Imaging of Temporal Bone Mass Lesions: A Pictorial Review
Abstract
1. Introduction
2. Review
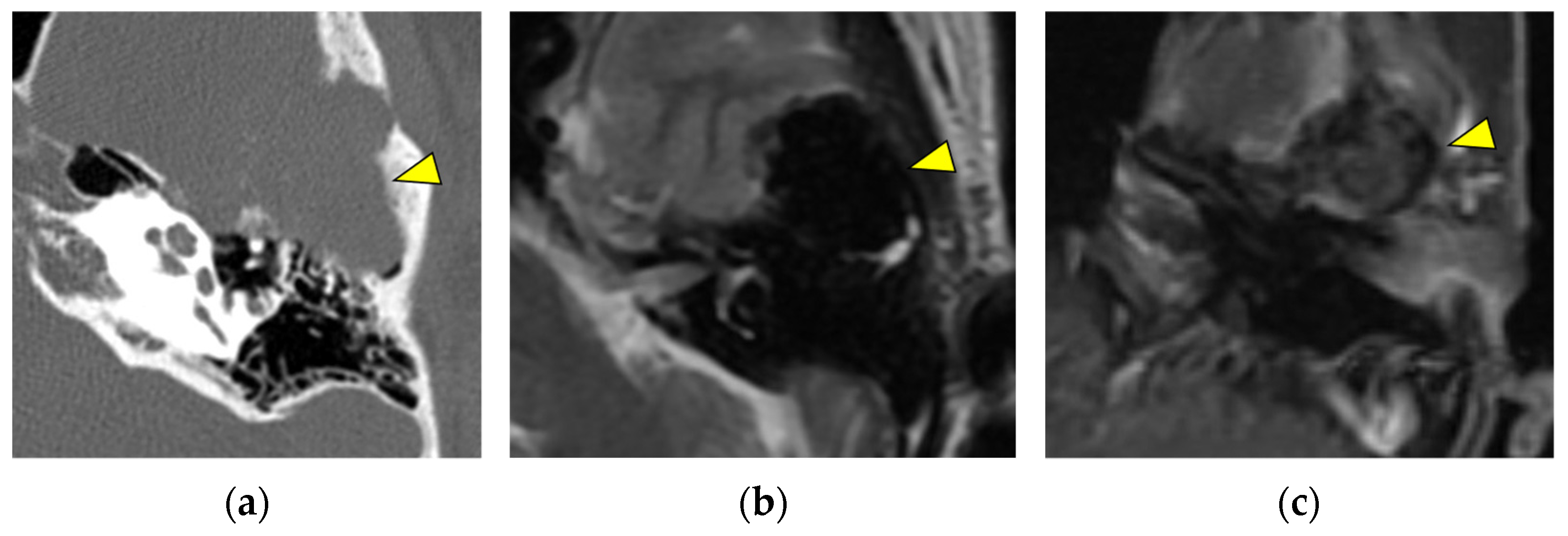
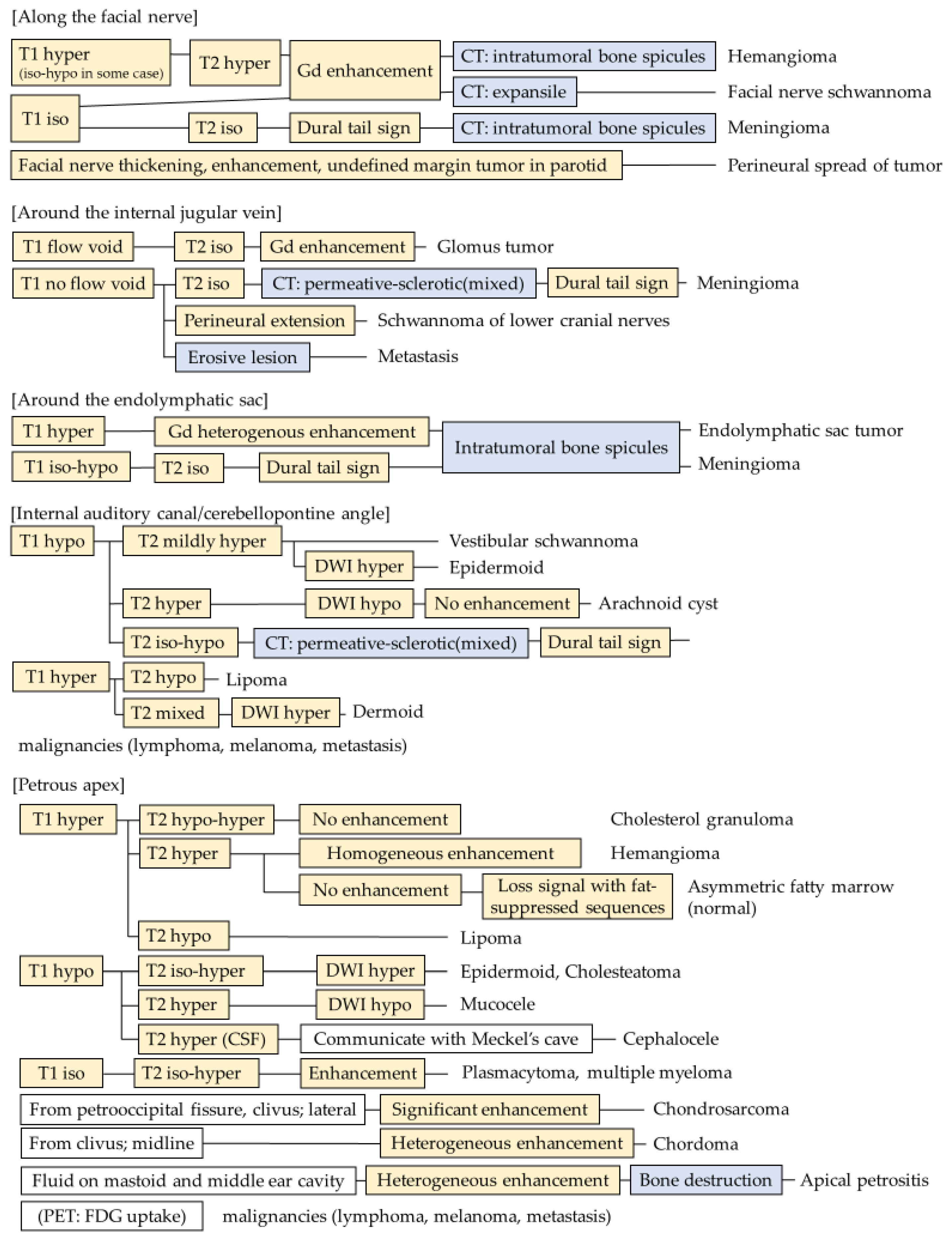
2.1. Along the Facial Nerve
2.2. Along the Internal Jugular Vein
2.3. Around the Endolymphatic Sac
2.4. Internal Auditory Canal/Cerebellopontine Angle
2.5. Petrous Apex
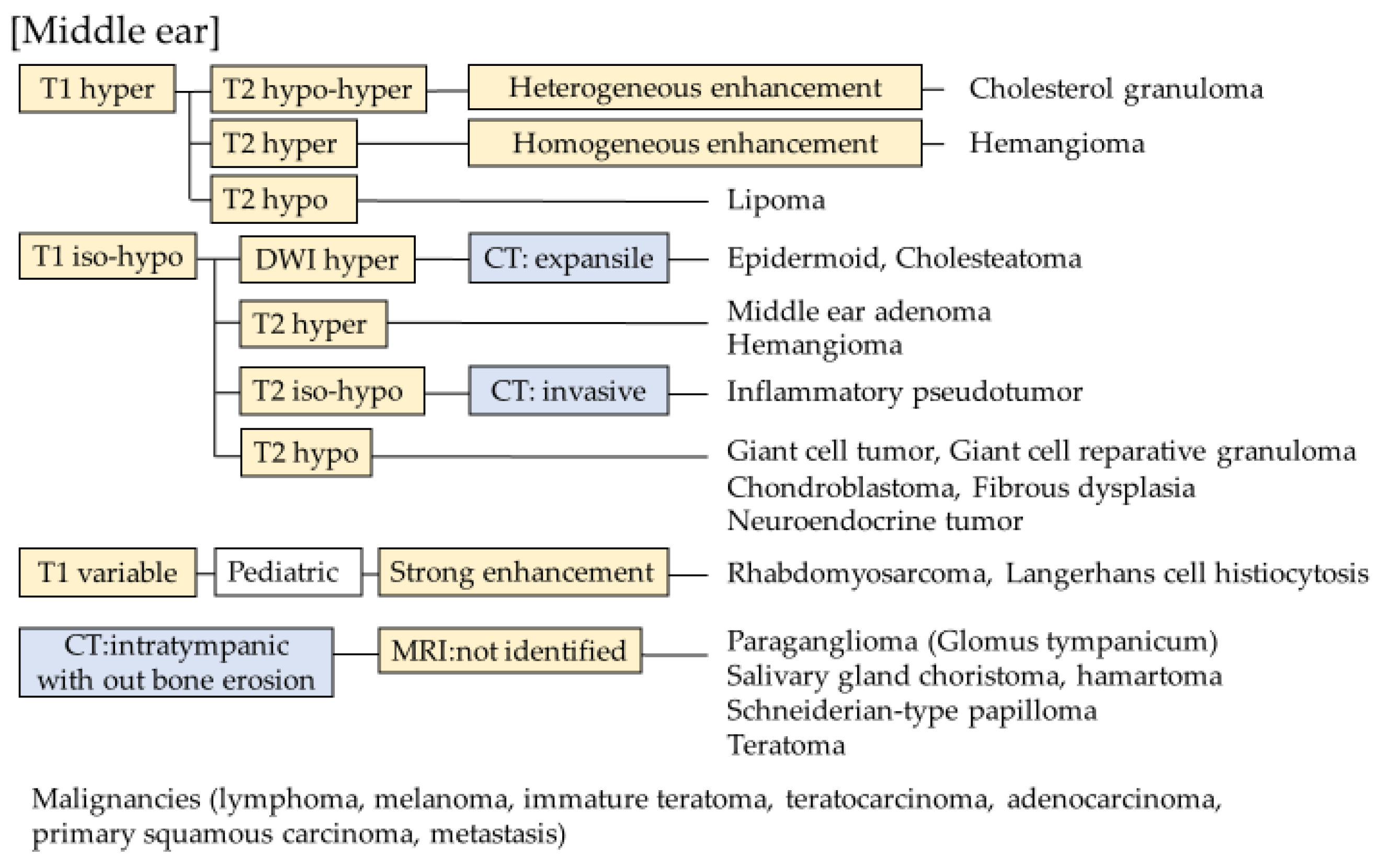
2.6. Middle Ear and Mastoid
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juliano, A.F.; Ginat, D.T.; Moonis, G. Imaging review of the temporal bone: Part I. Anatomy and inflammatory and neoplastic processes. Radiology 2013, 269, 17–33. [Google Scholar] [CrossRef]
- De Foer, B.; Kenis, C.; Vercruysse, J.P.; Somers, T.; Pouillon, M.; Offeciers, E.; Casselman, J.W. Imaging of temporal bone tumors. Neuroimaging Clin. N. Am. 2009, 19, 339–366. [Google Scholar] [CrossRef]
- Chapman, P.R.; Shah, R.; Curé, J.K.; Bag, A.K. Petrous apex lesions: Pictorial review. AJR Am. J. Roentgenol. 2011, 196, WS26–WS37. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Suh, M.W. Skull base surgery for removal of temporal bone tumors. Acta Otolaryngol. Suppl. 2007, 127, 4–14. [Google Scholar] [CrossRef]
