[18F]-FDHT PET for the Imaging of Androgen Receptor in Prostate and Breast Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
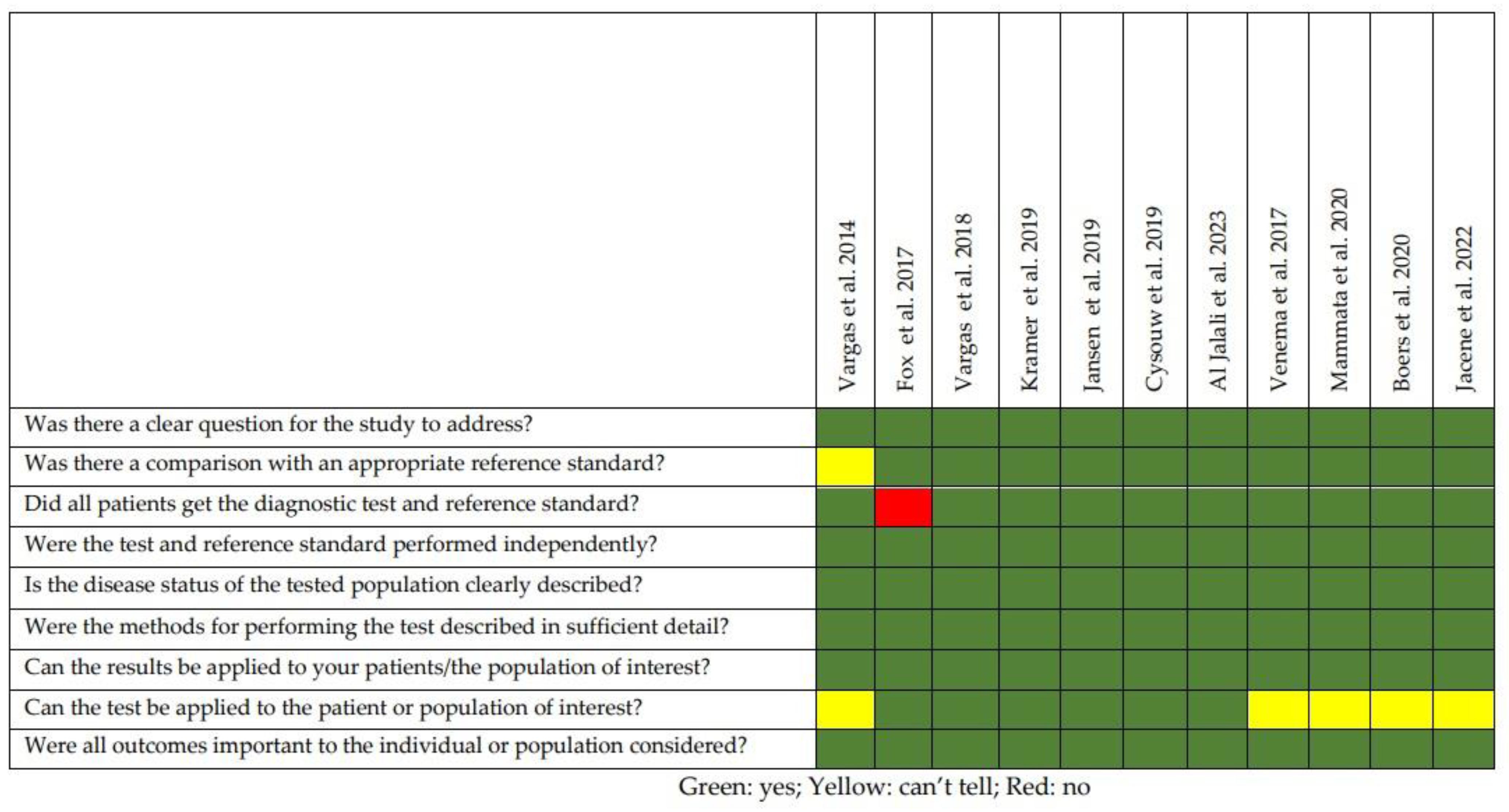
2.2. Quality of the Selected Studies
3. Results
3.1. Analysis of the Evidence
3.2. Prostate Cancer
3.3. Breast Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- De Silva, F.; Alcorn, J. A Tale of Two Cancers: A Current Concise Overview of Breast and Prostate Cancer. Cancers 2022, 14, 2954. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Naylor, M.J. The Influence of Modifiable Factors on Breast and Prostate Cancer Risk and Disease Progression. Front. Physiol. 2022, 13, 840826. [Google Scholar] [CrossRef] [PubMed]
- Michmerhuizen, A.R.; Spratt, D.E.; Pierce, L.J.; Speers, C.W. ARe We There yet? Understanding Androgen Receptor Signaling in Breast Cancer. NPJ Breast Cancer 2020, 6, 47. [Google Scholar] [CrossRef]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Knudsen, K.E.; Scher, H.I. Starving the Addiction: New Opportunities for Durable Suppression of AR Signaling in Prostate Cancer. Clin. Cancer Res. 2009, 15, 4792–4798. [Google Scholar] [CrossRef]
- Estrada, M.; Espinosa, A.; Müller, M.; Jaimovich, E. Testosterone Stimulates Intracellular Calcium Release and Mitogen-Activated Protein Kinases via a G Protein-Coupled Receptor in Skeletal Muscle Cells. Endocrinology 2003, 144, 3586–3597. [Google Scholar] [CrossRef]
- Imamura, Y.; Sadar, M.D. Androgen Receptor Targeted Therapies in Castration-Resistant Prostate Cancer: Bench to Clinic. Int. J. Urol. 2016, 23, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-Targeted Therapy in Men with Prostate Cancer: Evolving Practice and Future Considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38. [Google Scholar] [CrossRef]
- Tesei, A.; Castoria, G. Editorial: The Androgen Receptor in Breast Cancer. Front. Endocrinol. 2020, 11, 636480. [Google Scholar] [CrossRef]
- Barbet, J.; Bernaudin, M.; Payoux, P.; Cicone, F.; Gaugler, M.-H.; Kraeber-Bodéré, F. Editorial: Nuclear Medicine in the Context of Personalized Medicine. Front. Med. 2020, 7, 252. [Google Scholar] [CrossRef]
- Solnes, L.B.; Werner, R.A.; Jones, K.M.; Sadaghiani, M.S.; Bailey, C.R.; Lapa, C.; Pomper, M.G.; Rowe, S.P. Theranostics: Leveraging Molecular Imaging and Therapy to Impact Patient Management and Secure the Future of Nuclear Medicine. J. Nucl. Med. 2020, 61, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Cimini, A.; Ricci, M.; Chiaravalloti, A.; Filippi, L.; Schillaci, O. Theragnostic Aspects and Radioimmunotherapy in Pediatric Tumors. Int. J. Mol. Sci. 2020, 21, 3849. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Bagni, O. The Potential of PSMA-Targeted Alpha Therapy in the Management of Prostate Cancer. Expert Rev. Anticancer Ther. 2020, 20, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Kairemo, K.; Hodolic, M. Androgen Receptor Imaging in the Management of Hormone-Dependent Cancers with Emphasis on Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 8235. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Vargas, H.A.; Wassberg, C.; Fox, J.J.; Wibmer, A.; Goldman, D.A.; Kuk, D.; Gonen, M.; Larson, S.M.; Morris, M.J.; Scher, H.I.; et al. Bone Metastases in Castration-Resistant Prostate Cancer: Associations between Morphologic CT Patterns, Glycolytic Activity, and Androgen Receptor Expression on PET and Overall Survival. Radiology 2014, 271, 220–229. [Google Scholar] [CrossRef]
- Fox, J.J.; Gavane, S.C.; Blanc-Autran, E.; Nehmeh, S.; Gönen, M.; Beattie, B.; Vargas, H.A.; Schöder, H.; Humm, J.L.; Fine, S.W.; et al. Positron Emission Tomography/Computed Tomography-Based Assessments of Androgen Receptor Expression and Glycolytic Activity as a Prognostic Biomarker for Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2018, 4, 217–224. [Google Scholar] [CrossRef]
- Vargas, H.A.; Kramer, G.M.; Scott, A.M.; Weickhardt, A.; Meier, A.A.; Parada, N.; Beattie, B.J.; Humm, J.L.; Staton, K.D.; Zanzonico, P.B.; et al. Reproducibility and Repeatability of Semiquantitative 18F-Fluorodihydrotestosterone Uptake Metrics in Castration-Resistant Prostate Cancer Metastases: A Prospective Multicenter Study. J. Nucl. Med. 2018, 59, 1516–1523. [Google Scholar] [CrossRef]
- Kramer, G.M.; Yaqub, M.; Vargas, H.A.; Schuit, R.C.; Windhorst, A.D.; van den Eertwegh, A.J.M.; van der Veldt, A.A.M.; Bergman, A.M.; Burnazi, E.M.; Lewis, J.S.; et al. Assessment of Simplified Methods for Quantification of 18F-FDHT Uptake in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2019, 60, 1221–1227. [Google Scholar] [CrossRef]
- Jansen, B.H.E.; Kramer, G.M.; Cysouw, M.C.F.; Yaqub, M.M.; de Keizer, B.; Lavalaye, J.; Booij, J.; Vargas, H.A.; Morris, M.J.; Vis, A.N.; et al. Healthy Tissue Uptake of 68Ga-Prostate-Specific Membrane Antigen, 18F-DCFPyL, 18F-Fluoromethylcholine, and 18F-Dihydrotestosterone. J. Nucl. Med. 2019, 60, 1111–1117. [Google Scholar] [CrossRef]
- Cysouw, M.C.F.; Kramer, G.M.; Heijtel, D.; Schuit, R.C.; Morris, M.J.; van den Eertwegh, A.J.M.; Voortman, J.; Hoekstra, O.S.; Oprea-Lager, D.E.; Boellaard, R. Sensitivity of 18F-Fluorodihydrotestosterone PET-CT to Count Statistics and Reconstruction Protocol in Metastatic Castration-Resistant Prostate Cancer. EJNMMI Res. 2019, 9, 70. [Google Scholar] [CrossRef]
