0.456, and 0.670, respectively; all p < 0.001) [139]. Furthermore, multiple studies reported that NLR has been considered the top biomarker to diagnose sepsis [140,141,142,143]. It has been reported that NLR levels are raised in any chronic systemic inflammatory state, including severe acute asthma, cancer, atherosclerosis, and endocrine stress [26,144,145,146]. Multiple studies reported that the integrated result of CRP level and NLR are considered propitious biomarkers for recognizing bronchial asthma [147,148,149]. The global prevalence of bronchial asthma is depicted in Figure 4.
Figure 4.
Diagram showing the prevalence of asthma worldwide [150]. This figure has been drawn using the premium version of BioRender with the license number AN25JJ2Y2S. Image Credit: Susmita Sinha.
8. Conclusions
It can be challenging for medical professionals to determine the cause of sepsis because it exhibits many clinical symptoms that are similar to other illnesses which might arise in intensive care unit (ICU) patients. The introduction of antimicrobial therapy during lower respiratory tract infections can be guided by the implementation of biomarkers (PCT or CRP), and the excessive use of prescribing antibiotics in health care can be decreased. PCT is a more applicable and accurate biomarker for identifying the bacterial (high PCT) as opposed to viral (low PCT) origin of lower respiratory tract infections than CRP. The advancement of PCT throughout time seems to be associated with patient mortality and prognosis. The primary biomarkers regularly employed in everyday clinical practice for diagnosing, phenotyping, and managing asthma are FeNO, blood eosinophils, and total IgE. The demand for the generation of biomarkers to aid doctors in managing asthma is growing due to the need to more accurately and rapidly phenotype asthma, foresee complications, and determine whether or not interventions are responding.
Author Contributions
(1) Conceptualization, Methodology, Validation: S.S., S.K., M.N., A.S. and M.H. (2) Investigation, Resources, Data curation: S.S., S.K., M.N., A.S. and M.H. (3) Writing—original draft preparation, Writing—review and editing, Visualization, Supervision: S.S., S.K., M.N., A.S. and M.H. (4) Finally all authors are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: S.S., S.K., M.N., A.S. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Information is taken from freely available sources for this review paper.
Conflicts of Interest
The author declares that they do not have any financial involvement or affiliations with any organization, association, or entity directly or indirectly with the subject matter or materials presented in this article. This includes honoraria, expert testimony, employment, ownership of stocks or options, patents or grants received or pending, or royalties.
References
- Bond, K.R.; Horsley, C.A.; Williams, A.B. Non-invasive ventilation use in status asthmaticus: 16 years of experience in a tertiary intensive care. Emerg. Med. Australas. 2018, 30, 187–192. [Google Scholar] [CrossRef]
- Adams, J.Y.; Sutter, M.E.; Albertson, T.E. The patient with asthma in the emergency department. Clin. Rev. Allergy Immunol. 2012, 43, 14–29. [Google Scholar] [CrossRef]
- D’Amato, G.; Vitale, C.; Molino, A.; Stanziola, A.; Sanduzzi, A.; Vatrella, A.; Mormile, M.; Lanza, M.; Calabrese, G.; Antonicelli, L.; et al. Asthma-related deaths. Multidiscip. Respir. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- D’Amato, G.; Vitale, C.; Lanza, M.; Sanduzzi, A.; Molino, A.; Mormile, M.; Vatrella, A.; Bilò, M.B.; Antonicelli, L.; Bresciani, M.; et al. Near fatal asthma: Treatment and prevention. Eur. Ann. Allergy Clin. Immunol. 2016, 48, 116–122. [Google Scholar] [PubMed]
- Fernandes, A.G.; Souza-Machado, C.; Coelho, R.C.; Franco, P.A.; Esquivel, R.M.; Souza-Machado, A.; Cruz, A.A. Risk factors for death in patients with severe asthma. J. Bras. Pneumol. 2014, 40, 364–372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McFadden, E.R., Jr. Acute severe asthma. Am. J. Respir. Crit. Care Med. 2003, 168, 740–759. [Google Scholar] [CrossRef] [PubMed]
- Normansell, R.; Sayer, B.; Waterson, S.; Dennett, E.J.; Del Forno, M.; Dunleavy, A. Antibiotics for exacerbations of asthma. Cochrane Database Syst. Rev. 2018, 6, CD002741. [Google Scholar] [CrossRef]
