Advanced Cardiac Imaging and Women’s Chest Pain: A Question of Gender
Abstract
1. Introduction
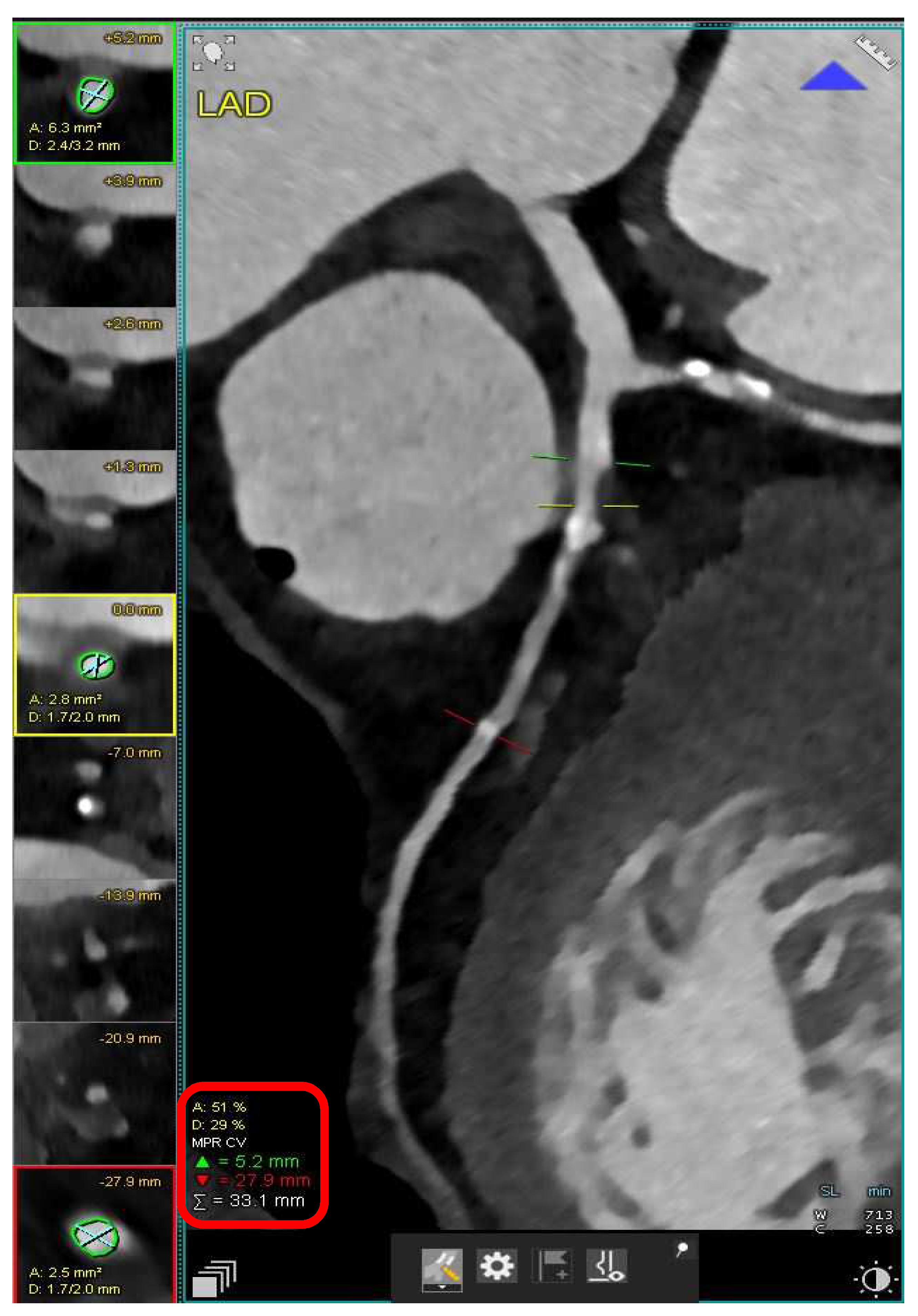
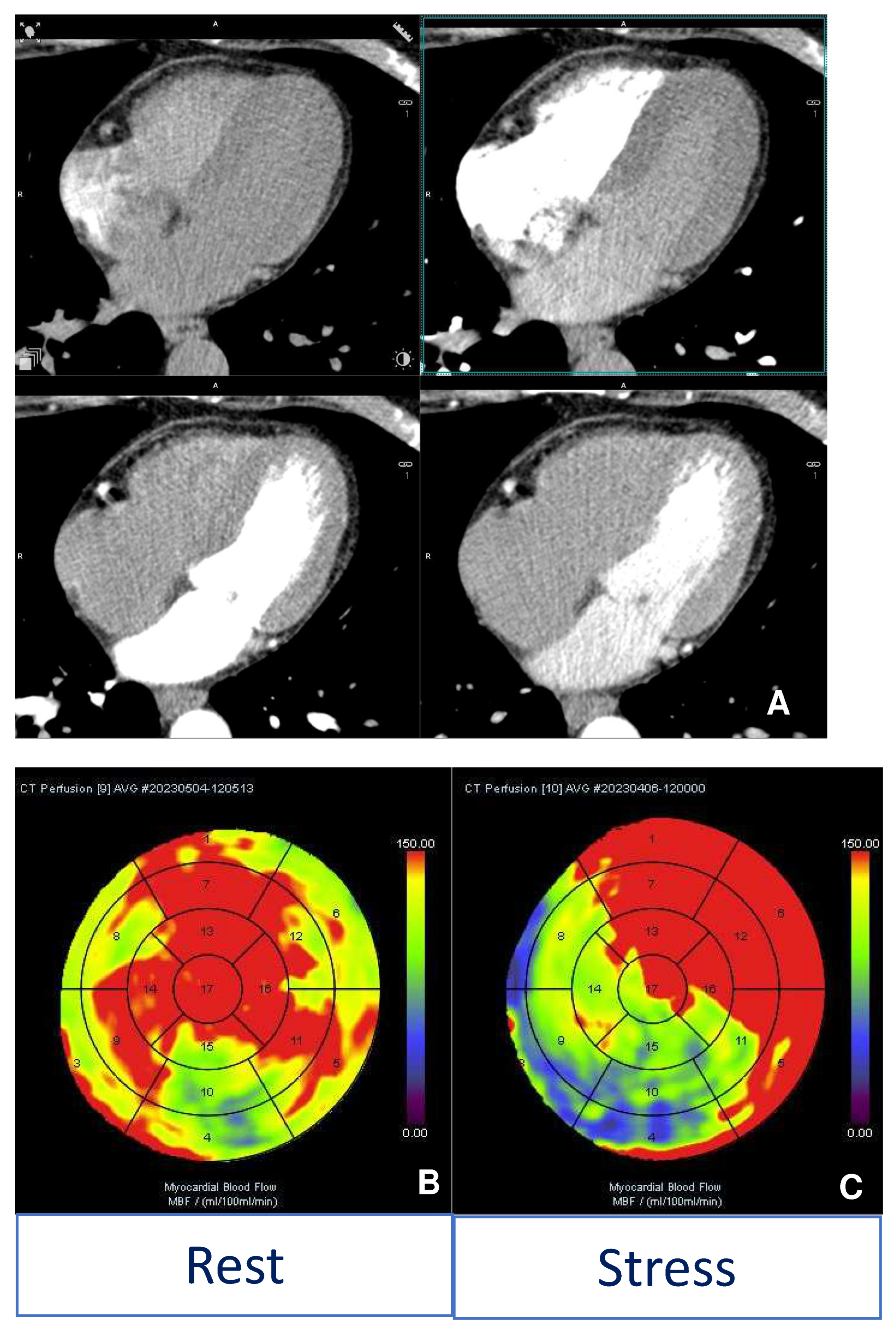
2. Acute Coronary Syndrome
3. Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA)
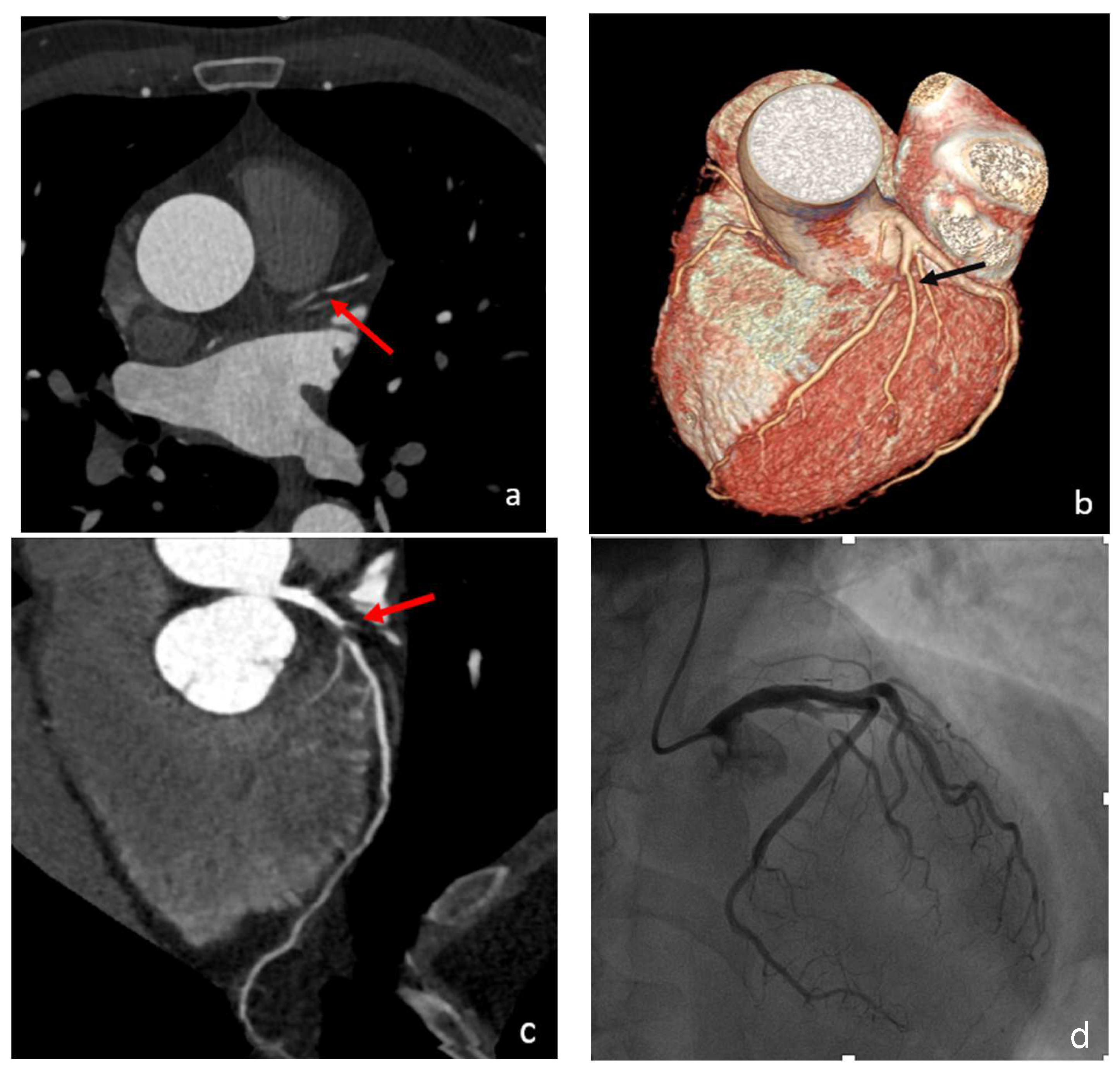
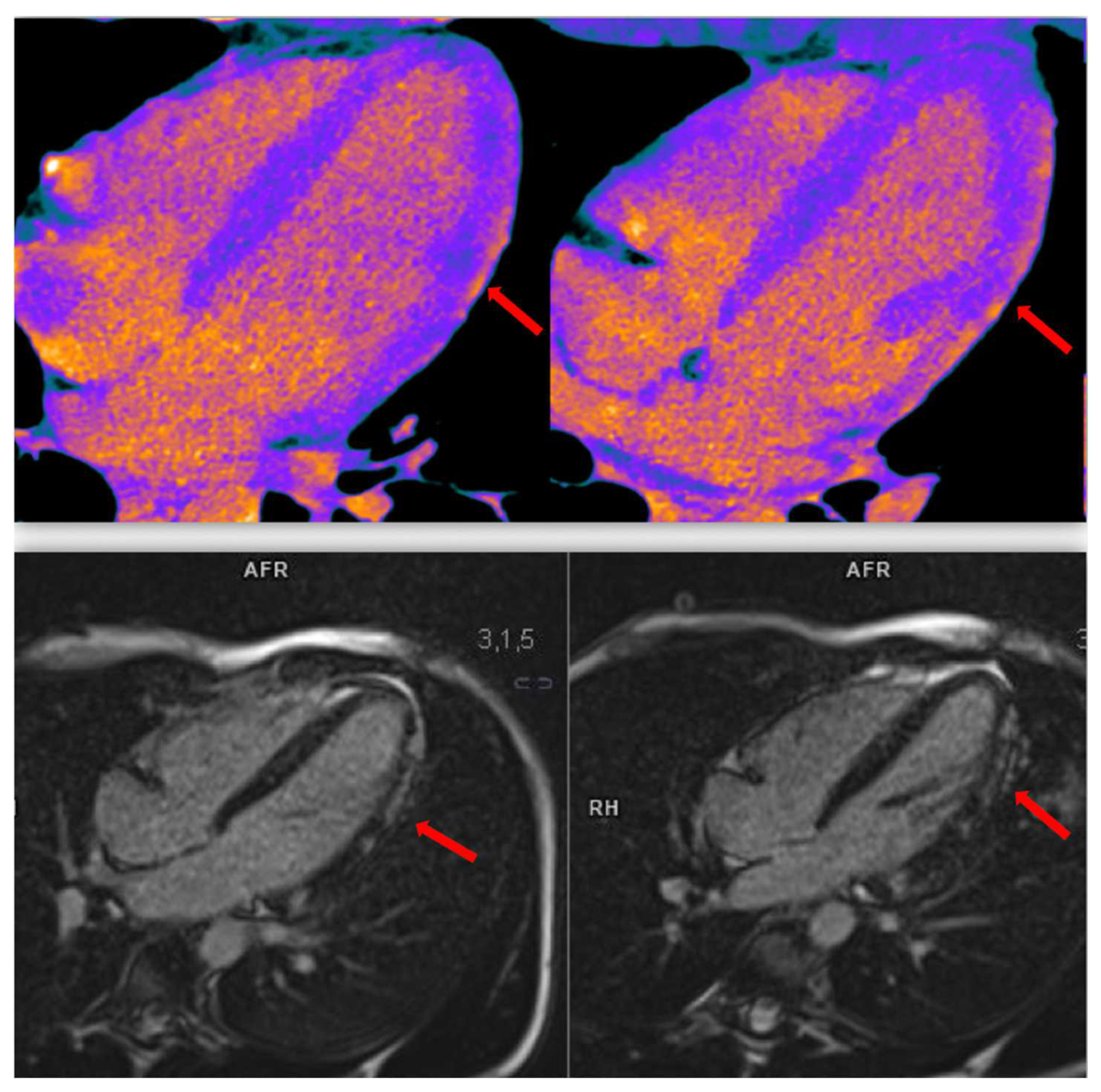
4. Spontaneous Coronary Artery Dissection (SCAD)
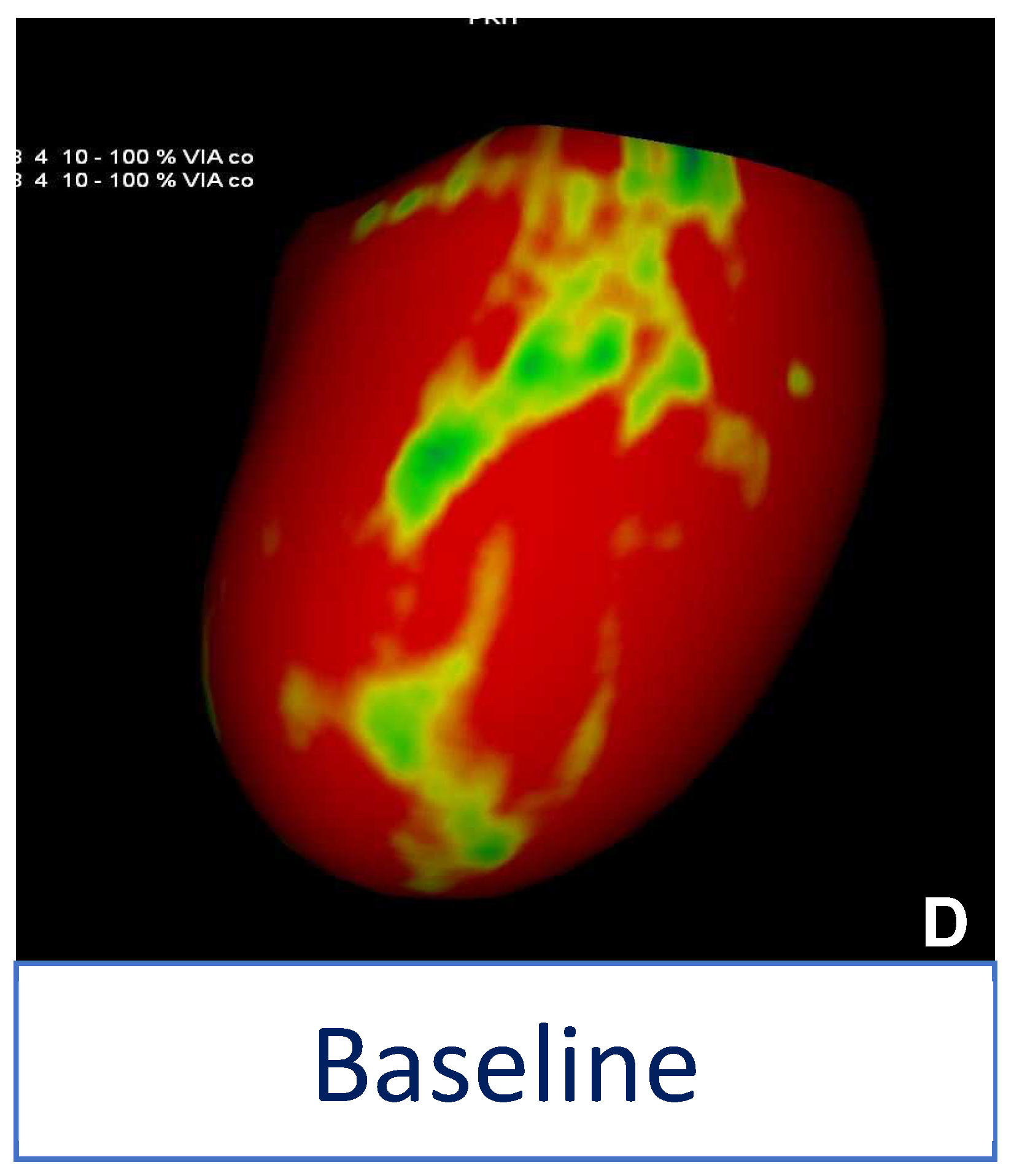
5. Takotsubo Syndrome (TTS)
6. Myocarditis
7. Pericarditis
8. Cardiac Hybrid Imaging
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.B.; Buring, J.E.; Manson, J.E. Cardiovascular Disease in Women: Clinical Perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Masci, P.G.; Dymarkowski, S.; Rademakers, F.E.; Bogaert, J. Determination of Regional Ejection Fraction in Patients with Myocardial Infarction by Using Merged Late Gadolinium Enhancement and Cine MR: Feasibility Study. Radiology 2009, 250, 50–60. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Paradisi, G.; Cronin, J.; Crowde, K.; Hempfling, A.; Hook, G.; Baron, A.D. Type II Diabetes Abrogates Sex Differences in Endothelial Function in Premenopausal Women. Circulation 2000, 101, 2040–2046. [Google Scholar] [CrossRef]
- Lu, Y.-W.; Tsai, C.-T.; Chou, R.-H.; Tsai, Y.-L.; Kuo, C.-S.; Huang, P.-H.; Lin, S.-J. Sex Difference in the Association of the Triglyceride Glucose Index with Obstructive Coronary Artery Disease. Sci. Rep. 2023, 13, 9652. [Google Scholar] [CrossRef]
- Pradhan, A.D. Sex Differences in the Metabolic Syndrome: Implications for Cardiovascular Health in Women. Clin. Chem. 2014, 60, 44–52. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the Epidemiology of Cardiovascular Disease: A Historical Perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef]
- Andersson, C.; Nayor, M.; Tsao, C.W.; Levy, D.; Vasan, R.S. Framingham Heart Study: JACC Focus Seminar, 1/8. J. Am. Coll. Cardiol. 2021, 77, 2680–2692. [Google Scholar] [CrossRef]
- Slater, K.; Colyvas, K.; Taylor, R.; Collins, C.E.; Hutchesson, M. Primary and Secondary Cardiovascular Disease Prevention Interventions Targeting Lifestyle Risk Factors in Women: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 1010528. [Google Scholar] [CrossRef]
- Maas, A.H.E.M.; Appelman, Y.E.A. Gender Differences in Coronary Heart Disease. Neth. Heart J. 2010, 18, 598–602. [Google Scholar] [CrossRef]
- Mosca, L.; Linfante, A.H.; Benjamin, E.J.; Berra, K.; Hayes, S.N.; Walsh, B.W.; Fabunmi, R.P.; Kwan, J.; Mills, T.; Simpson, S.L. National Study of Physician Awareness and Adherence to Cardiovascular Disease Prevention Guidelines. Circulation 2005, 111, 499–510. [Google Scholar] [CrossRef]
- Chou, A.F.; Scholle, S.H.; Weisman, C.S.; Bierman, A.S.; Correa-de-Araujo, R.; Mosca, L. Gender Disparities in the Quality of Cardiovascular Disease Care in Private Managed Care Plans. Women’s Health Issues 2007, 17, 120–130. [Google Scholar] [CrossRef]
- Brown, C.J.; Chang, L.-S.; Hosomura, N.; Malmasi, S.; Morrison, F.; Shubina, M.; Lan, Z.; Turchin, A. Assessment of Sex Disparities in Nonacceptance of Statin Therapy and Low-Density Lipoprotein Cholesterol Levels Among Patients at High Cardiovascular Risk. JAMA Netw. Open 2023, 6, e231047. [Google Scholar] [CrossRef]
- Soriano-Maldonado, C.; Lopez-Pineda, A.; Orozco-Beltran, D.; Quesada, J.A.; Alfonso-Sanchez, J.L.; Pallarés-Carratalá, V.; Navarro-Perez, J.; Gil-Guillen, V.F.; Martin-Moreno, J.M.; Carratala-Munuera, C. Gender Differences in the Diagnosis of Dyslipidemia: ESCARVAL-GENERO. Int. J. Environ. Res. Public Health 2021, 18, 12419. [Google Scholar] [CrossRef]
- Saeed, A.; Kampangkaew, J.; Nambi, V. Prevention of Cardiovascular Disease in Women. Methodist Debakey Cardiovasc. J. 2017, 13, 185–192. [Google Scholar] [CrossRef]
- Shufelt, C.L.; Pacheco, C.; Tweet, M.S.; Miller, V.M. Sex-Specific Physiology and Cardiovascular Disease. In Sex-Specific Analysis of Cardiovascular Function; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1065, pp. 433–454. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Shaw, L.J.; Min, J.K.; Spertus, J.A.; Chaitman, B.R.; Berman, D.S.; Picard, M.H.; Kwong, R.Y.; Bairey-Merz, C.N.; Cyr, D.D.; et al. Association of Sex with Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients With Moderate or Severe Ischemia: Secondary Analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol. 2020, 5, 773–786. [Google Scholar] [CrossRef]
- McClelland, R.L.; Chung, H.; Detrano, R.; Post, W.; Kronmal, R.A. Distribution of Coronary Artery Calcium by Race, Gender, and Age: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006, 113, 30–37. [Google Scholar] [CrossRef]