- Al Jalali, V.; Wasinger, G.; Rasul, S.; Grubmüller, B.; Wulkersdorfer, B.; Balber, T.; Mitterhauser, M.; Simon, J.; Hacker, M.; Shariat, S.; et al. Consecutive Prostate-Specific Membrane Antigen (PSMA) and Antigen Receptor (AR) PET Imaging Shows Positive Correlation with AR and PSMA Protein Expression in Primary Hormone-Naïve Prostate Cancer. J. Nucl. Med. 2023, 64, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Venema, C.M.; Mammatas, L.H.; Schröder, C.P.; van Kruchten, M.; Apollonio, G.; Glaudemans, A.W.J.M.; Bongaerts, A.H.H.; Hoekstra, O.S.; Verheul, H.M.W.; Boven, E.; et al. Androgen and Estrogen Receptor Imaging in Metastatic Breast Cancer Patients as a Surrogate for Tissue Biopsies. J. Nucl. Med. 2017, 58, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Mammatas, L.H.; Venema, C.M.; Schröder, C.P.; de Vet, H.C.W.; van Kruchten, M.; Glaudemans, A.W.J.M.; Yaqub, M.M.; Verheul, H.M.W.; Boven, E.; van der Vegt, B.; et al. Visual and Quantitative Evaluation of [18F]FES and [18F]FDHT PET in Patients with Metastatic Breast Cancer: An Interobserver Variability Study. EJNMMI Res. 2020, 10, 40. [Google Scholar] [CrossRef]
- Boers, J.; Venema, C.M.; de Vries, E.F.J.; Hospers, G.A.P.; Boersma, H.H.; Rikhof, B.; Dorbritz, C.; Glaudemans, A.W.J.M.; Schröder, C.P. Serial [18F]-FDHT-PET to Predict Bicalutamide Efficacy in Patients with Androgen Receptor Positive Metastatic Breast Cancer. Eur. J. Cancer 2021, 144, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Jacene, H.; Liu, M.; Cheng, S.-C.; Abbott, A.; Dubey, S.; McCall, K.; Young, D.; Johnston, M.; Van den Abbeele, A.D.; Overmoyer, B. Imaging Androgen Receptors in Breast Cancer with 18F-Fluoro-5α-Dihydrotestosterone PET: A Pilot Study. J. Nucl. Med. 2022, 63, 22–28. [Google Scholar] [CrossRef]
- Kaalep, A.; Sera, T.; Oyen, W.; Krause, B.J.; Chiti, A.; Liu, Y.; Boellaard, R. EANM/EARL FDG-PET/CT Accreditation—Summary Results from the First 200 Accredited Imaging Systems. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 412–422. [Google Scholar] [CrossRef] [PubMed]
- van Sluis, J.; van Snick, J.H.; Brouwers, A.H.; Noordzij, W.; Dierckx, R.A.J.O.; Borra, R.J.H.; Slart, R.H.J.A.; Lammertsma, A.A.; Glaudemans, A.W.J.M.; Boellaard, R.; et al. EARL Compliance and Imaging Optimisation on the Biograph Vision Quadra PET/CT Using Phantom and Clinical Data. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4652–4660. [Google Scholar] [CrossRef]
- Urso, L.; Frantellizzi, V.; De Vincentis, G.; Schillaci, O.; Filippi, L.; Evangelista, L. Clinical Applications of Long Axial Field-of-View PET/CT Scanners in Oncology. Clin. Transl. Imaging 2023, 11, 365–380. [Google Scholar] [CrossRef]
- Alongi, P.; Laudicella, R.; Lanzafame, H.; Farolfi, A.; Mapelli, P.; Picchio, M.; Burger, I.A.; Iagaru, A.; Minutoli, F.; Evangelista, L. PSMA and Choline PET for the Assessment of Response to Therapy and Survival Outcomes in Prostate Cancer Patients: A Systematic Review from the Literature. Cancers 2022, 14, 1770. [Google Scholar] [CrossRef]