- Iikura, M.; Hojo, M.; Koketsu, R.; Watanabe, S.; Sato, A.; Chino, H.; Ro, S.; Masaki, H.; Hirashima, J.; Ishii, S.; et al. The importance of bacterial and viral infections associated with adult asthma exacerbations in clinical practice. PLoS ONE 2015, 10, e0123584. [Google Scholar] [CrossRef] [PubMed]
- Majellano, E.C.; Clark, V.L.; Winter, N.A.; Gibson, P.G.; McDonald, V.M. Approaches to the assessment of severe asthma: Barriers and strategies. J. Asthma Allergy 2019, 12, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Narumoto, O. Adult bronchial asthma, definition, cause, and severity assessment. Nihon Rinsho. Jpn. J. Clin. Med. 2016, 74, 1617–1621. [Google Scholar]
- Cheong, A.T.; Lee, P.Y.; Shariff-Ghazali, S.; Salim, H.; Hussein, N.; Ramli, R.; Pinnock, H.; Liew, S.M.; Hanafi, N.S.; Abu Bakar, A.I.; et al. Implementing asthma management guidelines in public primary care clinics in Malaysia. NPJ Prim. Care Respir. Med. 2021, 31, 47. [Google Scholar] [CrossRef] [PubMed]
- Saglani, S.; Fleming, L.; Sonnappa, S.; Bush, A. Advances in the etiology, management, and prevention of acute asthma attacks in children. Lancet Child. Adolesc. Health 2019, 3, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Kaza, V.; Bandi, V.; Guntupalli, K.K. Acute severe asthma: Recent advances. Curr. Opin. Pulm. Med. 2007, 13, 1–7. [Google Scholar] [CrossRef]
- Klingenberg, C.; Kornelisse, R.F.; Buonocore, G.; Maier, R.F.; Stocker, M. Culture-Negative Early-Onset Neonatal Sepsis—At the Crossroad between Efficient Sepsis Care and Antimicrobial Stewardship. Front. Pediatr. 2018, 6, 285. [Google Scholar] [CrossRef] [PubMed]
- Lever, A.; Mackenzie, I. Sepsis: Definition, epidemiology, and diagnosis. BMJ 2007, 335, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Peters, M.J.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schlapbach, L.J.; Tasker, R.C.; Argent, A.C.; et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020, 46 (Suppl. S1), 10–67. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Lubell, Y.; Blacksell, S.D.; Dunachie, S.; Tanganuchitcharnchai, A.; Althaus, T.; Watthanaworawit, W.; Paris, D.H.; Mayxay, M.; Peto, T.J.; Dondorp, A.M.; et al. Performance of C-reactive protein and procalcitonin to distinguish viral from bacterial and malarial causes of fever in Southeast Asia. BMC Infect. Dis. 2015, 15, 511. [Google Scholar] [CrossRef]
- Lamrous, A.; Repetto, E.; Depp, T.; Jimenez, C.; Chua, A.C.; Kanapathipillai, R.; Jensen, T.O. C-reactive protein and procalcitonin use in adults in low- and middle-income countries: A narrative review. JAC Antimicrob. Resist. 2023, 5, dlad057. [Google Scholar] [CrossRef]
- Lassere, M.N.; Johnson, K.R.; Boers, M.; Tugwell, P.; Brooks, P.; Simon, L.; Strand, V.; Conaghan, P.G.; Ostergaard, M.; Maksymowych, W.P.; et al. Definitions and validation criteria for biomarkers and surrogate endpoints: Development and testing of a quantitative hierarchical levels of evidence schema. J. Rheumatol. 2007, 34, 607–615. [Google Scholar]
- Aabenhus, R.; Jensen, J.U.; Jørgensen, K.J.; Hróbjartsson, A.; Bjerrum, L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst. Rev. 2014, 11, CD010130. [Google Scholar] [CrossRef]
- Pijnenburg, M.W. The Role of FeNO in Predicting Asthma. Front. Pediatr. 2019, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S. Severe asthma: From characteristics to phenotypes to endotypes. Clin. Exp. Allergy 2012, 42, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Huang, G.T.; Zhan, Q.M.; Chen, J.L.; Luo, W.T.; Wu, L.H.; Wu, L.Y.; Wu, L.Y.; Lu, Z.N.; Sun, Y.F. The neutrophil to lymphocyte ratio as a novel predictor of asthma and its exacerbation: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11719–11728. [Google Scholar] [CrossRef]
- Pan, R.; Ren, Y.; Li, Q.; Zhu, X.; Zhang, J.; Cui, Y.; Yin, H. Neutrophil-lymphocyte ratios in blood to distinguish children with asthma exacerbation from healthy subjects. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320221149849. [Google Scholar] [CrossRef]
- Arwas, N.; Shvartzman, S.U.; Goldbart, A.; Bari, R.; Hazan, I.; Horev, A.; Golan Tripto, I. Elevated Neutrophil-to-Lymphocyte Ratio Is Associated with Severe Asthma Exacerbation in Children. J. Clin. Med. 2023, 12, 3312. [Google Scholar] [CrossRef]