- Shaw, L.J.; Min, J.K.; Nasir, K.; Xie, J.X.; Berman, D.S.; Miedema, M.D.; Whelton, S.P.; Dardari, Z.A.; Rozanski, A.; Rumberger, J.; et al. Sex Differences in Calcified Plaque and Long-Term Cardiovascular Mortality: Observations from the CAC Consortium. Eur. Heart J. 2018, 39, 3727–3735. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.E.; Kwong, R.Y.; Salerno, M.; Greenwood, J.P.; Bucciarelli-Ducci, C. Society for Cardiovascular Magnetic Resonance Perspective on the 2021 AHA/ACC Chest Pain Guidelines. J. Cardiovasc. Magn. Reson. 2022, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Bigeh, A.; Shekar, C.; Gulati, M. Sex Differences in Coronary Artery Calcium and Long-Term CV Mortality. Curr. Cardiol. Rep. 2020, 22, 21. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Lopes, R.D.; Harrington, R.A. Diagnosis and Treatment of Acute Coronary Syndromes: A Review. JAMA 2022, 327, 662–675. [Google Scholar] [CrossRef]
- Mehilli, J.; Presbitero, P. Coronary Artery Disease and Acute Coronary Syndrome in Women. Heart 2020, 106, 487–492. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Apple, F.S.; Jaffe, A.S.; Collinson, P.; Mockel, M.; Ordonez-Llanos, J.; Lindahl, B.; Hollander, J.; Plebani, M.; Than, M.; Chan, M.H.M.; et al. IFCC Educational Materials on Selected Analytical and Clinical Applications of High Sensitivity Cardiac Troponin Assays. Clin. Biochem. 2015, 48, 201–203. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Ferencik, M.; Mayrhofer, T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Lu, M.T.; Meyersohn, N.M.; Ivanov, A.V.; Adami, E.C.; Patel, M.R.; et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients with Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 144–152. [Google Scholar] [CrossRef]
- Wenger, N.K. Clinical Presentation of CAD and Myocardial Ischemia in Women. J. Nucl. Cardiol. 2016, 23, 976–985. [Google Scholar] [CrossRef]
- Kenkre, T.S.; Malhotra, P.; Johnson, B.D.; Handberg, E.M.; Thompson, D.V.; Marroquin, O.C.; Rogers, W.J.; Pepine, C.J.; Bairey Merz, C.N.; Kelsey, S.F. Ten-Year Mortality in the WISE Study (Women’s Ischemia Syndrome Evaluation). Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003863. [Google Scholar] [CrossRef]
- Mangion, K.; Adamson, P.D.; Williams, M.C.; Hunter, A.; Pawade, T.; Shah, A.S.V.; Lewis, S.; Boon, N.A.; Flather, M.; Forbes, J.; et al. Sex Associations and Computed Tomography Coronary Angiography-Guided Management in Patients with Stable Chest Pain. Eur. Heart J. 2020, 41, 1337–1345. [Google Scholar] [CrossRef]
- Bharadwaj, A.S.; Vengrenyuk, Y.; Yoshimura, T.; Baber, U.; Hasan, C.; Narula, J.; Sharma, S.K.; Kini, A.S. Multimodality Intravascular Imaging to Evaluate Sex Differences in Plaque Morphology in Stable CAD. JACC Cardiovasc. Imaging 2016, 9, 400–407. [Google Scholar] [CrossRef]
- Plank, F.; Beyer, C.; Friedrich, G.; Wildauer, M.; Feuchtner, G. Sex Differences in Coronary Artery Plaque Composition Detected by Coronary Computed Tomography: Quantitative and Qualitative Analysis. Neth. Heart J. 2019, 27, 272–280. [Google Scholar] [CrossRef]
- Puchner, S.B.; Lu, M.T.; Mayrhofer, T.; Liu, T.; Pursnani, A.; Ghoshhajra, B.B.; Truong, Q.A.; Wiviott, S.D.; Fleg, J.L.; Hoffmann, U.; et al. High-Risk Coronary Plaque at Coronary CT Angiography Is Associated with Nonalcoholic Fatty Liver Disease, Independent of Coronary Plaque and Stenosis Burden: Results from the ROMICAT II Trial. Radiology 2015, 274, 693–701. [Google Scholar] [CrossRef]
- Lee, S.-E.; Sung, J.M.; Andreini, D.; Al-Mallah, M.H.; Budoff, M.J.; Cademartiri, F.; Chinnaiyan, K.; Choi, J.H.; Chun, E.J.; Conte, E.; et al. Sex Differences in Compositional Plaque Volume Progression in Patients with Coronary Artery Disease. JACC Cardiovasc. Imaging 2020, 13, 2386–2396. [Google Scholar] [CrossRef]
- Dreyer, R.P.; Tavella, R.; Curtis, J.P.; Wang, Y.; Pauspathy, S.; Messenger, J.; Rumsfeld, J.S.; Maddox, T.M.; Krumholz, H.M.; Spertus, J.A.; et al. Myocardial Infarction with Non-Obstructive Coronary Arteries as Compared with Myocardial Infarction and Obstructive Coronary Disease: Outcomes in a Medicare Population. Eur. Heart J. 2020, 41, 870–878. [Google Scholar] [CrossRef]
- Neumann, J.T.; Sörensen, N.A.; Rübsamen, N.; Ojeda, F.; Renné, T.; Qaderi, V.; Teltrop, E.; Kramer, S.; Quantius, L.; Zeller, T.; et al. Discrimination of Patients with Type 2 Myocardial Infarction. Eur. Heart J. 2017, 38, 3514–3520. [Google Scholar] [CrossRef]
