Abstract
Assessing coronary physiology after stent implantation facilitates the optimisation of percutaneous coronary intervention (PCI). Coronary artery disease (CAD) patterns can be characterised by the pullback pressure gradient (PPG) index. The impact of focal vs. diffuse disease on physiology-guided incremental optimisation strategy (PIOS) is unknown. This is a sub-study of the TARGET-FFR randomized clinical trial (NCT03259815). The study protocol directed that optimisation be attempted for patients in the PIOS arm when post-PCI FFR was <0.90. Overall, 114 patients (n = 61 PIOS and 53 controls) with both pre-PCI fractional flow reserve (FFR) pullbacks and post-PCI FFR were included. A PPG ≥ 0.74 defined focal CAD. The PPG correlated significantly with post-PCI FFR (r = 0.43; 95% CI 0.26 to 0.57; p-value < 0.001) and normalised delta FFR (r = 0.49; 95% CI 0.34 to 0.62; p-value < 0.001). PIOS was more frequently applied to vessels with diffuse CAD (6% focal vs. 42% diffuse; p-value = 0.006). In patients randomized to PIOS, those with focal disease achieved higher post-PCI FFR than patients with diffuse CAD (0.93 ± 0.05 vs. 0.83 ± 0.07, p < 0.001). There was a significant interaction between CAD patterns and the randomisation arm for post-PCI FFR (p-value for interaction = 0.004). Physiology-guided stent optimisation was applied more frequently to vessels with diffuse disease; however, patients with focal CAD at baseline achieved higher post-PCI FFR.
1. Introduction
The optimisation of percutaneous coronary intervention (PCI) is advocated to reduce stent failure and improve patient outcomes [1]. After stent implantation, intravascular imaging can be used to optimise stent expansion, correct malapposition, and detect residual disease [2,3]. Fractional flow reserve (FFR) can also be used to optimise PCI by identifying physiologically sub-optimal results and guide additional intervention [4,5]. Performing additional interventions in segments of residual pressure losses translates into higher post-PCI FFR values, which may positively influence prognosis [4,6,7]. Recently, the TARGET-FFR (Trial of Angiography vs. Pressure-Ratio-Guided Enhancement Techniques-Fractional Flow Reserve) trial assessed the efficacy of a routine post-PCI physiology-guided incremental optimisation strategy (PIOS) in achieving optimal post-PCI FFR results (FFR ≥ 0.90). Patients randomized to the PIOS arm with post-PCI FFR < 0.90 were treated according to a pre-specified protocol based on the findings of the post-PCI FFR pullback (Figure S1). Whilst PIOS improved post-PCI FFR and reduced the proportion of patients with a post-PCI FFR ≤ 0.80, there was no significant difference between groups in the proportion of patients achieving an optimal post-PCI FFR value of ≥0.90 [5]. The pullback pressure gradient (PPG) is a new metric derived from FFR pullbacks that can differentiate focal from diffuse coronary artery disease (CAD) [8]. Although CAD patterns impact post-PCI physiology, their influence of PCI optimisation is unknown. Thus, we sought to investigate the impact of baseline CAD patterns, defined by the PPG, on physiology-guided PCI optimisation in the TARGET-FFR trial.
2. Materials and Methods
2.1. Study Population
This study is a post hoc analysis of the TARGET-FFR study. Briefly, TARGET-FFR was a prospective, single-centre, randomized, controlled, parallel-group, blinded clinical trial conducted at the Golden Jubilee National Hospital in Glasgow, UK [5]. The study is registered at ClinicalTrials.gov identifier NCT03259815. Patients undergoing PCI for either stable angina, medically stabilised non-ST-segment elevation myocardial infarction (NSTEMI) or staged completion of non-culprit vessel revascularization following either NSTEMI or ST-segment elevation myocardial infarction (STEMI) were eligible for inclusion. A list of the inclusion and exclusion criteria is provided in Table S1. All patients signed informed consent before any study procedure. Patients were randomized to either PIOS or control group [5]. For the present analysis, patients with both pre-PCI FFR pullbacks and post-PCI FFR were considered for inclusion. Coronary physiology data were analysed by a core laboratory (CoreAalst BV, Aalst, Belgium).