- Landry, V.; Coburn, P.; Kost, K.; Liu, X.; Li-Jessen, N.Y.K. Diagnostic Accuracy of Liquid Biomarkers in Airway Diseases: Toward Point-of-Care Applications. Front. Med. 2022, 9, 855250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, M.; Wang, Y.; Su, X.; Lei, T.; Yu, H.; Liu, J. Diagnostic value of fractional exhaled nitric oxide in differentiating the asthma-COPD overlap from COPD: A systematic review and meta-analysis. Expert. Rev. Respir. Med. 2022, 16, 679–687. [Google Scholar] [CrossRef]
- Grover, H.L.; Higgins, B.G. GPs have a key role in improving outcomes in acute asthma. Practitioner 2016, 260, 15–19. [Google Scholar]
- Zelicof Paul, A.; Rutherford, K.A.; Abuso, S.M. Emergency department management of pediatric acute asthma: An evidence-based review. Pediatr. Emerg. Med. Pract. 2023, 20, 1–28. [Google Scholar] [PubMed]
- Aniapravan, R.; Pullattayil, A.; Al Ansari, K.; Powell, C.V.E. Question 5: Magnesium Sulphate for Acute Asthma in children. Paediatr. Respir. Rev. 2020, 36, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.; Sweeney, J.; Rumantir, M.; Coates, A.L.; Willan, A.R.; Stephens, D.; Atenafu, E.G.; Finkelstein, Y.; Thompson, G.; Zemek, R.; et al. Effect of Nebulized Magnesium vs Placebo Added to Albuterol on Hospitalization Among Children with Refractory Acute Asthma Treated in the Emergency Department: A Randomized Clinical Trial. JAMA 2020, 324, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Kwofie, K.; Wolfson, A.B. Intravenous Magnesium Sulfate for Acute Asthma Exacerbation in Children and Adults. Am. Fam. Physician 2021, 103, 245–246. [Google Scholar] [PubMed]
- Chavasse, R.; Scott, S. The Differences in Acute Management of Asthma in Adults and Children. Front. Pediatr. 2019, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Alangari, A.A. Corticosteroids in the treatment of acute asthma. Ann. Thorac. Med. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Kearns, N.; Maijers, I.; Harper, J.; Beasley, R.; Weatherall, M. Inhaled Corticosteroids in Acute Asthma: A Systemic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2020, 8, 605–617.e6. [Google Scholar] [CrossRef]
- Vázquez, Y.; González, L.; Noguera, L.; González, P.A.; Riedel, C.A.; Bertrand, P.; Bueno, S.M. Cytokines in the Respiratory Airway as Biomarkers of Severity and Prognosis for Respiratory Syncytial Virus Infection: An Update. Front. Immunol. 2019, 10, 1154. [Google Scholar] [CrossRef]
- Rubin, B.K.; Pohanka, V. Beyond the guidelines: Fatal and near-fatal asthma. Paediatr. Respir. Rev. 2012, 13, 106–111. [Google Scholar] [CrossRef]
- Pardue Jones, B.; Fleming, G.M.; Otillio, J.K.; Asokan, I.; Arnold, D.H. Pediatric acute asthma exacerbations: Evaluation and management from emergency department to intensive care unit. J. Asthma 2016, 53, 607–617. [Google Scholar] [CrossRef]
- Uffen, J.W.; Oosterheert, J.J.; Schweitzer, V.A.; Thursky, K.; Kaasjager, H.A.H.; Ekkelenkamp, M.B. Interventions for rapid recognition and treatment of sepsis in the emergency department: A narrative review. Clin. Microbiol. Infect. 2021, 27, 192–203. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensiv. Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Zawistowski, C.A. The management of sepsis. Curr. Probl. Pediatr. Adolesc. Health Care 2013, 43, 285–291. [Google Scholar] [CrossRef]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Tan, J.C.; Harvey, S.E.; Bell, D.; et al. Protocolised Management In Sepsis (ProMISe): A multicentre randomised controlled trial of the clinical effectiveness and cost-effectiveness of early, goal-directed, protocolised resuscitation for emerging septic shock. Health Technol. Assess. 2015, 19, 1–150. [Google Scholar] [CrossRef] [PubMed]
- Mangioni, D.; Viaggi, B.; Giani, T.; Arena, F.; D’Arienzo, S.; Forni, S.; Tulli, G.; Rossolini, G.M. Diagnostic stewardship for sepsis: The need for risk stratification to triage patients for fast microbiology workflows. Future Microbiol. 2019, 14, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Haktanir Abul, M.; Phipatanakul, W. Severe asthma in children: Evaluation and management. Allergol. Int. 2019, 68, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.C.; Levy, M.L.; Moreiras, J.; Fleming, L. Managing problematic severe asthma: Beyond the guidelines. Arch. Dis. Child. 2018, 103, 392–397. [Google Scholar] [CrossRef]
- Castagnoli, R.; Marseglia, A.; Brambilla, I.; Marseglia, G.L.; Licari, A. Severe uncontrolled asthma in children: Practical approach on diagnosis and management. Minerva Pediatr. 2020, 72, 196–205. [Google Scholar] [CrossRef]