- Chapman, A.R.; Shah, A.S.V.; Lee, K.K.; Anand, A.; Francis, O.; Adamson, P.; McAllister, D.A.; Strachan, F.E.; Newby, D.E.; Mills, N.L. Long-Term Outcomes in Patients with Type 2 Myocardial Infarction and Myocardial Injury. Circulation 2018, 137, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Hulten, E.; Ahmadi, A.; Blankstein, R. CT Assessment of Myocardial Perfusion and Fractional Flow Reserve. Prog. Cardiovasc. Dis. 2015, 57, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Yang, D.H.; Choo, K.S.; Lee, W. Beyond Coronary CT Angiography: CT Fractional Flow Reserve and Perfusion. J. Korean Soc. Radiol. 2022, 83, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Coenen, A.; Rossi, A.; Lubbers, M.M.; Kurata, A.; Kono, A.K.; Chelu, R.G.; Segreto, S.; Dijkshoorn, M.L.; Wragg, A.; van Geuns, R.-J.M.; et al. Integrating CT Myocardial Perfusion and CT-FFR in the Work-Up of Coronary Artery Disease. JACC Cardiovasc. Imaging 2017, 10, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, T.A.; Dobson, R.; Hurwitz-Koweek, L.; Matsuo, H.; Norgaard, B.L.; Rønnow Sand, N.P.; Nieman, K.; Bax, J.J.; Pontone, G.; Raff, G.; et al. Sex Differences in Coronary Computed Tomography Angiography-Derived Fractional Flow Reserve: Lessons From ADVANCE. JACC Cardiovasc. Imaging 2020, 13, 2576–2587. [Google Scholar] [CrossRef]
- Pasupathy, S.; Beltrame, J.F. Refining the Role of CMR Imaging in MINOCA. JACC Cardiovasc. Imaging 2021, 14, 1784–1786. [Google Scholar] [CrossRef]
- Baessato, F.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; Fusini, L.; Scafuri, S.; Babbaro, M.; Mollace, R.; Collevecchio, A.; Guaricci, A.I.; et al. Stress CMR in Known or Suspected CAD: Diagnostic and Prognostic Role. BioMed Res. Int. 2021, 2021, 6678029. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K.; Dall’Armellina, E.; Karamitsos, T.D.; Francis, J.M.; Choudhury, R.P.; Friedrich, M.G.; Robson, M.D.; Neubauer, S. Non-Contrast T1-Mapping Detects Acute Myocardial Edema with High Diagnostic Accuracy: A Comparison to T2-Weighted Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2012, 14, 42. [Google Scholar] [CrossRef]
- Taylor, A.J.; Al-Saadi, N.; Abdel-Aty, H.; Schulz-Menger, J.; Messroghli, D.R.; Friedrich, M.G. Detection of Acutely Impaired Microvascular Reperfusion after Infarct Angioplasty with Magnetic Resonance Imaging. Circulation 2004, 109, 2080–2085. [Google Scholar] [CrossRef]
- Singh, T.; Khan, H.; Gamble, D.T.; Scally, C.; Newby, D.E.; Dawson, D. Takotsubo Syndrome: Pathophysiology, Emerging Concepts, and Clinical Implications. Circulation 2022, 145, 1002–1019. [Google Scholar] [CrossRef]
- Assad, J.; Femia, G.; Pender, P.; Badie, T.; Rajaratnam, R. Takotsubo Syndrome: A Review of Presentation, Diagnosis and Management. Clin. Med. Insights Cardiol. 2022, 16, 5782. [Google Scholar] [CrossRef]
- Mollet, N.R.; Dymarkowski, S.; Volders, W.; Wathiong, J.; Herbots, L.; Rademakers, F.E.; Bogaert, J. Visualization of Ventricular Thrombi with Contrast-Enhanced Magnetic Resonance Imaging in Patients with Ischemic Heart Disease. Circulation 2002, 106, 2873–2876. [Google Scholar] [CrossRef]
- Pacheco, C.; Mullen, K.-A.; Coutinho, T.; Jaffer, S.; Parry, M.; Van Spall, H.G.C.; Clavel, M.-A.; Edwards, J.D.; Sedlak, T.; Norris, C.M.; et al. The Canadian Women’s Heart Health Alliance Atlas on the Epidemiology, Diagnosis, and Management of Cardiovascular Disease in Women—Chapter 5: Sex- and Gender-Unique Manifestations of Cardiovascular Disease. CJC Open 2022, 4, 243–262. [Google Scholar] [CrossRef]
- Pacheco Claudio, C.; Quesada, O.; Pepine, C.J.; Noel Bairey Merz, C. Why Names Matter for Women: MINOCA/INOCA (Myocardial Infarction/Ischemia and No Obstructive Coronary Artery Disease). Clin. Cardiol. 2018, 41, 185–193. [Google Scholar] [CrossRef]
- Occhipinti, G.; Bucciarelli-Ducci, C.; Capodanno, D. Diagnostic Pathways in Myocardial Infarction with Non-Obstructive Coronary Artery Disease (MINOCA). Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 813–822. [Google Scholar] [CrossRef]
- Safdar, B.; Spatz, E.S.; Dreyer, R.P.; Beltrame, J.F.; Lichtman, J.H.; Spertus, J.A.; Reynolds, H.R.; Geda, M.; Bueno, H.; Dziura, J.D.; et al. Presentation, Clinical Profile, and Prognosis of Young Patients with Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): Results From the VIRGO Study. J. Am. Heart Assoc. 2018, 7, e009174. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.P.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC Working Group Position Paper on Myocardial Infarction with Non-Obstructive Coronary Arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef]