2.2. Procedures
The details of the coronary physiology measurements and PCI have been described previously [5]. FFR measurements were performed using the PressureWire X Guidewire (Abbott Laboratories, Abbott Park, IL, USA). Following administration of a 200 μg bolus of intracoronary nitrate, the pressure wire sensor was positioned at the tip of the guide catheter and equalised with the aortic pressure. The pressure wire was then advanced to position the sensor in the distal third of the vessel. Hyperaemia was induced by infusion of adenosine into an antecubital vein at a rate of 140 μg/kg/min [9]. Pre-PCI FFR pullbacks were performed manually, and their use and interpretation were left to the operator’s discretion. After angiographically successful PCI, patients were randomized 1:1 to either the PIOS or control groups. In patients randomized to the PIOS group with post-PCI FFR < 0.90, operators planned additional interventions based on the findings of the post-PCI FFR pullback according to the incremental optimisation protocol (Figure S1). Following these additional optimisation measures, FFR was re-assessed, and the procedure was completed. In the control group, post-PCI FFR and pullback information were acquired but concealed from the operator. The use of adjunctive intravascular imaging was left to the operator’s discretion.
2.3. Characterisation of CAD Patterns
The PPG index was calculated off-line from the manual pre-PCI FFR pullbacks. Details of the PPG calculation have been described in detail elsewhere [8]. In brief, the PPG combines two parameters extracted from FFR pullback curves: (1) the maximal pressure gradient over 20% of the pullback duration and (2) the length of functional disease computed using a FFR threshold per unit of time. PPG values close to 1 represent focal disease, and values close to 0 characterise diffuse CAD. The PPG was calculated using a commercially available console (Coroflow v3.5, Coroventis Research AP, Uppsala, Sweden). Pressure tracings with ventricularisation, absence of a dicrotic notch, drift of more than 0.05 FFR units, unstable hyperaemic conditions during the pullback manoeuvre, pullback duration less than 15 s, and pullback curves with major artifacts were excluded. Delta FFR was normalised by pre-PCI FFR ((final post-PCI FFR minus pre-PCI FFR divided by one minus pre-PCI FFR) by a factor of one hundred). Post-PCI FFR pullbacks were also quantitatively analysed to determine the magnitude of residual focal pressure gradients. The residual PPG was defined as the maximal pressure gradient, in absolute FFR units, over 20% of the duration of the pullback curve (i.e., the first component of the PPG equation). To assess the outcomes of PIOS stratified by CAD patterns, the population was divided into tertiles according to the baseline PPG distribution, and the highest tertile was considered to be focal disease, whereas the intermediate and low tertiles, comprising patients with both mixed (focal and diffuse) and diffuse CAD, were considered to be diffuse disease [10]. The objective of the present analysis was to investigate the effect of PPG-defined focal vs. diffuse disease on the efficacy of PIOS in improving post-PCI FFR.
2.4. Statistical Analysis
Data are expressed as mean ± SD and median (interquartile range) for normally and non-normally distributed data, respectively. Categorical variables are expressed as frequencies and percentages (%). Continuous variables were compared using Student’s t-test (or Mann–Whitney tests as appropriate), and categorical variables were compared using the Chi-square test (or Fisher’s exact test as appropriate). Differences across the groups were compared with the Kruskal–Wallis H test. Predictors of the post-PCI FFR value were assessed using univariate and multivariate regression analyses. The variables included in the multivariate analysis were selected based on their known association with final post-PCI FFR [11,12]. Formal interaction testing was performed between the randomization arm (PIOS or controls) and the PPG for the outcome of post-PCI FFR. Receiver operating characteristic (ROC) curve analyses were used to assess the capacity of the residual PPG for predicting final post-PCI FFR ≥ 0.90. All analyses were performed in the intention-to-treat population in patients with pre-PCI FFR pullbacks suitable for PPG calculation. A two-sided p-value of 0.05 or less was considered statistically significant. All statistical analyses were performed with R statistical software (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Study Population
Between February 2018 and November 2019, 260 patients were randomized. Of these, 192 patients had pre-PCI FFR pullbacks (PIOS: n = 98; control: n = 94). Finally, 114 patients (61 patients in the PIOS group and 53 patients in the control group) with pullbacks of sufficient quality for PPG calculation were included in the analysis. The study flowchart is shown in Figure S2.
3.2. Baseline Characteristics
Baseline clinical, procedural, and functional characteristics stratified by the randomization arms are shown in Table 1. The mean age was 59.8 ± 8.1 years, 85.1% were male, and the LAD was the most frequently treated vessel in 63.2%. There were no significant differences in clinical, procedural, or functional baseline characteristics between PIOS and controls. Overall, the mean FFR increased after PCI from 0.62 ± 0.14 to 0.85 ± 0.07 (p < 0.001). There was no difference in the final post-PCI FFR between PIOS and controls (0.86 ± 0.08 vs. 0.85 ± 0.07; p-value = 0.27).