- Heffler, E.; Blasi, F.; Latorre, M.; Menzella, F.; Paggiaro, P.; Pelaia, G.; Senna, G.; Canonica, G.W.; SANI Network. The Severe Asthma Network in Italy: Findings and Perspectives. J. Allergy Clin. Immunol. Pract. 2019, 7, 1462–1468. [Google Scholar] [CrossRef]
- Agnihotri, N.T.; Saltoun, C. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2019, 40, 406–409. [Google Scholar] [CrossRef]
- Lofrese, J.J.; Tupper, C.; Denault, D.; Lappin, S.L. Physiology, Residual Volume. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Haddad, M.; Sharma, S. Physiology, Lung. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hammer, J. Acute respiratory failure in children. Paediatr. Respir. Rev. 2013, 14, 64–69. [Google Scholar] [CrossRef]
- Schneider, J.; Sweberg, T. Acute respiratory failure. Crit. Care Clin. 2013, 29, 167–183. [Google Scholar] [CrossRef]
- Trachsel, D.; Erb, T.O.; Hammer, J.; von Ungern-Sternberg, B.S. Developmental respiratory physiology. Paediatr. Anaesth. 2022, 32, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Grasso, S.; Karbing, D.S.; Fogagnolo, A.; Contoli, M.; Bollini, G.; Ragazzi, R.; Cinnella, G.; Verri, M.; Cavallesco, N.G.; et al. Physiologic Evaluation of Ventilation Perfusion Mismatch and Respiratory Mechanics at Different Positive End-expiratory Pressure in Patients Undergoing Protective One-lung Ventilation. Anesthesiology 2018, 128, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Powers, K.A.; Dhamoon, A.S. Physiology, Pulmonary Ventilation, and Perfusion. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rubin, B.K.; Priftis, K.N.; Schmidt, H.J.; Henke, M.O. Secretory hyperresponsiveness and pulmonary mucus hypersecretion. Chest 2014, 146, 496–507. [Google Scholar] [CrossRef]
- Evans, C.M.; Kim, K.; Tuvim, M.J.; Dickey, B.F. Mucus hypersecretion in asthma: Causes and effects. Curr. Opin. Pulm. Med. 2009, 15, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Papiris, S.A.; Manali, E.D.; Kolilekas, L.; Triantafillidou, C.; Tsangaris, I. Acute severe asthma: New approaches to assessment and treatment. Drugs 2009, 69, 2363–2391. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.E.; Leung, A.K.C. Medications and Recent Patents for Status Asthmaticus in Children. Recent. Pat. Inflamm. Allergy Drug Discov. 2017, 11, 12–21. [Google Scholar] [CrossRef]
- Lammers, S.; Scott, D.; Hunter, K.; Tan, W.; Shandas, R.; Stenmark, K.R. Mechanics and Function of the Pulmonary Vasculature: Implications for Pulmonary Vascular Disease and Right Ventricular Function. Compr. Physiol. 2012, 2, 295–319. [Google Scholar] [CrossRef]
- Dhand, R. Ventilator graphics and respiratory mechanics in the patient with obstructive lung disease. Respir. Care 2005, 50, 246–261. [Google Scholar]
- Hamzaoui, O.; Monnet, X.; Teboul, J.L. Pulsus paradoxus. Eur. Respir. J. 2013, 42, 1696–1705. [Google Scholar] [CrossRef]
- Sarkar, M.; Bhardwaz, R.; Madabhavi, I.; Modi, M. Physical signs in patients with chronic obstructive pulmonary disease. Lung India 2019, 36, 38–47. [Google Scholar] [CrossRef]
- Giacomelli, R.; Afeltra, A.; Alunno, A.; Bartoloni-Bocci, E.; Berardicurti, O.; Bombardieri, M.; Bortoluzzi, A.; Caporali, R.; Caso, F.; Cervera, R.; et al. Guidelines for biomarkers in autoimmune rheumatic diseases—Evidence-based analysis. Autoimmun. Rev. 2019, 18, 93–106. [Google Scholar] [CrossRef]
- Burke, H.B. Predicting Clinical Outcomes Using Molecular Biomarkers. Biomark. Cancer 2016, 8, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.D.; McCabe, S.; White, N.; Clancy, R.L. Biomarkers: An important clinical assessment tool. Am. J. Nurs. 2012, 112, 52–58. [Google Scholar] [CrossRef] [PubMed]
- di Palmo, E.; Cantarelli, E.; Catelli, A.; Ricci, G.; Gallucci, M.; Miniaci, A.; Pession, A. The Predictive Role of Biomarkers and Genetics in Childhood Asthma Exacerbations. Int. J. Mol. Sci. 2021, 22, 4651. [Google Scholar] [CrossRef]