- Lindahl, B.; Baron, T.; Albertucci, M.; Prati, F. Myocardial Infarction with Non-Obstructive Coronary Artery Disease. EuroIntervention 2021, 17, e875–e887. [Google Scholar] [CrossRef]
- Wingate, S. Cardiovascular Anatomy and Physiology in the Female. Crit. Care Nurs. Clin. N. Am. 1997, 9, 447–452. [Google Scholar] [CrossRef]
- Patel, M.R.; Chen, A.Y.; Peterson, E.D.; Newby, L.K.; Pollack, C.V.; Brindis, R.G.; Gibson, C.M.; Kleiman, N.S.; Saucedo, J.F.; Bhatt, D.L.; et al. Prevalence, Predictors, and Outcomes of Patients with Non-ST-Segment Elevation Myocardial Infarction and Insignificant Coronary Artery Disease: Results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress ADverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) Initiative. Am. Heart J. 2006, 152, 641–647. [Google Scholar] [CrossRef]
- Dastidar, A.G.; Rodrigues, J.C.L.; Johnson, T.W.; De Garate, E.; Singhal, P.; Baritussio, A.; Scatteia, A.; Strange, J.; Nightingale, A.K.; Angelini, G.D.; et al. Myocardial Infarction With Nonobstructed Coronary Arteries: Impact of CMR Early After Presentation. JACC Cardiovasc. Imaging 2017, 10, 1204–1206. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.Y.; Chiang, J.B.; Ng, P.P.; Chow, B.C.K.; Cheng, Y.W.; Wong, C.Y. Utility of Cardiac Magnetic Resonance Imaging in Troponin-Positive Chest Pain with Non-Obstructive Coronary Arteries: Literature Review. Hong Kong Med. J. 2021, 27, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.N.; Kim, E.S.H.; Saw, J.; Adlam, D.; Arslanian-Engoren, C.; Economy, K.E.; Ganesh, S.K.; Gulati, R.; Lindsay, M.E.; Mieres, J.H.; et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e523–e557. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Meyersohn, N.M.; Wood, M.J.; Steigner, M.L.; Blankstein, R.; Ghoshhajra, B.B.; Hedgire, S.S. Role of Coronary CT Angiography in Spontaneous Coronary Artery Dissection. Radiol. Cardiothorac. Imaging 2020, 2, e200364. [Google Scholar] [CrossRef]
- Franke, K.B.; Nerlekar, N.; Marshall, H.; Psaltis, P.J. Systematic Review and Meta-Analysis of the Clinical Characteristics and Outcomes of Spontanous Coronary Artery Dissection. Int. J. Cardiol. 2021, 322, 34–39. [Google Scholar] [CrossRef]
- Aslam, A.; Stojanovska, J.; Khokhar, U.S.; Weinberg, R.L.; Ganesh, S.K.; Labounty, T.; Sutton, N.R.; Patel, S. Spontaneous Coronary Artery Dissection: An Underdiagnosed Clinical Entity-A Primer for Cardiac Imagers. RadioGraphics 2021, 41, 1897–1915. [Google Scholar] [CrossRef]
- Teruzzi, G.; Santagostino Baldi, G.; Gili, S.; Guarnieri, G.; Montorsi, P.; Trabattoni, D. Spontaneous Coronary Artery Dissections: A Systematic Review. J. Clin. Med. 2021, 10, 5925. [Google Scholar] [CrossRef]
- Marrazzo, G.; Palermi, S.; Pastore, F.; Ragni, M.; De Luca, M.; Gambardella, M.; Quaranta, G.; Messalli, G.; Riegler, L.; Pergola, V.; et al. Multimodality Imaging Approach to Spontaneous Coronary Artery Dissection. J. Clin. Med. 2022, 12, 154. [Google Scholar] [CrossRef]
- Ito, H.; Taylor, L.; Bowman, M.; Fry, E.T.A.; Hermiller, J.B.; Van Tassel, J.W. Presentation and Therapy of Spontaneous Coronary Artery Dissection and Comparisons of Postpartum versus Nonpostpartum Cases. Am. J. Cardiol. 2011, 107, 1590–1596. [Google Scholar] [CrossRef]
- Tweet, M.S.; Hayes, S.N.; Codsi, E.; Gulati, R.; Rose, C.H.; Best, P.J.M. Spontaneous Coronary Artery Dissection Associated with Pregnancy. J. Am. Coll. Cardiol. 2017, 70, 426–435. [Google Scholar] [CrossRef]
- Tweet, M.S.; Hayes, S.N.; Pitta, S.R.; Simari, R.D.; Lerman, A.; Lennon, R.J.; Gersh, B.J.; Khambatta, S.; Best, P.J.M.; Rihal, C.S.; et al. Clinical Features, Management, and Prognosis of Spontaneous Coronary Artery Dissection. Circulation 2012, 126, 579–588. [Google Scholar] [CrossRef]
- Alfonso, F.; Paulo, M.; Lennie, V.; Dutary, J.; Bernardo, E.; Jiménez-Quevedo, P.; Gonzalo, N.; Escaned, J.; Bañuelos, C.; Pérez-Vizcayno, M.J.; et al. Spontaneous Coronary Artery Dissection: Long-Term Follow-up of a Large Series of Patients Prospectively Managed with a “Conservative” Therapeutic Strategy. JACC Cardiovasc. Interv. 2012, 5, 1062–1070. [Google Scholar] [CrossRef]
- Sun, Y.; Mao, D.; Lu, F.; Chen, Y.; Shi, K.; Qi, L.; Guo, X.; Hua, Y. Diagnosis of Dissection of the Coronary Artery Dissection by Multidetector Computed Tomography: A Comparative Study with Coronary Angiology. J. Comput. Assist. Tomogr. 2015, 39, 572–577. [Google Scholar] [CrossRef]
- Roura, G.; Ariza-Solé, A.; Rodriguez-Caballero, I.F.; Gomez-Lara, J.; Ferreiro, J.L.; Romaguera, R.; Teruel, L.; de Albert, M.; Gomez-Hospital, J.A.; Cequier, A. Noninvasive Follow-Up of Patients with Spontaneous Coronary Artery Dissection with CT Angiography. JACC Cardiovasc. Imaging 2016, 9, 896–897. [Google Scholar] [CrossRef]