Table 1.
Baseline characteristics.
3.3. Baseline CAD Patterns and Final Post-PCI FFR
The mean PPG value was 0.65 ± 0.14, and focal disease was defined as PPG ≥ 0.74 (highest tertile). The PPG was moderately correlated with normalised delta FFR and final post-PCI FFR (Figure 1). Patients with focal disease achieved significantly larger changes in FFR after PCI leading to higher final post-PCI FFR than patients with diffuse CAD (normalised delta FFR 72.0 ± 20.3% in focal vs. 52.5 ± 19.2% in diffuse, p < 0.001). In addition, patients with focal CAD achieved higher post-PCI CFR (Table S2). The proportion of patients achieving optimal final post-PCI FFR ≥ 0.90 was significantly higher in focal disease (52.6% vs. 15.8%; p-value < 0.001). In the multivariate analysis, pre-PCI FFR and the PPG were independently associated with final post-PCI FFR (Table S3).
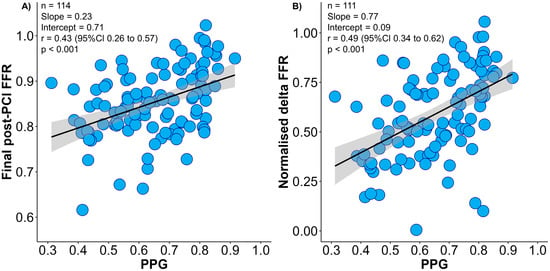
Figure 1.
Correlation between pull-back pressure gradient (PPG) and final post-PCI fractional frow reserve (FFR) and normalised delta FFR. Panel (A) shows the relationship between the PPG (x-axis) and final post-PCI FFR (y-axis). Panel (B) shows the relationship between the PPG (x-axis) and normalised delta FFR (y-axis). PPG had significant correlations with both final post-PCI FFR and normalised delta FFR. Delta FFR was normalised by pre-PCI FFR (post-PCI FFR minus pre-PCI FFR divided by 1 − pre-PCI FFR). FFR = Fractional flow reserve; PCI = percutaneous coronary intervention; PPG = pullback pressure gradient.
3.4. Stent Optimisation in Focal and Diffuse Disease
Post-PCI physiological results stratified by CAD patterns and the randomisation arm are shown in Table 2. Immediately after stenting, patients with focal CAD had higher post-PCI FFR compared to diffuse disease (0.93 ± 0.05 focal PIOS vs. 0.83 ± 0.07 diffuse PIOS vs. 0.87 ± 0.07 focal controls vs. 0.83 ± 0.07 diffuse controls). In the PIOS arm, optimisation was applied more frequently to vessels with diffuse CAD (6% (1/18) vs. 42% (18/43); p-value = 0.006). In the 18 patients with diffuse CAD in the PIOS group who received additional optimisation, PIOS, FFR improved from 0.76 ± 0.09 to 0.83 ± 0.05 (p-value < 0.01; Figure 2 and Table S4). However, when comparing post-PCI FFR values in patients with diffuse disease, PIOS did not result in higher post-PCI FFR compared to controls (0.83 ± 0.07 PIOS vs. 0.83 ± 0.07 control; p-value = 0.90, Figure S3). In contrast, patients with focal CAD achieved higher post-PCI FFR, and those with focal CAD randomized to PIOS achieved significantly higher final post-PCI FFR (0.93 ± 0.05 focal PIOS vs. 0.83 ± 0.07 diffuse PIOS vs. 0.87 ± 0.07 focal controls vs. 0.83 ± 0.07 diffuse controls; p-value < 0.001; Figure S3). Moreover, there was a significant interaction between PPG and the randomization arm for post-PCI FFR (p-value for interaction = 0.004; Figure 3). The proportion of patients achieving post-PCI FFR ≥ 0.90 stratified by CAD patterns and the randomization arm is shown in Figure S4.

Table 2.
Comparison of functional characteristics between randomized groups stratified by physiological pattern of CAD.