- Wu, A.C.; Kiley, J.P.; Noel, P.J.; Amur, S.; Burchard, E.G.; Clancy, J.P.; Galanter, J.; Inada, M.; Jones, T.K.; Kropski, J.A.; et al. Current Status and Future Opportunities in Lung Precision Medicine Research with a Focus on Biomarkers. An American Thoracic Society/National Heart, Lung, and Blood Institute Research Statement. Am. J. Respir. Crit. Care Med. 2018, 198, e116–e136. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Baines, K.J.; Pavord, I.D.; Gibson, P.G. The role of biomarkers in the management of airways disease. Int. J. Tuberc. Lung Dis. 2014, 18, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.F.; Ko, F.W.; Wong, G.W. Recent advances in asthma biomarker research. Ther. Adv. Respir. Dis. 2013, 7, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Koczulla, A.R.; Vogelmeier, C.F.; Garn, H.; Renz, H. New concepts in asthma: Clinical phenotypes and pathophysiological mechanisms. Drug Discov. Today 2017, 22, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Poon, A.H.; Hamid, Q. Asthma phenotypes and endotypes. Curr. Opin. Pulm. Med. 2013, 19, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, K.; Van Den Heuvel, R.; Govarts, E.; Hooyberghs, J.; Nelen, V.; Witters, E.; Desager, K.; Schoeters, G. A new approach to study exhaled proteins as potential biomarkers for asthma. Clin. Exp. Allergy 2011, 41, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Bartminski, G.; Crossley, M.; Turcanu, V. Novel biomarkers for asthma stratification and personalized therapy. Expert. Rev. Mol. Diagn. 2015, 15, 415–430. [Google Scholar] [CrossRef]
- Richards, L.B.; Neerincx, A.H.; van Bragt, J.J.M.H.; Sterk, P.J.; Bel, E.H.D.; Maitland-van der Zee, A.H. Biomarkers and asthma management: Analysis and potential applications. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 96–108. [Google Scholar] [CrossRef]
- Arnold, R.J.; Massanari, M.; Lee, T.A.; Brooks, E. A Review of the Utility and Cost Effectiveness of Monitoring Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management. Manag. Care 2018, 27, 34–41. [Google Scholar]
- Rao, D.R.; Phipatanakul, W. An Overview of Fractional Exhaled Nitric Oxide and Children with Asthma. Expert. Rev. Clin. Immunol. 2016, 12, 521–530. [Google Scholar] [CrossRef]
- Zhou, A.; Zhou, Z.; Deng, D.; Zhao, Y.; Duan, J.; Cheng, W.; Liu, C.; Chen, P. The Value of FENO Measurement for Predicting Treatment Response in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct Pulmon Dis. 2020, 15, 2257–2266. [Google Scholar] [CrossRef]
- Licari, A.; Manti, S.; Castagnoli, R.; Leonardi, S.; Marseglia, G.L. Measuring inflammation in pediatric severe asthma: Biomarkers in clinical practice. Breathe 2020, 16, 190301. [Google Scholar] [CrossRef]
- Guida, G.; Bagnasco, D.; Carriero, V.; Bertolini, F.; Ricciardolo, F.L.M.; Nicola, S.; Brussino, L.; Nappi, E.; Paoletti, G.; Canonica, G.W.; et al. Critical evaluation of asthma biomarkers in clinical practice. Front. Med. 2022, 9, 969243. [Google Scholar] [CrossRef]
- Kim, H.; Ellis, A.K.; Fischer, D.; Noseworthy, M.; Olivenstein, R.; Chapman, K.R.; Lee, J. Asthma biomarkers in the age of biologics. Allergy Asthma Clin. Immunol. 2017, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qiu, R.; Yang, Z.; Li, J.; Chung, K.F.; Zhong, N.; Zhang, Q. Sputum microbiota in severe asthma patients: Relationship to eosinophilic inflammation. Respir. Med. 2017, 131, 192–198. [Google Scholar] [CrossRef]
- Li, W.; Gao, P.; Zhi, Y.; Xu, W.; Wu, Y.; Yin, J.; Zhang, J. Periostin: Its role in asthma and its potential as a diagnostic or therapeutic target. Respir. Res. 2015, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Refaat, M.M.; El Sayed, E.; Abd El-Fattah, W.; Elbanna, A.H.; Sayed, H.M.E. Relationship between sputum periostin level and inflammatory asthma phenotypes in Egyptian patients. J. Asthma 2021, 58, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Bobolea, I.; Barranco, P.; Del Pozo, V.; Romero, D.; Sanz, V.; López-Carrasco, V.; Canabal, J.; Villasante, C.; Quirce, S. Sputum periostin in patients with different severe asthma phenotypes. Allergy 2015, 70, 540–546. [Google Scholar] [CrossRef]
- Bruijnzeel, P.L.; Uddin, M.; Koenderman, L. Targeting neutrophilic inflammation in severe neutrophilic asthma: Can we target the disease-relevant neutrophil phenotype? J. Leukoc. Biol. 2015, 98, 549–556. [Google Scholar] [CrossRef]
- Kostakou, E.; Kaniaris, E.; Filiou, E.; Vasileiadis, I.; Katsaounou, P.; Tzortzaki, E.; Koulouris, N.; Koutsoukou, A.; Rovina, N. Acute Severe Asthma in Adolescent and Adult Patients: Current Perspectives on Assessment and Management. J. Clin. Med. 2019, 8, 1283. [Google Scholar] [CrossRef]
- Kostikas, K.; Brindicci, C.; Patalano, F. Blood Eosinophils as Biomarkers to Drive Treatment Choices in Asthma and COPD. Curr. Drug Targets 2018, 19, 1882–1896. [Google Scholar] [CrossRef]