- Tweet, M.S.; Akhtar, N.J.; Hayes, S.N.; Best, P.J.; Gulati, R.; Araoz, P.A. Spontaneous Coronary Artery Dissection: Acute Findings on Coronary Computed Tomography Angiography. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 467–475. [Google Scholar] [CrossRef]
- Bin Saeedan, M.; Ramchand, J.; Bolen, M. Role of Computed Tomography Angiography in Setting of Spontaneous Coronary Artery Dissection. Curr. Probl. Diagn. Radiol. 2021, 50, 532–539. [Google Scholar] [CrossRef]
- Saw, J.; Aymong, E.; Sedlak, T.; Buller, C.E.; Starovoytov, A.; Ricci, D.; Robinson, S.; Vuurmans, T.; Gao, M.; Humphries, K.; et al. Spontaneous Coronary Artery Dissection: Association with Predisposing Arteriopathies and Precipitating Stressors and Cardiovascular Outcomes. Circ. Cardiovasc. Interv. 2014, 7, 645–655. [Google Scholar] [CrossRef]
- Tweet, M.S.; Eleid, M.F.; Best, P.J.M.; Lennon, R.J.; Lerman, A.; Rihal, C.S.; Holmes, D.R.; Hayes, S.N.; Gulati, R. Spontaneous Coronary Artery Dissection: Revascularization versus Conservative Therapy. Circ. Cardiovasc. Interv. 2014, 7, 777–786. [Google Scholar] [CrossRef]
- Lettieri, C.; Zavalloni, D.; Rossini, R.; Morici, N.; Ettori, F.; Leonzi, O.; Latib, A.; Ferlini, M.; Trabattoni, D.; Colombo, P.; et al. Management and Long-Term Prognosis of Spontaneous Coronary Artery Dissection. Am. J. Cardiol. 2015, 116, 66–73. [Google Scholar] [CrossRef]
- Main, A.; Prakash, R.; Starovoytov, A.; Sabbaghan, A.; Aymong, E.; Mancini, G.B.J.; Saw, J. Characteristics of Extension and de Novo Recurrent Spontaneous Coronary Artery Dissection. EuroIntervention 2017, 13, e1454–e1459. [Google Scholar] [CrossRef]
- Tan, N.Y.; Hayes, S.N.; Young, P.M.; Gulati, R.; Tweet, M.S. Usefulness of Cardiac Magnetic Resonance Imaging in Patients with Acute Spontaneous Coronary Artery Dissection. Am. J. Cardiol. 2018, 122, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, N.; Briasoulis, A.; Zouganeli, V.; Dimopoulos, S.; Kalpakos, D.; Kourek, C. Spontaneous Coronary Artery Dissection: A Review of Diagnostic Methods and Management Strategies. World J. Cardiol. 2022, 14, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.N.; Tweet, M.S.; Adlam, D.; Kim, E.S.H.; Gulati, R.; Price, J.E.; Rose, C.H. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Citro, R.; Schneider, B.; Morel, O.; Ghadri, J.R.; Templin, C.; Omerovic, E. Pathophysiology of Takotsubo Syndrome: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 902–921. [Google Scholar] [CrossRef]
- Boyd, B.; Solh, T. Takotsubo Cardiomyopathy: Review of Broken Heart Syndrome. JAAPA 2020, 33, 24–29. [Google Scholar] [CrossRef]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current State of Knowledge on Takotsubo Syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical Ballooning Syndrome (Tako-Tsubo or Stress Cardiomyopathy): A Mimic of Acute Myocardial Infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef]
- Citro, R.; Okura, H.; Ghadri, J.R.; Izumi, C.; Meimoun, P.; Izumo, M.; Dawson, D.; Kaji, S.; Eitel, I.; Kagiyama, N.; et al. Multimodality Imaging in Takotsubo Syndrome: A Joint Consensus Document of the European Association of Cardiovascular Imaging (EACVI) and the Japanese Society of Echocardiography (JSE). J. Echocardiogr. 2020, 18, 199–224. [Google Scholar] [CrossRef]
- Samul-Jastrzębska, J.; Roik, M.; Wretowski, D.; Łabyk, A.; Ślubowska, A.; Bizoń, A.; Paczyńska, M.; Kurnicka, K.; Pruszczyk, P.; Ciurzyński, M. Evaluation of the InterTAK Diagnostic Score in Differentiating Takotsubo Syndrome from Acute Coronary Syndrome. A Single Center Experience. Cardiol. J. 2021, 28, 416–422. [Google Scholar] [CrossRef]
- Amin, H.Z.; Amin, L.Z.; Pradipta, A. Takotsubo Cardiomyopathy: A Brief Review. J. Med. Life 2020, 13, 3–7. [Google Scholar] [CrossRef]
- Nakano, T.; Onoue, K.; Nakada, Y.; Nakagawa, H.; Kumazawa, T.; Ueda, T.; Nishida, T.; Soeda, T.; Okayama, S.; Watanabe, M.; et al. Alteration of β-Adrenoceptor Signaling in Left Ventricle of Acute Phase Takotsubo Syndrome: A Human Study. Sci. Rep. 2018, 8, 12731. [Google Scholar] [CrossRef]
- Matta, A.G.; Carrié, D. Epidemiology, Pathophysiology, Diagnosis, and Principles of Management of Takotsubo Cardiomyopathy: A Review. Med. Sci. Monit. 2023, 29, e939020. [Google Scholar] [CrossRef]
- Khaja, M.; Stastka, P.; Kandhi, S.; Itare, V.; Latif, A.; Dileep, A. A Rare Case of Reverse Takotsubo Cardiomyopathy in a 28-Year-Old Female in Peripartum Period. Cureus 2022, 14, e30504. [Google Scholar] [CrossRef]