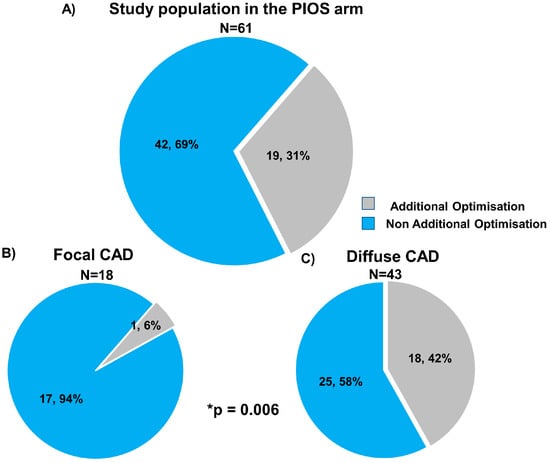
Figure 2.
Use of physiology-guided optimisation stratified by focal and diffuse coronary artery disease. Panel (A) shows the frequency of additional optimisation in overall population in the PIOS arm (grey colour). Panel (B) shows the frequency of additional optimisation in focal CAD, and panel (C) shows the frequency of additional optimisation in diffuse CAD. CAD = Coronary artery disease; PIOS = physiology-guided incremental optimisation strategy. * Comparison frequency of additional optimisation between focal and diffuse CAD. Categorical variables are expressed as number and percentage n, %.
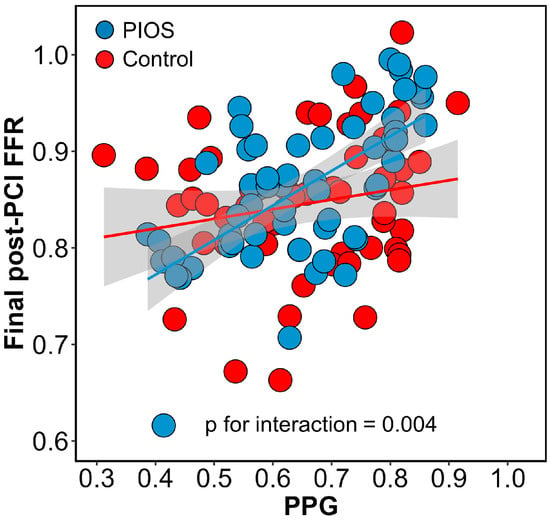
Figure 3.
Interaction between pullback pressure gradient (PPG) and randomization arms. Interaction between PPG and randomization arms for final post-PCI FFR. The blue dots and line represent patients in the PIOS arm, and the red dots and line represent the control arm. There was a significant interaction between randomization groups. FFR = Fractional flow reserve; PCI = percutaneous coronary intervention; PIOS = physiology-guided incremental optimisation strategy; PPG = pullback pressure gradient.
3.5. Residual PPG and Final Post-PCI FFR
The mean residual diameter stenosis was 15.0 ± 8.8%, and there was no difference between focal and diffuse CAD (16.0 ± 9.9% focal vs. 14.5 ± 8.2% diffuse; p-value = 0.41) or between PIOS and controls (14.5 ± 9.2% PIOS vs. 15.6 ± 8.4% control; p-value = 0.50). The mean residual PPG derived from post-PCI FFR pullbacks was 0.07 ± 0.04. The residual PPG conditioned final post-PCI FFR (R2 = 0.64, 95% CI 0.50 to 0.74, p < 0.001; Figure 4A). The residual PPG was not significantly different between patients randomized to PIOS versus controls (0.06 ± 0.04 PIOS vs. 0.07 ± 0.04 controls; p-value = 0.06). However, patients with focal CAD randomized to PIOS had the lowest residual PPG (0.04 ± 0.02 focal PIOS vs. 0.07 ± 0.04 focal control, 0.07 ± 0.04 diffuse PIOS vs. 0.08 ± 0.04 diffuse control; ANOVA p-value = 0.008; Figure 4B). The residual PPG predicted final post-PCI FFR ≥ 0.90 with an AUC of 0.93 (95% CI: 0.87–0.99; p < 0.001; Figure 4C).
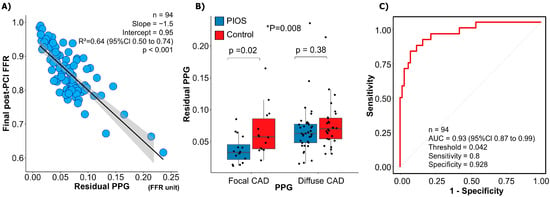
Figure 4.