- Campion, T.; Taranath, A.; Pinelli, L.; Ugga, L.; Nash, R.; Talenti, G.; Dahmoush, H.; D’Arco, F. Imaging of temporal bone inflammations in children: A pictorial review. Neuroradiology 2019, 61, 959–970. [Google Scholar] [CrossRef]
- Touska, P.; Juliano, A.F. Temporal Bone Tumors: An Imaging Update. Neuroimaging Clin. N. Am. 2019, 29, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Sterkers, O.; Nahum, H. Haemangioma of the petrous bone: MRI. Neuroradiology 1992, 34, 420–422. [Google Scholar] [CrossRef]
- Friedman, O.; Neff, B.A.; Willcox, T.O.; Kenyon, L.C.; Sataloff, R.T. Temporal bone hemangiomas involving the facial nerve. Otol. Neurotol. 2002, 23, 760–766. [Google Scholar] [CrossRef]
- Lee, D.W.; Byeon, H.K.; Chung, H.P.; Choi, E.C.; Kim, S.H.; Park, Y.M. Diagnosis and surgical outcomes of intraparotid facial nerve schwannoma showing normal facial nerve function. Int. J. Oral Maxillofac. Surg. 2013, 42, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.H.; Huang, D.L.; Han, D.Y.; Dai, P.; Young, W.Y.; Yang, S.M. Surgical treatment of endolymphatic sac tumor. Acta Otolaryngol. 2012, 132, 329–336. [Google Scholar] [CrossRef]