- Mogensen, I.; James, A.; Malinovschi, A. Systemic and breath biomarkers for asthma: An update. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 71–79. [Google Scholar] [CrossRef]
- Lv, M.Y.; Qiang, L.X.; Li, Z.H.; Jin, S.D. The lower the eosinophils, the stronger the inflammatory response? The relationship of different levels of eosinophils with the degree of inflammation in acute exacerbation chronic obstructive pulmonary disease (AECOPD). J. Thorac. Dis. 2021, 13, 232–243. [Google Scholar] [CrossRef]
- Naumova, V.; Beltyukov, E.; Niespodziana, K.; Errhalt, P.; Valenta, R.; Karaulov, A.; Kiseleva, D. Cumulative IgE-levels specific for respiratory allergens as biomarker to predict efficacy of anti-IgE-based treatment of severe asthma. Front. Immunol. 2022, 13, 941492. [Google Scholar] [CrossRef]
- Pelham, C.J.; Agrawal, D.K. Emerging roles for triggering receptor expressed on myeloid cells receptor family signaling in inflammatory diseases. Expert. Rev. Clin. Immunol. 2014, 10, 243–256. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, H.; Fu, X.; Han, L.; Zhang, H.; Zhang, L.; Zhao, J.; Xiao, D.; Li, H.; Li, P. Autophagy-driven neutrophil extracellular traps: The dawn of sepsis. Pathol. Res. Pract. 2022, 234, 153896. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.F.; Cao, K.; Jiang, J.P.; Guan, W.X.; Du, J.F. Neutrophil dysregulation during sepsis: An overview and update. J. Cell Mol. Med. 2017, 21, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Zhou, M.; Chen, G.B.; Du, J.; Wang, Y.; Ye, C. The value of lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio in differentiating pneumonia from upper respiratory tract infection (URTI) in children: A cross-sectional study. BMC Pediatr. 2021, 21, 545. [Google Scholar] [CrossRef]
- Tsang, M.S.; Hou, T.; Chan, B.C.; Wong, C.K. Immunological Roles of NLR in Allergic Diseases and Its Underlying Mechanisms. Int. J. Mol. Sci. 2021, 22, 1507. [Google Scholar] [CrossRef]
- Zuo, H.; Xie, X.; Peng, J.; Wang, L.; Zhu, R. Predictive Value of Novel Inflammation-Based Biomarkers for Pulmonary Hypertension in the Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Anal. Cell. Pathol. 2019, 2019, 5189165. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Teng, S.; Li, K. Prediction of sepsis mortality using metabolite biomarkers in the blood: A meta-analysis of death-related pathways and prospective validation. BMC Med. 2020, 18, 83. [Google Scholar] [CrossRef]
- Pregernig, A.; Müller, M.; Held, U.; Beck-Schimmer, B. Prediction of mortality in adult patients with sepsis using six biomarkers: A systematic review and meta-analysis. Ann. Intensive Care 2019, 9, 125. [Google Scholar] [CrossRef]
- Vincent, J.L. The Clinical Challenge of Sepsis Identification and Monitoring. PLoS Med. 2016, 13, e1002022. [Google Scholar] [CrossRef] [PubMed]
- van Engelen, T.S.R.; Wiersinga, W.J.; Scicluna, B.P.; van der Poll, T. Biomarkers in Sepsis. Crit. Care Clin. 2018, 34, 139–152. [Google Scholar] [CrossRef]
- Li, H.X.; Liu, Z.M.; Zhao, S.J.; Zhang, D.; Wang, S.J.; Wang, Y.S. Measuring both procalcitonin and C-reactive protein for a diagnosis of sepsis in critically ill patients. J. Int. Med. Res. 2014, 42, 1050–1059. [Google Scholar] [CrossRef]
- Tan, M.; Lu, Y.; Jiang, H.; Zhang, L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J. Cell Biochem. 2019, 120, 5852–5859. [Google Scholar] [CrossRef] [PubMed]
- Hamade, B.; Huang, D.T. Procalcitonin: Where Are We Now? Crit. Care Clin. 2020, 36, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Naot, D.; Musson, D.S.; Cornish, J. The Activity of Peptides of the Calcitonin Family in Bone. Physiol. Rev. 2019, 99, 781–805. [Google Scholar] [CrossRef]
- Xie, J.; Guo, J.; Kanwal, Z.; Wu, M.; Lv, X.; Ibrahim, N.A.; Li, P.; Buabeid, M.A.; Arafa, E.A.; Sun, Q. Calcitonin and Bone Physiology: In Vitro, In Vivo, and Clinical Investigations. Int. J. Endocrinol. 2020, 2020, 3236828. [Google Scholar] [CrossRef]
- Angeletti, S.; Spoto, S.; Fogolari, M.; Cortigiani, M.; Fioravanti, M.; De Florio, L.; Curcio, B.; Cavalieri, D.; Costantino, S.; Dicuonzo, G. Diagnostic and prognostic role of procalcitonin (PCT) and MR-pro-Adrenomedullin (MR-proADM) in bacterial infections. APMIS 2015, 123, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Kip, M.M.; Kusters, R.; IJzerman, M.J.; Steuten, L.M. A PCT algorithm for discontinuation of antibiotic therapy is a cost-effective way to reduce antibiotic exposure in adult intensive care patients with sepsis. J. Med. Econ. 2015, 18, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Mustafić, S.; Brkić, S.; Prnjavorac, B.; Sinanović, A.; Porobić Jahić, H.; Salkić, S. Diagnostic and prognostic value of procalcitonin in patients with sepsis. Med. Glas. 2018, 15, 93–100. [Google Scholar] [CrossRef]