Residual pullback pressure gradient (PPG) and post-PCI fractional flow reserve (FFR). Panel (A) shows the relationship between the residual PPG (x-axis) and final post-PCI FFR (y-axis). Residual PPG had a significant correlation with final post-PCI FFR. Panel (B) demonstrates differences in residual FFR between the focal PIOS, diffuse PIOS, focal control, and diffuse control groups. * Focal CAD PIOS vs. diffuse CAD PIOS vs. focal CAD controls vs. diffuse CAD controls. Panel (C) shows ROC curve analysis for the capacity of residual PPG for predicting final post-PCI FFR ≥ 0.90. AUC = Area under curve; CAD = coronary artery disease; FFR = fractional flow reserve; PIOS = physiology-guided incremental optimisation strategy; PPG = pullback pressure gradient; ROC = receiver operating characteristic.
3.6. Clinical Outcomes
At a median follow-up of 2 years, only one target vessel failure (TVF) occurred. This patient, randomized to PIOS with focal disease, suffered a presumed cardiac death 17 months after the procedure. There were no differences in TVF between PIOS and controls or between patients with focal or diffuse CAD.
4. Discussion
The main findings of the present study can be summarized as follows: (1) pathophysiological CAD patterns, defined by the PPG index, correlated with post-PCI FFR and delta FFR. Patients with focal disease achieved higher post-PCI FFR compared to those with diffuse CAD. (2) Physiology-guided PCI optimisation was more frequently applied to patients with diffuse CAD (as the incidence of post-PCI FFR < 0.90 was higher). However, despite the higher use of optimisation, final FFR values in patients with diffuse disease did not differ between PIOS and controls. (3) There was a significant interaction between the PPG and the randomization arm for post-PCI FFR. Patients with focal disease randomized to PIOS achieved the highest post-PCI FFR. (4) Post-PCI FFR was largely determined by focal residual pressure gradients and the residual PPG index, which was significantly lower in patients with focal disease randomized to stent optimisation.
The PPG is the first quantitative metric to differentiate focal from diffuse disease. In line with previous publications, the PPG correlated with post-PCI and delta FFR [13]. Patients with a high PPG (focal disease) achieved significantly higher post-PCI FFR and delta FFR. We demonstrated that improvement in coronary blood flow with PCI partially depends on the baseline pattern of disease. The higher post-PCI FFR in focal disease was observed independent of the application of physiology-guided stent optimisation. In contrast, in diffuse disease, additional optimisation had limited value in terms of post-PCI FFR, and, in this group, the final FFR was similar between patients randomized to PIOS and controls. The advent of the PPG, a reproducible metric for quantifying the physiological pattern of CAD, will facilitate the study of treatment options stratified by disease patterns.
TARGET-FFR was the first randomized trial assessing the feasibility of routine post-PCI FFR-guided optimisation strategy [14]. As shown in this study, physiologic outcomes after PCI are influenced by the baseline CAD pattern. By design, TARGET-FFR applied physiology-guided stent optimisation to patients with predominantly diffuse disease because these additional manoeuvres were triggered by low post-PCI FFR, a proxy of diffuse disease. In addition, the high proportion of vessels with diffuse CAD in the study limited the efficacy of PIOS in improving the post-PCI FFR. Similarly, the mechanism leading to higher post-PCI FFR in patients with focal CAD randomized to PIOS is likely unrelated to the application of optimisation manoeuvres since only one patient with focal CAD in the PIOS group received an additional intervention. Nonetheless, it can be hypothesised that patients with focal disease pre-PCI and a suboptimal functional result after stenting might have additional targets for optimisation (e.g., unmasked focal pressure gradients arising from the untreated segments), which may lead to improved post-PCI physiology. The hypothesis that functionally guided PCI optimisation is more effective in focal vs. diffuse disease requires further investigation.