- Onoda, K.; Kawaguchi, A.; Takaya, Y.; Inoue, Y.; Nakazato, I.; Saito, Y.; Ishikawa, H.; Oyama, K.; Oshima, Y.; Saito, K.; et al. A Case of Dermoid Cyst Arising in the Temporal Lobe. NMC Case Rep. J. 2021, 8, 529–534. [Google Scholar] [CrossRef]
- Cristobal, R.; Oghalai, J.S. Peripetrosal arachnoid cysts. Curr. Opin. Otolaryngol. Head. Neck Surg. 2007, 15, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.M.; Siripurapu, R. Imaging of Petrous Apex Lesions. Neuroimaging Clin. N. Am. 2021, 31, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Lingam, R.K.; Kumar, R.; Vaidhyanath, R. Inflammation of the Temporal Bone. Neuroimaging Clin. N. Am. 2019, 29, 1–17. [Google Scholar] [CrossRef]
- Isaacson, B.; Kutz, J.W.; Roland, P.S. Lesions of the petrous apex: Diagnosis and management. Otolaryngol. Clin. N. Am. 2007, 40, 479–519. [Google Scholar] [CrossRef]
- Devaney, K.O.; Rinaldo, A.; Ferlito, A. Teratoma of the middle ear: A real entity or a non-entity? Acta Otolaryngol. 