- Ozger, H.S.; Senol, E. Use of infection biomarkers in the emergency department. Turk. J. Emerg. Med. 2022, 22, 169–176. [Google Scholar] [CrossRef]
- Hausfater, P. Biomarkers and infection in the emergency unit. Med. Mal. Infect. 2014, 44, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.; Bigham, M.T.; Giuliano, J.S., Jr. Use of procalcitonin for the prediction and treatment of acute bacterial infection in children. Curr. Opin. Pediatr. 2014, 26, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Long, W.; Yan, L.; Zhang, Y.; Xie, J.; Lu, G.; Yang, C. Procalcitonin guided antibiotic therapy of acute exacerbations of asthma: A randomized controlled trial. BMC Infect. Dis. 2013, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.T.; Sun, L.C.; Jia, H.B.; Gao, W.; Yang, J.P.; Zhang, G.Q. Procalcitonin levels in bloodstream infections caused by different sources and species of bacteria. Am. J. Emerg. Med. 2017, 35, 579–583. [Google Scholar] [CrossRef]
- Carbonell, R.; Moreno, G.; Martín-Loeches, I.; Gomez-Bertomeu, F.; Sarvisé, C.; Gómez, J.; Bodí, M.; Díaz, E.; Papiol, E.; Trefler, S.; et al. Prognostic Value of Procalcitonin and C-Reactive Protein in 1608 Critically Ill Patients with Severe Influenza Pneumonia. Antibiotics 2021, 10, 350. [Google Scholar] [CrossRef]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Pal, M.; Febbraio, M.A.; Whitham, M. From cytokine to myokine: The emerging role of interleukin-6 in metabolic regulation. Immunol. Cell Biol. 2014, 92, 331–339. [Google Scholar] [CrossRef]
- von Witting, E.; Lindbo, S.; Lundqvist, M.; Möller, M.; Wisniewski, A.; Kanje, S.; Rockberg, J.; Tegel, H.; Åstrand, M.; Uhlén, M.; et al. Small Bispecific Affinity Proteins for Simultaneous Target Binding and Albumin-Associated Half-Life Extension. Mol. Pharm. 2021, 18, 328–337. [Google Scholar] [CrossRef]
- Wildes, D.M.; Chisale, M.; Drew, R.J.; Harrington, P.; Watson, C.J.; Ledwidge, M.T.; Gallagher, J. A Systematic Review of Clinical Prediction Rules to Predict Hospitalisation in Children with Lower Respiratory Infection in Primary Care and their Validation in a New Cohort. EClinicalMedicine 2021, 41, 101164. [Google Scholar] [CrossRef]
- Williams, S.J.; Halls, A.V.; Tonkin-Crine, S.; Moore, M.V.; Latter, S.E.; Little, P.; Eyles, C.; Postle, K.; Leydon, G.M. General practitioner and nurse prescriber experiences of prescribing antibiotics for respiratory tract infections in UK primary care out-of-hours services (the UNITE study). J. Antimicrob. Chemother. 2018, 73, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.S.; Shieh, M.S.; Spitzer, K.A.; Pekow, P.S.; Krishnan, J.A.; Au, D.H.; Lindenauer, P.K. Association of Antibiotic Treatment with Outcomes in Patients Hospitalized for an Asthma Exacerbation Treated with Systemic Corticosteroids. JAMA Intern. Med. 2019, 179, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Koatz, A.M.; Coe, N.A.; Cicerán, A.; Alter, A.J. Clinical and Immunological Benefits of OM-85 Bacterial Lysate in Patients with Allergic Rhinitis, Asthma, and COPD and Recurrent Respiratory Infections. Lung 2016, 194, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.X.; Zhang, J.R.; Cao, Z.G.; Li, Y.; Wang, R.T. A decreased mean platelet volume is associated with stable and exacerbated asthma. Respiration 2014, 88, 31–37. [Google Scholar] [CrossRef]
- Couto, R.C.; Barbosa, J.A.; Pedrosa, T.M.; Biscione, F.M. C-reactive protein-guided approach may shorten length of antimicrobial treatment of culture-proven late-onset sepsis: An intervention study. Braz. J. Infect. Dis. 2007, 11, 240–245. [Google Scholar] [CrossRef]
- Krishack, P.A.; Louviere, T.J.; Decker, T.S.; Kuzel, T.G.; Greenberg, J.A.; Camacho, D.F.; Hrusch, C.L.; Sperling, A.I.; Verhoef, P.A. Protection against Staphylococcus aureus bacteremia-induced mortality depends on ILC2s and eosinophils. JCI Insight 2019, 4, e124168. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.; Wang, F.; Liang, J.; Chen, Q.; Lin, Z. Association between comorbid asthma and prognosis of critically ill patients with severe sepsis: A cohort study. Sci. Rep. 2021, 11, 15395. [Google Scholar] [CrossRef]