- Urso, L.; Lancia, F.; Ortolan, N.; Frapoli, M.; Rauso, M.; Artioli, P.; Cittanti, C.; Uccelli, L.; Frassoldati, A.; Evangelista, L.; et al. 18F-Choline PET/CT or PET/MR and the Evaluation of Response to Systemic Therapy in Prostate Cancer: Are We Ready? Clin. Transl. Imaging 2022, 10, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.C.; Cole, K.S.; Marotti, J.D.; Hu, R.; Schnitt, S.J.; Tamimi, R.M. Androgen Receptor Expression in Breast Cancer in Relation to Molecular Phenotype: Results from the Nurses’ Health Study. Mod. Pathol. 2011, 24, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Dalton, J.T. Androgen Receptor: A Complex Therapeutic Target for Breast Cancer. Cancers 2016, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Venema, C.M.; Bense, R.D.; Steenbruggen, T.G.; Nienhuis, H.H.; Qiu, S.-Q.; van Kruchten, M.; Brown, M.; Tamimi, R.M.; Hospers, G.A.P.; Schröder, C.P.; et al. Consideration of Breast Cancer Subtype in Targeting the Androgen Receptor. Pharmacol. Ther. 2019, 200, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.R.; Scher, H.I. Prostate Cancer: A Dynamic Illness with Shifting Targets. Lancet Oncol. 2003, 4, 407–414. [Google Scholar] [CrossRef]
- Mkango, S.B.; Shaban, N.; Mureithi, E.; Ngoma, T. Dynamics of Breast Cancer under Different Rates of Chemoradiotherapy. Comput. Math. Methods Med. 2019, 2019, 5216346. [Google Scholar] [CrossRef]
- De Vries, E.F.J.; Elsinga, P.H.; Tsoumpas, C. Will Extended Field-of-View PET/CT Depopulate the Graveyard of Failed PET Radiopharmaceuticals? Cancer Imaging 2022, 22, 70. [Google Scholar] [CrossRef]
- Filippi, L.; Dimitrakopoulou-Strauss, A.; Evangelista, L.; Schillaci, O. Long Axial Field-of-View PET/CT Devices: Are We Ready for the Technological Revolution? Expert Rev. Med. Devices 2022, 19, 739–743. [Google Scholar] [CrossRef]
- Filippi, L.; Urso, L.; Bianconi, F.; Palumbo, B.; Marzola, M.C.; Evangelista, L.; Schillaci, O. Radiomics and Theranostics with Molecular and Metabolic Probes in Prostate Cancer: Toward a Personalized Approach. Expert Rev. Mol. Diagn. 2023, 23, 243–255. [Google Scholar] [CrossRef]
- Filippi, L.; Bagni, O.; Schillaci, O. Digital PET/CT with 18F-FACBC in Early Castration-Resistant Prostate Cancer: Our Preliminary Results. Expert Rev. Med. Devices 2022, 19, 591–598. [Google Scholar] [CrossRef]
- Bauckneht, M.; Marini, C.; Cossu, V.; Campi, C.; Riondato, M.; Bruno, S.; Orengo, A.M.; Vitale, F.; Carta, S.; Chiola, S.; et al. Gene’s Expression Underpinning the Divergent Predictive Value of [18F]F-Fluorodeoxyglucose and Prostate-Specific Membrane Antigen Positron Emission Tomography in Primary Prostate Cancer: A Bioinformatic and Experimental Study. J. Transl. Med. 2023, 21, 3. [Google Scholar] [CrossRef] [PubMed]