2005, 125, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Pisaneschi, M.J.; Langer, B. Congenital cholesteatoma and cholesterol granuloma of the temporal bone: Role of magnetic resonance imaging. Top. Magn. Reson. Imaging 2000, 11, 87–97. [Google Scholar] [CrossRef]
- Ganaha, A.; Outa, S.; Kyuuna, A.; Matayoshi, S.; Yonaha, A.; Oyadomari, M.; Miyara, T.; Tono, T.; Suzuki, M. Efficacy of diffusion-weighted magnetic resonance imaging in the diagnosis of middle ear cholesteatoma. Auris Nasus Larynx 2011, 38, 329–334. [Google Scholar] [CrossRef] [PubMed]
- van Egmond, S.L.; Stegeman, I.; Grolman, W.; Aarts, M.C. A Systematic Review of Non-Echo Planar Diffusion-Weighted Magnetic Resonance Imaging for Detection of Primary and Postoperative Cholesteatoma. Otolaryngol.–Head Neck Surg. 2016, 154, 233–240. [Google Scholar] [CrossRef]
- Imamura, K.; Hosoya, M.; Kasuya, K.; Shimanuki, M.N.; Shinden, S.; Ogawa, K.; Oishi, N. Labyrinthine destruction caused by inflammatory pseudotumor of the temporal bone: A report of three cases and review of the literature. Laryngoscope Investig. Otolaryngol. 2021, 6, 857–865. [Google Scholar] [CrossRef]