- Yang, W.-I.; Moon, J.Y.; Shim, M.; Yang, P.-S.; Kang, S.H.; Kim, S.-H.; Kim, W.-J.; Sung, J.-H.; Kim, I.J.; Lim, S.-W.; et al. Clinical Features Differentiating Takotsubo Cardiomyopathy in the Peripartum Period from Peripartum Cardiomyopathy. Heart Vessel. 2020, 35, 665–671. [Google Scholar] [CrossRef]
- Citro, R.; Giudice, R.; Mirra, M.; Petta, R.; Baldi, C.; Bossone, E.; Piscione, F. Is Tako-Tsubo Syndrome in the Postpartum Period a Clinical Entity Different from Peripartum Cardiomyopathy? J. Cardiovasc. Med. 2013, 14, 568–575. [Google Scholar] [CrossRef]
- Helman, T.J.; Headrick, J.P.; Stapelberg, N.J.C.; Braidy, N. The Sex-Dependent Response to Psychosocial Stress and Ischaemic Heart Disease. Front. Cardiovasc. Med. 2023, 10, 1072042. [Google Scholar] [CrossRef]
- Sattar, Y.; Siew, K.S.W.; Connerney, M.; Ullah, W.; Alraies, M.C. Management of Takotsubo Syndrome: A Comprehensive Review. Cureus 2020, 12, e6556. [Google Scholar] [CrossRef]
- Mirmoeeni, S.; Azari Jafari, A.; Lacci, J.V.; Seifi, A. The Incidence of Takotsubo Cardiomyopathy in Patients with Intracerebral Hemorrhage: A US Nationwide Study. Neurocrit. Care 2023, 38, 288–295. [Google Scholar] [CrossRef]
- Bossone, E.; Lyon, A.; Citro, R.; Athanasiadis, A.; Meimoun, P.; Parodi, G.; Cimarelli, S.; Omerovic, E.; Ferrara, F.; Limongelli, G.; et al. Takotsubo Cardiomyopathy: An Integrated Multi-Imaging Approach. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 366–377. [Google Scholar] [CrossRef]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018, 39, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Murugiah, K.; Wang, Y.; Desai, N.R.; Spatz, E.S.; Nuti, S.V.; Dreyer, R.P.; Krumholz, H.M. Trends in Short- and Long-Term Outcomes for Takotsubo Cardiomyopathy Among Medicare Fee-for-Service Beneficiaries, 2007 to 2012. JACC Heart Fail. 2016, 4, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Citro, R.; Rigo, F.; D’Andrea, A.; Ciampi, Q.; Parodi, G.; Provenza, G.; Piccolo, R.; Mirra, M.; Zito, C.; Giudice, R.; et al. Echocardiographic Correlates of Acute Heart Failure, Cardiogenic Shock, andin-Hospital Mortality in Tako-Tsubo Cardiomyopathy. JACC Cardiovasc. Imaging 2014, 7, 119–129. [Google Scholar] [CrossRef]
- Eitel, I.; Friedrich, M.G. T2-Weighted Cardiovascular Magnetic Resonance in Acute Cardiac Disease. J. Cardiovasc. Magn. Reson. 2011, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Scally, C.; Rudd, A.; Mezincescu, A.; Wilson, H.; Srivanasan, J.; Horgan, G.; Broadhurst, P.; Newby, D.E.; Henning, A.; Dawson, D.K. Persistent Long-Term Structural, Functional, and Metabolic Changes After Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2018, 137, 1039–1048. [Google Scholar] [CrossRef]
- Arcari, L.; Camastra, G.; Ciolina, F.; Limite, L.R.; Danti, M.; Sclafani, M.; Ansalone, G.; Musumeci, M.B.; Nagel, E.; Puntmann, V.; et al. Myocardial Oedema Contributes to Interstitial Expansion and Associates with Mechanical and Electrocardiographic Changes in Takotsubo Syndrome: A CMR T1 and T2 Mapping Study. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1082–1091. [Google Scholar] [CrossRef]
- Ojha, V.; Khurana, R.; Ganga, K.P.; Kumar, S. Advanced Cardiac Magnetic Resonance Imaging inTakotsubo Cardiomyopathy. Br. J. Radiol. 2020, 93, 20200514. [Google Scholar] [CrossRef]
- Cooper, L.T. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef]
- Ammirati, E.; Moslehi, J.J. Diagnosis and Treatment of Acute Myocarditis: A Review. JAMA 2023, 329, 1098–1113. [Google Scholar] [CrossRef]
- Golpour, A.; Patriki, D.; Hanson, P.J.; McManus, B.; Heidecker, B. Epidemiological Impact of Myocarditis. J. Clin. Med. 2021, 10, 603. [Google Scholar] [CrossRef]
- Sagar, S.; Liu, P.P.; Cooper, L.T. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef]
- Koenig, A.; Buskiewicz, I.; Huber, S.A. Age-Associated Changes in Estrogen Receptor Ratios Correlate with Increased Female Susceptibility to Coxsackievirus B3-Induced Myocarditis. Front. Immunol. 2017, 8, 1585. [Google Scholar] [CrossRef]
- Di Florio, D.N.; Sin, J.; Coronado, M.J.; Atwal, P.S.; Fairweather, D. Sex Differences in Inflammation, Redox Biology, Mitochondria and Autoimmunity. Redox Biol. 2020, 31, 101482. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; Musigk, N.; Heidecker, B.; Lyle, M.A.; Cooper, L.T.; Bruno, K.A. Sex and Gender Differences in Myocarditis and Dilated Cardiomyopathy: An Update. Front. Cardiovasc. Med. 2023, 10, 1129348. [Google Scholar] [CrossRef]