- Karampela, I.; Vallianou, N.G.; Tsilingiris, D.; Christodoulatos, G.S.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Armaganidis, A.; Dalamaga, M. Diagnostic and Prognostic Value of Serum Omentin-1 in Sepsis: A Prospective Study in Critically Ill Patients. Medicina 2023, 59, 833. [Google Scholar] [CrossRef]
- Suzuki, Y.; Saito, J.; Munakata, M.; Shibata, Y. Hydrogen sulfide as a novel biomarker of asthma and chronic obstructive pulmonary disease. Allergol. Int. 2021, 70, 181–189. [Google Scholar] [CrossRef]
- Li, H.; Hou, X.; Ding, Y.; Nie, L.; Zhou, H.; Nie, Z.; Tang, Y.; Chen, L.; Zheng, Y. Effects of H2S on the central regulation of respiration in adult rats. Neuroreport 2014, 25, 358–366. [Google Scholar] [CrossRef]
- Bazhanov, N.; Ansar, M.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Hydrogen Sulfide: A Novel Player in Airway Development, Pathophysiology of Respiratory Diseases, and Antiviral Defenses. Am. J. Respir. Cell Mol. Biol. 2017, 57, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Liu, S.J.; Tang, X.L.; Duan, G.L.; Ni, X.; Zhu, X.Y.; Liu, Y.J.; Wang, C.N. H2S Attenuates LPS-Induced Acute Lung Injury by Reducing Oxidative/Nitrative Stress and Inflammation. Cell Physiol. Biochem. 2016, 40, 1603–1612. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, G.; Wondimu, T.; Ross, B.; Wang, R. Hydrogen sulfide and asthma. Exp. Physiol. 2011, 96, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jaswani, P.; Sharma, R.K.; Agrawal, S.; Prasad, N.; Sahu, C.; Gupta, A.; Prasad, K.N. Procalcitonin as a diagnostic biomarker of sepsis: A tertiary care centre experience. J. Infect. Public Health 2019, 12, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Yu, F. Value of CRP, PCT, and NLR in Prediction of Severity and Prognosis of Patients with Bloodstream Infections and Sepsis. Front. Surg. 2022, 9, 857218. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wei, B.; Zhang, X.; Hu, L.; Ye, X. Value of Neutrophil:Lymphocyte Ratio Combined with Sequential Organ Failure Assessment Score in Assessing the Prognosis of Sepsis Patients. Int. J. Gen. Med. 2022, 15, 1901–1908. [Google Scholar] [CrossRef]
- Drăgoescu, A.N.; Pădureanu, V.; Stănculescu, A.D.; Chiuțu, L.C.; Tomescu, P.; Geormăneanu, C.; Pădureanu, R.; Iovănescu, V.F.; Ungureanu, B.S.; Pănuș, A.; et al. Neutrophil to Lymphocyte Ratio (NLR)—A Useful Tool for the Prognosis of Sepsis in the ICU. Biomedicines 2021, 10, 75. [Google Scholar] [CrossRef]
- Rehman, F.U.; Khan, A.; Aziz, A.; Iqbal, M.; Mahmood, S.B.Z.; Ali, N. Neutrophils to Lymphocyte Ratio: Earliest and Efficacious Markers of Sepsis. Cureus 2020, 12, e10851. [Google Scholar] [CrossRef]
- Omran, A.; Maaroof, A.; Mohammad, M.H.S.; Abdelwahab, A. Salivary C-reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. J. Pediatr. 2018, 94, 82–87. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef]
- Imtiaz, F.; Shafique, K.; Mirza, S.S.; Ayoob, Z.; Vart, P.; Rao, S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int. Arch. Med. 2012, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Lee, K.O.; Choi, J.W.; Kim, N.K.; Kim, O.J.; Kim, S.H.; Oh, S.H.; Kim, W.C. Blood Neutrophil/Lymphocyte Ratio Is Associated with Cerebral Large-Artery Atherosclerosis but not with Cerebral Small-Vessel Disease. Front. Neurol. 2020, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, L.; Li, Q.; Pan, R.; Zhang, J.; Cui, Y. Combined score of C-reactive protein level and neutrophil-to-lymphocyte ratio: A novel marker in distinguishing children with exacerbated asthma. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211040641. [Google Scholar] [CrossRef] [PubMed]
- Yufei, Y.; Mingli, L.; Xuejiao, L.; Xuemei, D.; Yiming, J.; Qin, Q.; Hui, S.; Jie, G. Utility of the neutrophil-to-lymphocyte ratio and C-reactive protein level for coronavirus disease 2019 (COVID-19). Scand. J. Clin. Lab. Investig. 2020, 80, 536–540. [Google Scholar] [CrossRef]
- Cag, Y.; Pacal, Y.; Gunduz, M.; Isik, S.; Kertmen, B.A.; Toprak, N.; Ozaydin, S.E.; Ozcetin, M.; Kut, A. The effect of peripheral blood eosinophilia on inflammatory markers in asthmatic patients with lower respiratory tract infections. J. Int. Med. Res. 2019, 47, 2452–2460. [Google Scholar] [CrossRef]
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R.; Global Initiative for Asthma (GINA) Program. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).