- Park, S.B.; Lee, J.H.; Weon, Y.C. Imaging findings of head and neck inflammatory pseudotumor. AJR Am. J. Roentgenol. 2009, 193, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Boedeker, C.C.; Kayser, G.; Ridder, G.J.; Maier, W.; Schipper, J. Giant-cell reparative granuloma of the temporal bone: A case report and review of the literature. Ear Nose Throat J. 2003, 82, 926–937. [Google Scholar] [CrossRef]
- Martinez, A.P.; Torres-Mora, J. Selected Giant Cell Rich Lesions of the Temporal Bone. Head Neck Pathol. 2018, 12, 367–377. [Google Scholar] [CrossRef]
- Zagaria, A.; Nicastro, V.; Abita, P.; Freni, F.; Galletti, F. Adenomatous Neuroendocrine Tumors of the Middle Ear in a Young Man With Conductive Hearing Loss. J. Craniofacial Surg. 2021, 32, e432–e434. [Google Scholar] [CrossRef]
- Shishido, T.; Ikeda, R.; Suzuki, J.; Honkura, Y.; Koshiba, Y.; Watarai, G.; Kanbayashi, T.; Hatano, M.; Yamauchi, D.; Kawase, T.; et al. Middle ear adenoma with facial palsy: A case report and a review of the literature. Auris Nasus Larynx 2022, 49, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, J.; Onda, K.; Tanaka, R.; Takahashi, H. Giant cell reparative granuloma of the temporal bone: Neuroradiological and immunohistochemical findings. Neurol. Med.