There are mainly three mechanisms leading to unsatisfactory PCI results: first, the presence of residual pressure gradients within the stented segment, mainly associated with stent underexpansion or issues at the stent edges [15,16]; second, focal pressure gradients distal or proximal to the treated segment; and third, diffuse residual disease. The first two can potentially be addressed by additional post-dilation or PCI with a resultant improvement in intracoronary haemodynamics. In this study, half of the cases were optimised with additional stent post-dilation, and half underwent an additional PCI. Nonetheless, in the setting of diffuse disease optimisation manoeuvres will result in minor coronary physiology improvement as shown in this cohort by the small increase in FFR from 0.80 ± 0.09 immediately after stenting to 0.83 ± 0.07 after optimisation. In this work, we also introduced the residual PPG as a metric to quantify residual pressure gradients. Distinctively from the original study protocol, in which residual focal pressure gradients were assessed visually, the residual PPG provides an automatic quantification of residual focal gradients after stenting. This new approach leverages the original PPG formula adapted to the post-PCI setting to quantify focal pressure drops in absolute FFR units. As anticipated, the residual PPG was strongly associated with post-PCI FFR with an AUC of 0.93 (95% CI: 0.87–0.99). Interestingly, the residual PPG was significantly lower in patients with focal CAD assigned to PIOS highlighting the influence of the baseline pattern of disease and potentially the value of optimisation in the magnitude of residual focal gradients after PCI. The clinical relevance of focal residual translesional gradients using angiography-derived FFR software in the post-PCI phase has previously been suggested in one study [17]. Nonetheless, further studies are required to better understand the clinical implications of residual PPG better. The importance of this study is identifying which patients benefit from additional physiology-guided optimisation. Patients randomized to PIOS with focal CAD patterns achieved greater improvement in post-PCI FFR compared to patients with diffuse CAD. Therefore, using CAD patterns stratified by PPG before stenting identifies patients who are likely to achieve high post-PCI FFR.
This study has several limitations. First, pre-PCI FFR pullbacks were only performed in 74% (192/260) of cases, of which 59.3% (114/192) were deemed suitable for PPG calculation. However, this attrition of the sample size was equally distributed between the two arms, and the benefit of randomisation in terms of baseline risk distribution was preserved. Second, the study was not powered to assess clinical outcomes; the primary objective was post-PCI FFR, a surrogate endpoint of adverse events after PCI. Third, the PPG and residual PPG were calculated off-line, limiting the evaluation of the clinical utility of these metrics. Nonetheless, the quantification of pullback data has the potential to standardise diagnosis and treatment pathways, accounting for CAD patterns and residual disease. Further studies are required to better understand the pattern of CAD via non-invasive assessment using coronary blood flow models [18,19,20]. Finally, this is a post hoc analysis of randomized clinical trials, and all the findings presented in this study should be interpreted as hypothesis generating.
5. Conclusions
Baseline coronary artery disease patterns influence FFR after PCI. Patients with diffuse disease (low PPG) achieve lower post-PCI FFR compared to those with focal CAD. Physiology-guided stent optimisation was applied more frequently to vessels with diffuse disease; however, despite these patients with focal CAD at baseline achieved higher post-PCI FFR. Characterising coronary artery disease patterns with the PPG before stenting identifies patients likely to achieve high post-PCI FFR.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/diagnostics13152612/s1. Table S1: Inclusion criteria/exclusion criteria in TARGET FFR trial; Table S2: Comparison of functional characteristics between focal and diffuse coronary artery disease (CAD); Table S3: Multivariate regression of post-PCI fractional flow reserve (FFR) with pullback pressure gradient (PPG); Table S4: Comparison of fractional characteristics between focal and diffuse coronary artery disease in patients with additional optimisation; Figure S1: Physiology-guided incremental optimisation strategy; Figure S2: Study flowchart; Figure S3: Final post-PCI fractional flow reserve (FFR) stratified by randomization arm and PPG-defined focal or diffuse disease; Figure S4: The rate of optimal post-PCI fractional flow reserve (FFR) (≥0.90) stratified by randomization arm and coronary artery disease.
Author Contributions
Conceptualization, D.C., C.C. and T.M.; validation, T.M. and H.O.; writing—original draft preparation, H.O. and C.C.; writing—review and editing, M.D., K.S., M.A.-u.-R., D.M., P.M., T.J.F., M.L., A.S., P.R., R.B., S.W., M.M., R.G., K.R., P.O., A.D., A.K., S.H., H.E., T.A., J.S., C.B., K.G.O. and C.C.; visualization, T.M. and H.O.; supervision, D.C., C.C. and B.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by endowment funds at the Golden Jubilee National Hospital (NHS National Waiting Times Centre Board) and the British Heart Foundation Research Excellence Awards (RE/18/6/34217 to C.B.). TARGET-FFR was an investigator-initiated trial sponsored by the Golden Jubilee Research Institute (NHS National Waiting Times Centre Board). The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Institutional Review Board Statement
The study is registered at ClinicalTrials.gov identifier NCT03259815. The study received ethical approval from the West of Scotland Research Ethics Service (17/WS/0153) and was conducted in accordance with the International Conference on Harmonization Good Clinical Practice (ICH GCP) Guideline and the Declaration of Helsinki (64th World Medical Association General Assembly, Fortaleza, Brazil, October 2013).