| References | Location/Year | Study | N. of Patients | Setting | Median Age (Range) | Primary Endpoint | Device | Comment |
|---|---|---|---|---|---|---|---|---|
| Vargas et al. [16] | USA/2014 | Prospective, observational | 38 | mCRPC | 62.1 (43.1–76.0) | to compare CT, [18F]-FDHT, [18F]-FDG | PET/CT | The number of lesions detected on [18F]-FDG, [18F]-FDHT and CT has a prognostic impact on the OS, as well as the grade of [18F]-FDHT uptake in bone lesions. |
| Fox et al. [17] | USA/2017 | Prospective, observational | 133 | mCRPC | 68 (44–85) | To assess the prognostic role of [18F]-FDHT and [18F]-FDG in mCRPC submitted to ARSI | PET/CT | Four different phenotypes were identified. Lesions showing mismatch between [18F]-FDHT and [18F]-FDG (AR0Gly1 phenotype) had the poorest prognosis. |
| Vargas et al. [18] | USA/2018 | Prospective, observational | 27 | mCRPC | ---- | To assess reproducibility and repeatability of [18F]-FDHT PET/CT | PET/CT | Uptake metrics (particularly the SUVmax mean peak) derived from [18F]-FDHT PET/CT showed high reproducibility and repeatability. |
| Kramer et al. [19] | USA/2019 | Prospective, observational | 17 | mCRPC | 69 (58–85) | To investigate if simplified metrics can be used to measure [18F]-FDHT uptake in lesions | PET/CT | SUVBW corrected for sex hormone-binding globulin levels (SUVSHBG) may be used to quantify tracer uptake in lesions. |
| Jansen et al. [20] | the Netherlands/2019 | Centralized analysis of a multicenter data | 27 | mCRPC | 67 (64–69) | To assess the interpatient Variability of [18F]-FDHT uptake in healthy tissue | PET/CT | Low uptake variability was observed in all tissues, except the lungs. In particular, liver may be used as a reference region to characterize malignancies and standardize image interpretation |
| Cysouw et al. [21] | the Netherlands/2019 | Prospective | 14 | mCRPC | ---- | To investigate how count statistics and reconstruction protocol affect lesion [18F]-FDHT PET quality image | PET/CT | Count reduction resulted in higher intrascan variability, regardless of the reconstruction method (EARL-1 or -2). However, the count statistics could be reduced without impacting lesions’ detectability. |
| Al Jalali et al. [22] | 2023/Austria | Prospective, exploratory | 10 | mCRPC | 60 (54–67) | To measure the correlation between [18F]-FDHT and [68Ga]-PSMA-11 uptake in PC and the expression of AR and PSMA at immunohistochemical analysis | PET/MRI | Although [18F]-FDHT was less sensitive than [68Ga]-PSMA-11 for the detection of the primary PC, a strong and significant correlation was found between the [18F]-FDHT imaging signal and AR density. [18F]-FDHT PET may be helpful to monitor the changes in AR expression during targeted therapies. |