-Chir. 2002, 42, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Maerki, J.; Riddle, N.D.; Newman, J.; Husson, M.A.; Lee, J.Y. Giant cell granuloma of the temporal bone in a mixed martial arts fighter. J. Neurol. Surg. Rep. 2012, 73, 60–63. [Google Scholar] [CrossRef]
- Takata, Y.; Hidaka, H.; Ishida, K.; Kobayashi, T. Giant cell reparative granuloma of the temporal bone successfully resected with preservation of hearing. J. Laryngol. Otol. 2013, 127, 716–720. [Google Scholar] [CrossRef]
- Williams, J.C.; Thorell, W.E.; Treves, J.S.; Fidler, M.E.; Moore, G.F.; Leibrock, L.G. Giant cell reparative granuloma of the petrous temporal bone: A case report and literature review. Skull Base Surg. 2000, 10, 89–93. [Google Scholar] [CrossRef]
- Menge, M.; Maier, W.; Feuerhake, F.; Kaminsky, J.; Pfeiffer, J. Giant cell reparative granuloma of the temporal bone. Acta Neurochir. 2009, 151, 397–399. [Google Scholar] [CrossRef]
- Hu, H.; Lim, W.Y.; Tan, T.Y.; Yuen, H.W. Neuroendocrine adenoma of middle ear with new bone formation and review of literature. Am. J. Otolaryngol. 2016, 37, 108–111. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y. Salivary gland choristoma of the middle ear. Ear Nose Throat J. 2015, 94, E9–E12. [Google Scholar] [CrossRef] [PubMed]
- Jančíková, J.; Šikolová, S.; Machač, J.; Ježová, M.; Pavlovská, D.; Urík, M. Salivary Gland Choristoma of the Middle Ear in a Child: A Case Report. Ear Nose Throat J. 2021, 100, 356s–359s. [Google Scholar] [CrossRef]
- Fujima, N.; Andreu-Arasa, V.C.; Onoue, K.; Weber, P.C.; Hubbell, R.D.; Setty, B.N.; Sakai, O. Utility of deep learning for the diagnosis of otosclerosis on temporal bone CT. Eur. Radiol. 2021, 31, 5206–5211. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, O.; Eroğlu, Y.; Yıldırım, M.; Karlıdag, T.; Çınar, A.; Akyiğit, A.; Kaygusuz, İ.; Yıldırım, H.; Keleş, E.; Yalçın, Ş. Is it useful to use computerized tomography image-based artificial intelligence modelling in the differential diagnosis of chronic otitis media with and without cholesteatoma? Am. J. Otolaryngol. 2022, 43, 103395. [Google Scholar] [CrossRef] [PubMed]