Informed Consent Statement
All patients signed informed consent before any study procedure.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to patient privacy concerns.
Conflicts of Interest
D.C. reports honoraria from Abbott. T.M. reports receiving consulting fees from Zeon Medical and HeartFlow and speaker fees from Abbott Vascular. D.M. reports research grants provided by the CardioPath Ph.D program and speaker fees from Abbott Vascular. T.J.F. reports consulting fees from BioExcel and honoraria from Abbott, Boehringer, and Novartis. P.R. reports participation on a trial steering committee for the TACTIC-R study. S.W. reports honoraria from Abbott, AstraZeneca, Biosensors, Boston Scientific, Daiichi Sankyo, GE Healthcare, and ShockWave Medical and support for meeting attendance from Biosensors and Boston Scientific. K.R. reports honoraria from AstraZeneca and support for meeting attendance from Abbott. P.O.B. reports honoraria from AstraZeneca, Boston Scientific, and Novartis and participates on a data safety monitoring/advisory board for AstraZeneca. M.M.E. reports consulting fees from Abbott, Boston Scientific, and ShockWave Medical; honoraria from International Medical Device Solutions and Medtronic; and participation on an advisory board for Teleflex. C.B. reports institutional payments, including grants/contracts from Abbott, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, HeartFlow, Novartis, and Siemens Healthcare; consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, HeartFlow, Menarini, and Novartis; honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, HeartFlow and Valo Health; travel support from Philips; receipt of equipment for clinical research from Abbott, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and HeartFlow. He is an executive editor for the European Heart Journal. K.G.O. reports honoraria from Abbott, Biosensors International, and Boston Scientific; an unrestricted institutional research grant from Boston Scientific, which supported the present manuscript; and participation on a data safety monitoring board for the SYNTAX II trial and has been a full-time employee of Biosensors International since May 2020. B.D.B. reports receiving consultancy fees from Boston Scientific and Abbott and receiving research grants from Coroventis Research, Pie Medical Imaging, Cathworks, Boston Scientific, Siemens, HeartFlow Inc., and Abbott Vascular. C.C. reports receiving research grants from Biosensor, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, and Abbott Vascular and consultancy fees from HeartFlow, OpSens, Abbott Vascular, and Philips Volcano. All other authors declare no competing interests.
References
- Raber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.W.; Raber, L.; Di Mario, C.; Bourantas, C.V.; Jia, H.; Mattesini, A.; Gonzalo, N.; de la Torre Hernandez, J.M.; Prati, F.; Koskinas, K.C. Clinical use of intracoronary imaging. Part2: Acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 2019, 15, 434–451. [Google Scholar] [PubMed]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.-Y.; Lee, S.-J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J. Intravascular Imaging–Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.P.; Toth, G.G.; Lai, D.; Zhu, H.; Acar, G.; Agostoni, P.; Appelman, Y.; Arslan, F.; Barbato, E.; Chen, S.L. Prognostic value of fractional flow reserve: Linking physiologic severity to clinical outcomes. J. Am. Coll. Cardiol. 2014, 64, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Collison, D.; Didagelos, M.; Aetesam-Ur-Rahman, M.; Copt, S.; McDade, R.; McCartney, P.; Ford, T.J.; McClure, J.; Lindsay, M.; Shaukat, A.; et al. Post-stenting fractional flow reserve vs coronary angiography for optimization of percutaneous coronary intervention (TARGET-FFR). Eur. Heart J. 2021, 42, 4656–4668. [Google Scholar] [CrossRef] [PubMed]