| Venema et al. [23] | 2017/Austria | Feasibility trial | 13 | mBC | To gauge the correlation between [18F]-FDHT and [18F]-FES imaging and the expression of AR and ER in tissues at at immunohistochemical analysis | PET/CT | A good correlation was found between the PET signal and AR density in tissues. The optimal cutoff for AR- positive lesions was an SUVmax of 1.94 for [18F]-FDHT PET. | |
| Mammatas et al. [24] | the Netherlands/2020 | Prospective | 10 | mBC | 67 * (48–79) | To compare the visual and quantitative variability of [18F]-FES PET and [18F]-FDHT PET interpretation | PET/CT | [18F]-FDHT PET/CT showed lower visual agreement for a lesion’s detection than [18F]-FES but a good quantitative concordance. |
| Boers et al. [25] | the Netherlands/2021 | Prospective | 21 | mBC (AR+/HER2-) | 65 * | To investigate the usefulness of [18F]-FDHT PET for monitoring mBC treated with bicalutamide | PET/CT | Although a bicalutamide-induced [18F]-FDHT reduction was found on the follow-up PET/CT in mBC patients, this change was not predictive of the response. |
| Jacene et al. [26] | USA/2022 | Prospective | 11 | mBC | 59 (47–73) | To assess the role of [18F]-FDHT PET for monitoring mBC submitted to selective androgen receptor modulators (SARMs). | PET/CT | Patients showing a clinical benefit from SARMs showed a trend towards a progressive reduction in lesions’ [18F]-FDHT uptake on longitudinal PET/CT studies, with respect to subjects with a progressive disease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippi, L.; Urso, L.; Schillaci, O.; Evangelista, L. [18F]-FDHT PET for the Imaging of Androgen Receptor in Prostate and Breast Cancer: A Systematic Review. Diagnostics 2023, 13, 2613. https://doi.org/10.3390/diagnostics13152613
Filippi L, Urso L, Schillaci O, Evangelista L. [18F]-FDHT PET for the Imaging of Androgen Receptor in Prostate and Breast Cancer: A Systematic Review. Diagnostics. 2023; 13(15):2613. https://doi.org/10.3390/diagnostics13152613
Chicago/Turabian StyleFilippi, Luca, Luca Urso, Orazio Schillaci, and Laura Evangelista. 2023. "[18F]-FDHT PET for the Imaging of Androgen Receptor in Prostate and Breast Cancer: A Systematic Review" Diagnostics 13, no. 15: 2613. https://doi.org/10.3390/diagnostics13152613
APA StyleFilippi, L., Urso, L., Schillaci, O., & Evangelista, L. (2023). [18F]-FDHT PET for the Imaging of Androgen Receptor in Prostate and Breast Cancer: A Systematic Review. Diagnostics, 13(15), 2613. https://doi.org/10.3390/diagnostics13152613