- Kotanidis, C.P.; Bazmpani, M.-A.; Haidich, A.-B.; Karvounis, C.; Antoniades, C.; Karamitsos, T.D. Diagnostic Accuracy of Cardiovascular Magnetic Resonance in Acute Myocarditis: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2018, 11, 1583–1590. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- Hausvater, A.; Smilowitz, N.R.; Li, B.; Redel-Traub, G.; Quien, M.; Qian, Y.; Zhong, J.; Nicholson, J.M.; Camastra, G.; Bière, L.; et al. Myocarditis in Relation to Angiographic Findings in Patients with Provisional Diagnoses of MINOCA. JACC Cardiovasc. Imaging 2020, 13, 1906–1913. [Google Scholar] [CrossRef]
- Eichhorn, C.; Greulich, S.; Bucciarelli-Ducci, C.; Sznitman, R.; Kwong, R.Y.; Gräni, C. Multiparametric Cardiovascular Magnetic Resonance Approach in Diagnosing, Monitoring, and Prognostication of Myocarditis. JACC Cardiovasc. Imaging 2022, 15, 1325–1338. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K.; Dall’Armellina, E.; Karamitsos, T.D.; Francis, J.M.; Ntusi, N.; Holloway, C.; Choudhury, R.P.; Kardos, A.; Robson, M.D.; et al. T(1) Mapping for the Diagnosis of Acute Myocarditis Using CMR: Comparison to T2-Weighted and Late Gadolinium Enhanced Imaging. JACC Cardiovasc. Imaging 2013, 6, 1048–1058. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Walls, M.; Giri, S.; Verhaert, D.; Rajagopalan, S.; Moore, S.; Simonetti, O.P.; Raman, S.V. Improved Detection of Myocardial Involvement in Acute Inflammatory Cardiomyopathies Using T2 Mapping. Circ. Cardiovasc. Imaging 2012, 5, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; Arai, A.E.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 Mapping and Extracellular Volume Quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology Consensus Statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Topriceanu, C.-C.; Fornasiero, M.; Seo, H.; Webber, M.; Keenan, K.E.; Stupic, K.F.; Bruehl, R.; Ittermann, B.; Price, K.; McGrath, L.; et al. Developing a Medical Device-Grade T2 Phantom Optimized for Myocardial T2 Mapping by Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2023, 25, 19. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.A.; Lee, Y.J.; Salerno, M. Diagnostic Performance of Extracellular Volume, Native T1, and T2 Mapping Versus Lake Louise Criteria by Cardiac Magnetic Resonance for Detection of Acute Myocarditis: A Meta-Analysis. Circ. Cardiovasc. Imaging 2018, 11, e007598. [Google Scholar] [CrossRef] [PubMed]
- Blissett, S.; Chocron, Y.; Kovacina, B.; Afilalo, J. Diagnostic and Prognostic Value of Cardiac Magnetic Resonance in Acute Myocarditis: A Systematic Review and Meta-Analysis. Int. J. Cardiovasc. Imaging 2019, 35, 2221–2229. [Google Scholar] [CrossRef]
- Brett, N.J.; Strugnell, W.E.; Slaughter, R.E. Acute Myocarditis Demonstrated on CT Coronary Angiography with MRI Correlation. Circ. Cardiovasc. Imaging 2011, 4, e5–e6. [Google Scholar] [CrossRef]
- Dambrin, G.; Laissy, J.P.; Serfaty, J.M.; Caussin, C.; Lancelin, B.; Paul, J.F. Diagnostic Value of ECG-Gated Multidetector Computed Tomography in the Early Phase of Suspected Acute Myocarditis. A Preliminary Comparative Study with Cardiac MRI. Eur. Radiol. 2007, 17, 331–338. [Google Scholar] [CrossRef]
- Citro, R.; Pontone, G.; Bellino, M.; Silverio, A.; Iuliano, G.; Baggiano, A.; Manka, R.; Iesu, S.; Vecchione, C.; Asch, F.M.; et al. Role of Multimodality Imaging in Evaluation of Cardiovascular Involvement in COVID-19. Trends Cardiovasc. Med. 2021, 31, 8–16. [Google Scholar] [CrossRef]
- Imazio, M.; Gaita, F.; LeWinter, M. Evaluation and Treatment of Pericarditis: A Systematic Review. JAMA 2015, 314, 1498–1506. [Google Scholar] [CrossRef]
- Dababneh, E.; Siddique, M.S. Pericarditis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lazarou, E.; Tsioufis, P.; Vlachopoulos, C.; Tsioufis, C.; Lazaros, G. Acute Pericarditis: Update. Curr. Cardiol. Rep. 2022, 24, 905–913. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P. The 2015 ESC Guidelines on the Diagnosis and Management of Pericardial Diseases. Eur. Heart J. 2015, 36, 2873–2874. [Google Scholar] [CrossRef]
- Prepoudis, A.; Koechlin, L.; Nestelberger, T.; Boeddinghaus, J.; Lopez-Ayala, P.; Wussler, D.; Zimmermann, T.; Rubini Giménez, M.; Strebel, I.; Puelacher, C.; et al. Incidence, Clinical Presentation, Management, and Outcome of Acute Pericarditis and Myopericarditis. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 137–147. [Google Scholar] [CrossRef]
- Cepas-Guillén, P.L.; Borrego-Rodriguez, J.; Flores-Umanzor, E.; Echarte-Morales, J.; Fernandez-Valledor, A.; Menendez-Suarez, P.; Vazquez, S.; Alonso, N.; Ortiz, J.T.; Regueiro, A.; et al. Outcomes of Nonagenarians with ST Elevation Myocardial Infarction. Am. J. Cardiol. 2020, 125, 11–18. [Google Scholar] [CrossRef]