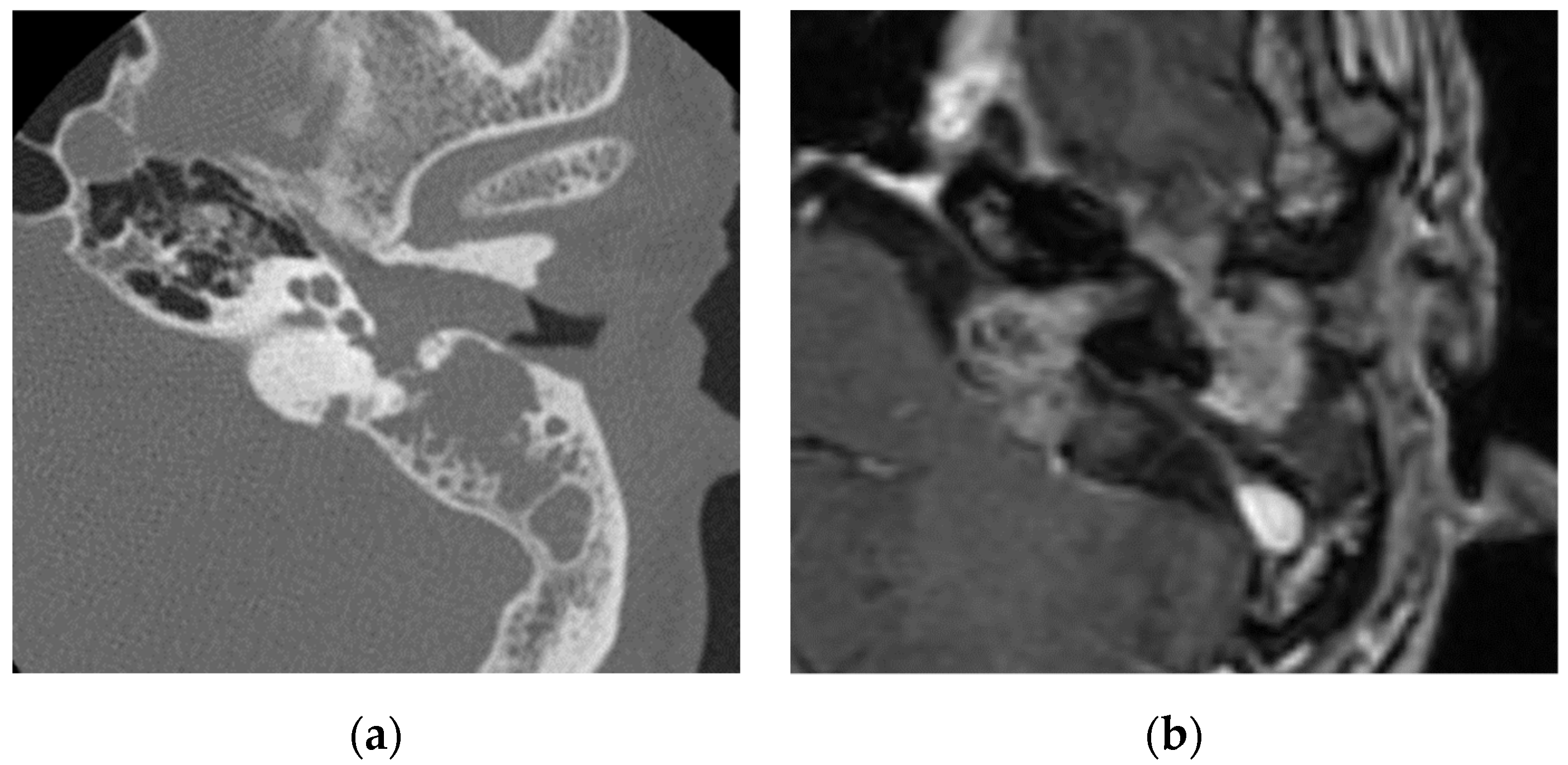
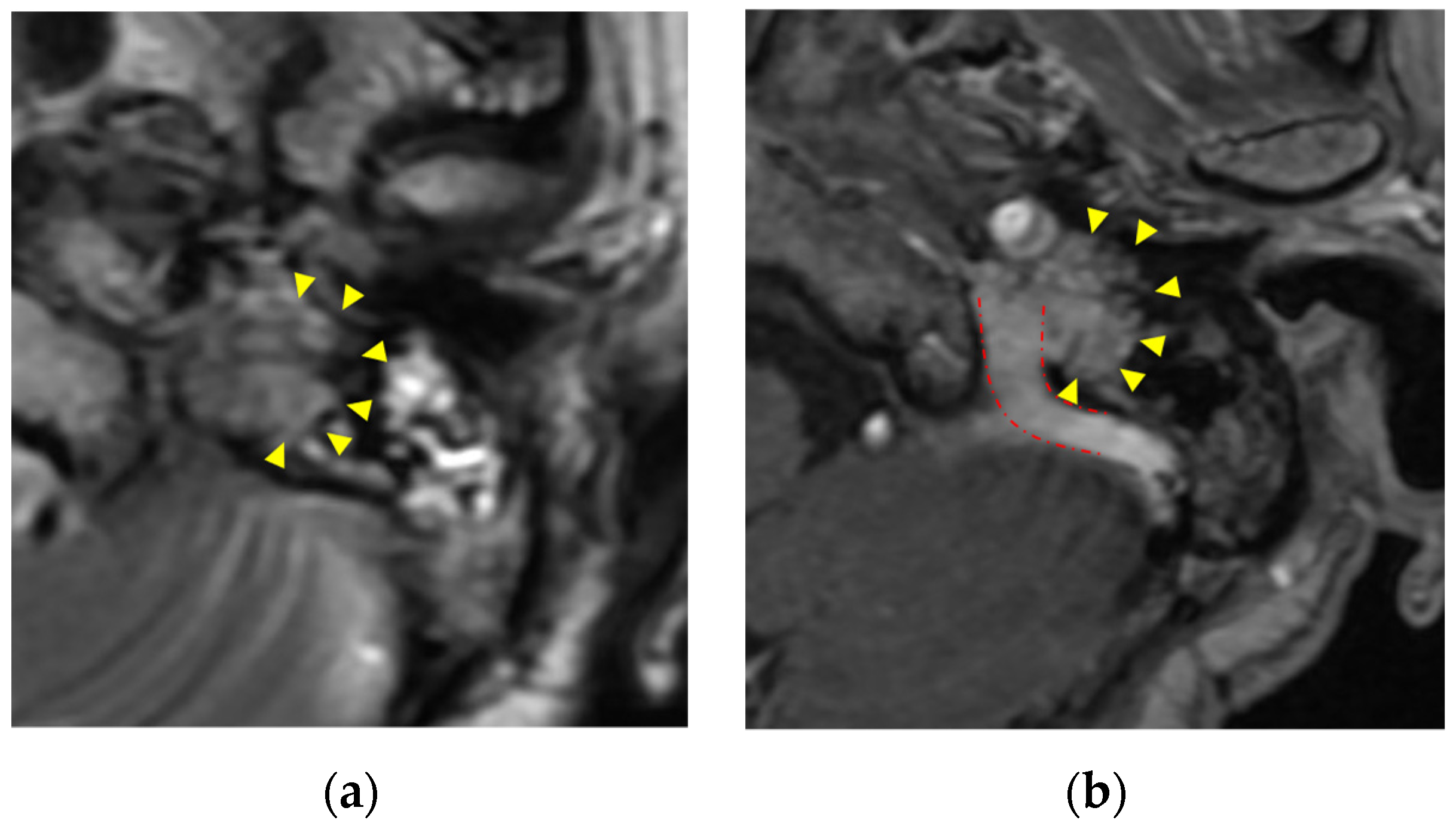
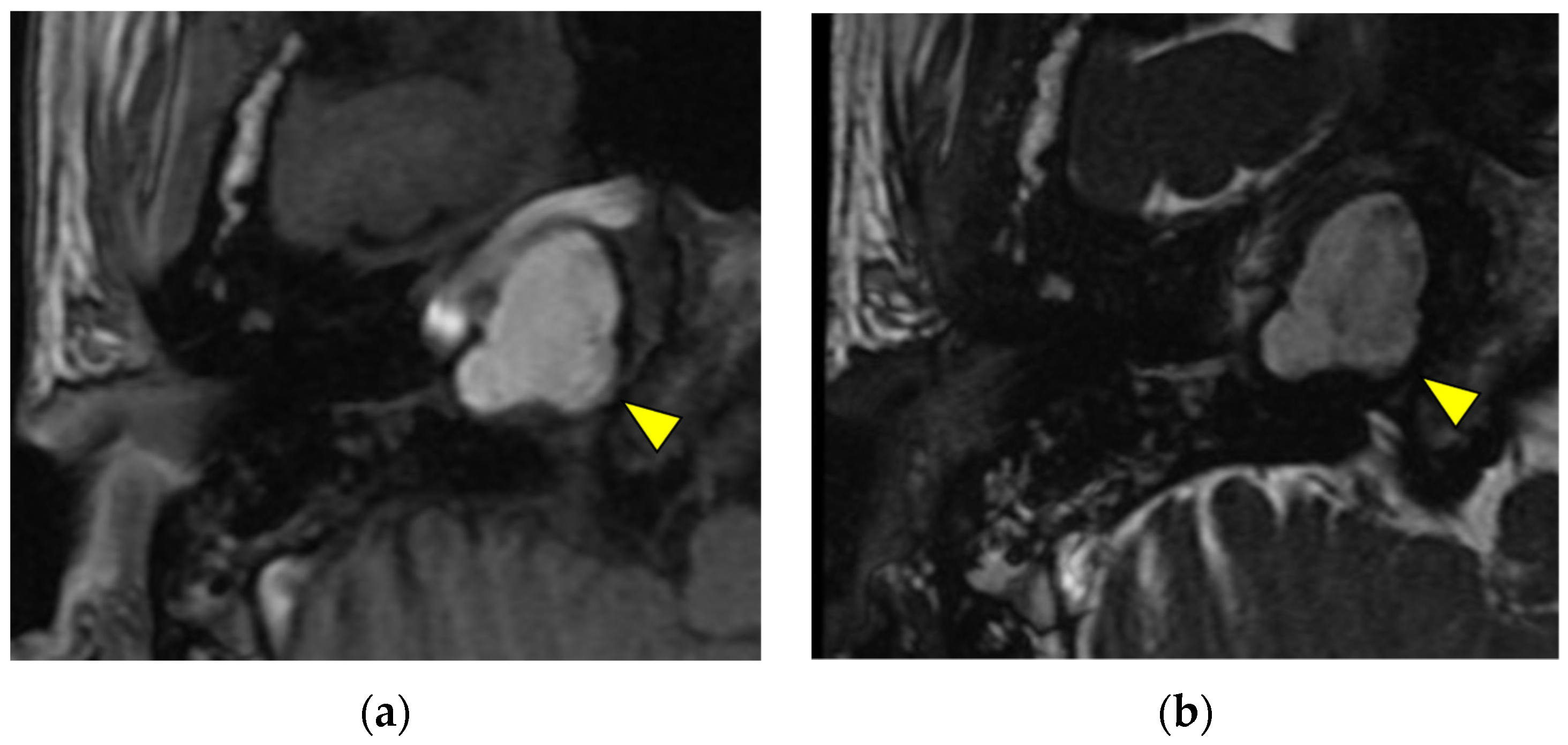
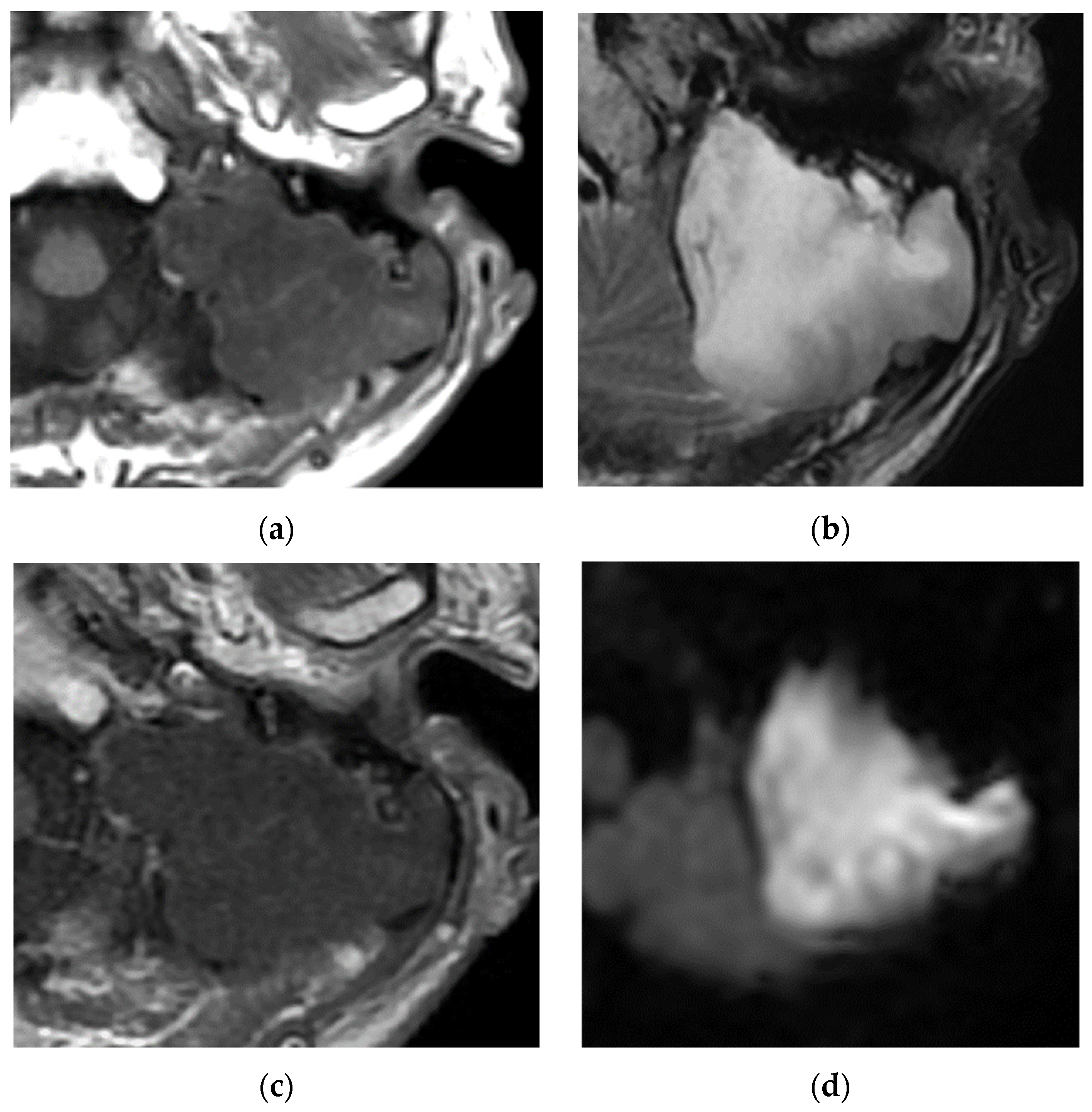
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimanuki, M.N.; Nishiyama, T.; Hosoya, M.; Wakabayashi, T.; Ozawa, H.; Oishi, N. Imaging of Temporal Bone Mass Lesions: A Pictorial Review. Diagnostics 2023, 13, 2665. https://doi.org/10.3390/diagnostics13162665
Shimanuki MN, Nishiyama T, Hosoya M, Wakabayashi T, Ozawa H, Oishi N. Imaging of Temporal Bone Mass Lesions: A Pictorial Review. Diagnostics. 2023; 13(16):2665. https://doi.org/10.3390/diagnostics13162665
Chicago/Turabian StyleShimanuki, Marie N., Takanori Nishiyama, Makoto Hosoya, Takeshi Wakabayashi, Hiroyuki Ozawa, and Naoki Oishi. 2023. "Imaging of Temporal Bone Mass Lesions: A Pictorial Review" Diagnostics 13, no. 16: 2665. https://doi.org/10.3390/diagnostics13162665
APA StyleShimanuki, M. N., Nishiyama, T., Hosoya, M., Wakabayashi, T., Ozawa, H., & Oishi, N. (2023). Imaging of Temporal Bone Mass Lesions: A Pictorial Review. Diagnostics, 13(16), 2665. https://doi.org/10.3390/diagnostics13162665