- Rimac, G.; Fearon, W.F.; De Bruyne, B.; Ikeno, F.; Matsuo, H.; Piroth, Z.; Costerousse, O.; Bertrand, O.F. Clinical value of post-percutaneous coronary intervention fractional flow reserve value: A systematic review and meta-analysis. Am. Heart J. 2017, 183, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Koo, B.K.; Zhang, J.; Park, J.; Yang, S.; Kim, M.; Yun, J.P.; Lee, J.M.; Nam, C.W.; Shin, E.S.; et al. Prognostic Implications of Fractional Flow Reserve after Coronary Stenting: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2232842. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Sonck, J.; Vandeloo, B.; Mizukami, T.; Roosens, B.; Lochy, S.; Argacha, J.F.; Schoors, D.; Colaiori, I.; Di Gioia, G.; et al. Measurement of Hyperemic Pullback Pressure Gradients to Characterize Patterns of Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2019, 74, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Layland, J.; Carrick, D.; Lee, M.; Oldroyd, K.; Berry, C. Adenosine: Physiology, pharmacology, and clinical applications. JACC Cardiovasc. Interv. 2014, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, C.A.; Shun-Shin, M.; Seligman, H.; Ahmad, Y.; Warisawa, T.; Cook, C.M.; Howard, J.P.; Ganesananthan, S.; Amarin, L.; Khan, C.; et al. Placebo-Controlled Efficacy of Percutaneous Coronary Intervention for Focal and Diffuse Patterns of Stable Coronary Artery Disease. Circ. Cardiovasc. Interv. 2021, 14, e009891. [Google Scholar] [CrossRef] [PubMed]
- Samady, H.; McDaniel, M.; Veledar, E.; De Bruyne, B.; Pijls, N.H.; Fearon, W.F.; Vaccarino, V. Baseline fractional flow reserve and stent diameter predict optimal post-stent fractional flow reserve and major adverse cardiac events after bare-metal stent deployment. JACC Cardiovasc. Interv. 2009, 2, 357–363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piroth, Z.; Toth, G.G.; Tonino, P.A.L.; Barbato, E.; Aghlmandi, S.; Curzen, N.; Rioufol, G.; Pijls, N.H.J.; Fearon, W.F.; Juni, P.; et al. Prognostic Value of Fractional Flow Reserve Measured Immediately after Drug-Eluting Stent Implantation. Circ. Cardiovasc. Interv. 2017, 10, e005233. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, T.; Sonck, J.; Sakai, K.; Ko, B.; Maeng, M.; Otake, H.; Koo, B.K.; Nagumo, S.; Norgaard, B.; Leipsic, J.; et al. Procedural Outcomes after Percutaneous Coronary Interventions in Focal and Diffuse Coronary Artery Disease. J. Am. Heart Assoc. 2022, 11, e026960. [Google Scholar] [CrossRef] [PubMed]
- Erlinge, D.; Gotberg, M. We need intracoronary physiology guidance before percutaneous coronary intervention, but do we need it post-stenting? Eur. Heart J. 2021, 42, 4669–4670. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.; Klauss, V.; Siebert, U.; Powers, E.; Takazawa, K.; Fearon, W.F.; Escaned, J.; Tsurumi, Y.; Akasaka, T.; Samady, H.; et al. Fractional Flow Reserve Post-Stent Registry, I. Coronary pressure measurement after stenting predicts adverse events at follow-up: A multicenter registry. Circulation 2002, 105, 2950–2954. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Matsumura, M.; Witzenbichler, B.; Metzger, D.C.; Rinaldi, M.J.; Duffy, P.L.; Weisz, G.; Stuckey, T.D.; Ali, Z.A.; Zhou, Z.; et al. Stent Expansion Indexes to Predict Clinical Outcomes: An IVUS Substudy From ADAPT-DES. JACC Cardiovasc. Interv. 2021, 14, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Biscaglia, S.; Tebaldi, M.; Brugaletta, S.; Cerrato, E.; Erriquez, A.; Passarini, G.; Ielasi, A.; Spitaleri, G.; Di Girolamo, D.; Mezzapelle, G.; et al. Prognostic Value of QFR Measured Immediately after Successful Stent Implantation: The International Multicenter Prospective HAWKEYE Study. JACC Cardiovasc. Interv. 2019, 12, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Choi, G.; Koo, B.K.; Hwang, D.; Park, J.; Zhang, J.; Kim, K.J.; Tong, Y.; Kim, H.J.; Grady, L. Identification of High-Risk Plaques Destined to Cause Acute Coronary Syndrome Using Coronary Computed Tomographic Angiography and Computational Fluid Dynamics. JACC Cardiovasc. Imaging 2019, 12, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, C.; Rao, S.; Zhang, Y.; Ghista, D.; Gao, Z.; Yang, G. Physiologically personalized coronary blood flow model to improve the estimation of noninvasive fractional flow reserve. Med. Phys. 2022, 49, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, X.; Sun, S.; Wu, D.; Bai, J.; Yin, Y.; Liu, X.; Zhang, H.; de Albuquerque, V.H.C. Learning physical properties in complex visual scenes: An intelligent machine for perceiving blood flow dynamics from static CT angiography imaging. Neural. Netw. 2020, 123, 82–93. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).