- Bouriche, F.; Toro, A.; Negre, V.; Yvorra, S. Acute Pericarditis: Aetiologic Diagnosis and Practical Aspect of the Management. Curr. Probl. Cardiol. 2021, 46, 100769. [Google Scholar] [CrossRef]
- Chetrit, M.; Xu, B.; Verma, B.R.; Klein, A.L. Multimodality Imaging for the Assessment of Pericardial Diseases. Curr. Cardiol. Rep. 2019, 21, 41. [Google Scholar] [CrossRef]
- Imazio, M.; Pivetta, E.; Palacio Restrepo, S.; Sormani, P.; Pedrotti, P.; Quarta, G.; Brucato, A.; Bubbico, E.; Dal Corso, M.; Milazzo, A.; et al. Usefulness of Cardiac Magnetic Resonance for Recurrent Pericarditis. Am. J. Cardiol. 2020, 125, 146–151. [Google Scholar] [CrossRef]
- Kiraç, S. The role of advanced cardiac imaging methods in coronary artery disease. Anatol. J. Cardiol. 2008, 8 (Suppl. 1), 1–4. [Google Scholar]
- Russell, R.R.; Zaret, B.L. Nuclear Cardiology: Present and Future. Curr. Probl. Cardiol. 2006, 31, 557–629. [Google Scholar] [CrossRef]
- Elfigih, I.A.; Henein, M.Y. Non-Invasive Imaging in Detecting Myocardial Viability: Myocardial Function versus Perfusion. Int. J. Cardiol. Heart Vasc. 2014, 5, 51–56. [Google Scholar] [CrossRef]
- Slomka, P.; Berman, D.S.; Alexanderson, E.; Germano, G. The Role of PET Quantification in Cardiovascular Imaging. Clin. Transl. Imaging 2014, 2, 343–358. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Elvas, F.; De Schepper, S.; Kennedy, J.A.; Israel, O. SPECT/CT: Standing on the Shoulders of Giants, It Is Time to Reach for the Sky! J. Nucl. Med. 2020, 61, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Rischpler, C.; Nekolla, S.G.; Heusch, G.; Umutlu, L.; Rassaf, T.; Heusch, P.; Herrmann, K.; Nensa, F. Cardiac PET/MRI-an Update. Eur. J. Hybrid Imaging 2019, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Leucker, T.M. Applications of PET-MR Imaging in Cardiovascular Disorders. PET Clin. 2020, 15, 509–520. [Google Scholar] [CrossRef]
- Ritt, P. Recent Developments in SPECT/CT. Semin. Nucl. Med. 2022, 52, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.H.; De Cecco, C.N.; Schoepf, U.J.; Spandorfer, A.; Eid, M.; De Santis, D.; Varga-Szemes, A.; van Assen, M.; von Knebel-Doeberitz, P.L.; Tesche, C.; et al. Dual-Energy CT of the Heart Current and Future Status. Eur. J. Radiol. 2018, 105, 110–118. [Google Scholar] [CrossRef]
- Chellini, D.; Kinman, K. Dual-Energy CT Principles and Applications. Radiol. Technol. 2020, 91, 561CT–576CT. [Google Scholar]
- Tarkowski, P.; Czekajska-Chehab, E. Dual-Energy Heart CT: Beyond Better Angiography-Review. J. Clin. Med. 2021, 10, 5193. [Google Scholar] [CrossRef]
- Dell’Aversana, S.; Ascione, R.; De Giorgi, M.; De Lucia, D.R.; Cuocolo, R.; Boccalatte, M.; Sibilio, G.; Napolitano, G.; Muscogiuri, G.; Sironi, S.; et al. Dual-Energy CT of the Heart: A Review. J. Imaging 2022, 8, 236. [Google Scholar] [CrossRef]
- Schwarz, F.; Ruzsics, B.; Schoepf, U.J.; Bastarrika, G.; Chiaramida, S.A.; Abro, J.A.; Brothers, R.L.; Vogt, S.; Schmidt, B.; Costello, P.; et al. Dual-Energy CT of the Heart--Principles and Protocols. Eur. J. Radiol. 2008, 68, 423–433. [Google Scholar] [CrossRef]
- Miller, C.A.; Naish, J.H.; Bishop, P.; Coutts, G.; Clark, D.; Zhao, S.; Ray, S.G.; Yonan, N.; Williams, S.G.; Flett, A.S.; et al. Comprehensive Validation of Cardiovascular Magnetic Resonance Techniques for the Assessment of Myocardial Extracellular Volume. Circ. Cardiovasc. Imaging 2013, 6, 373–383. [Google Scholar] [CrossRef]
- Lee, H.-J.; Im, D.J.; Youn, J.-C.; Chang, S.; Suh, Y.J.; Hong, Y.J.; Kim, Y.J.; Hur, J.; Choi, B.W. Myocardial Extracellular Volume Fraction with Dual-Energy Equilibrium Contrast-Enhanced Cardiac CT in Nonischemic Cardiomyopathy: A Prospective Comparison with Cardiac MR Imaging. Radiology 2016, 280, 49–57. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-Counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. J. Imaging 2022, 8, 112. [Google Scholar] [CrossRef]
- Esquivel, A.; Ferrero, A.; Mileto, A.; Baffour, F.; Horst, K.; Rajiah, P.S.; Inoue, A.; Leng, S.; McCollough, C.; Fletcher, J.G. Photon-Counting Detector CT: Key Points Radiologists Should Know. Korean J. Radiol. 2022, 23, 854–865. [Google Scholar] [CrossRef]
- Nasir, K.; Sharma, G.; Blumenthal, R.S. Sex Differences in Coronary Plaque Composition and Progression: Will It Influence Clinical Management? JACC Cardiovasc. Imaging 2020, 13, 2397–2399. [Google Scholar] [CrossRef]





| CCTA | CMR | |
|---|---|---|
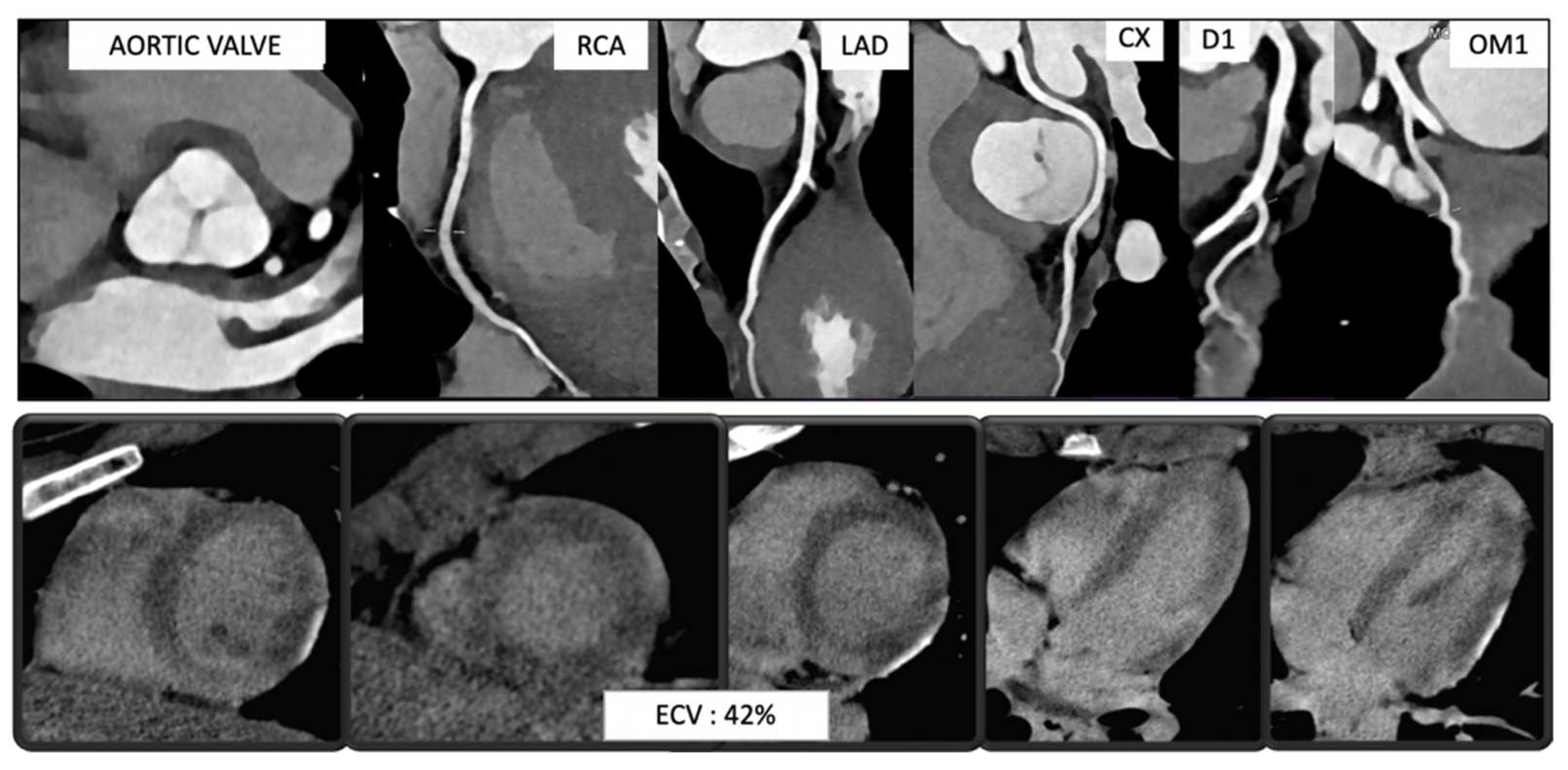
| Acute Coronary Syndrome |
|
|
| MINOCA |
|
|
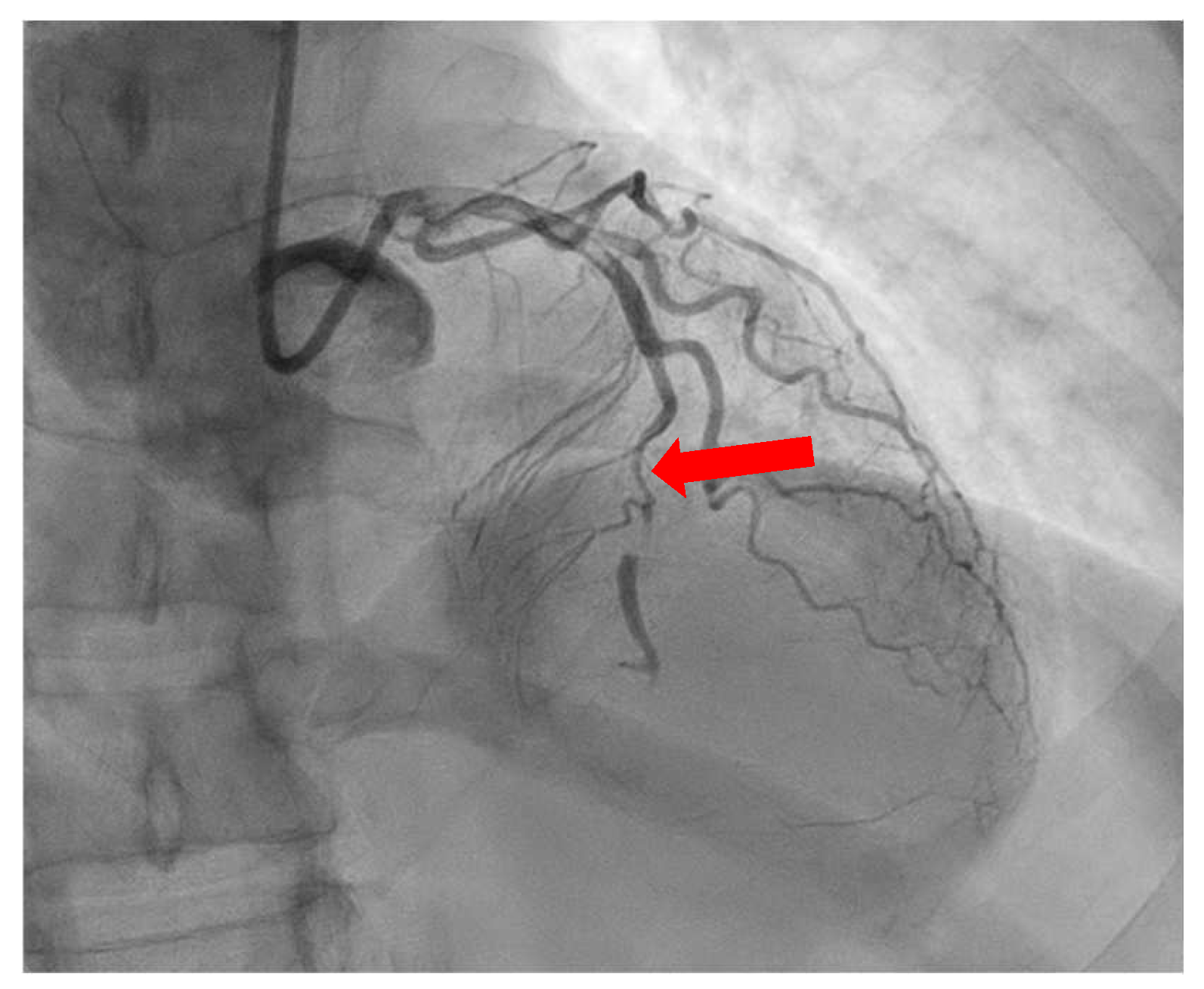
| SCAD |
|
|
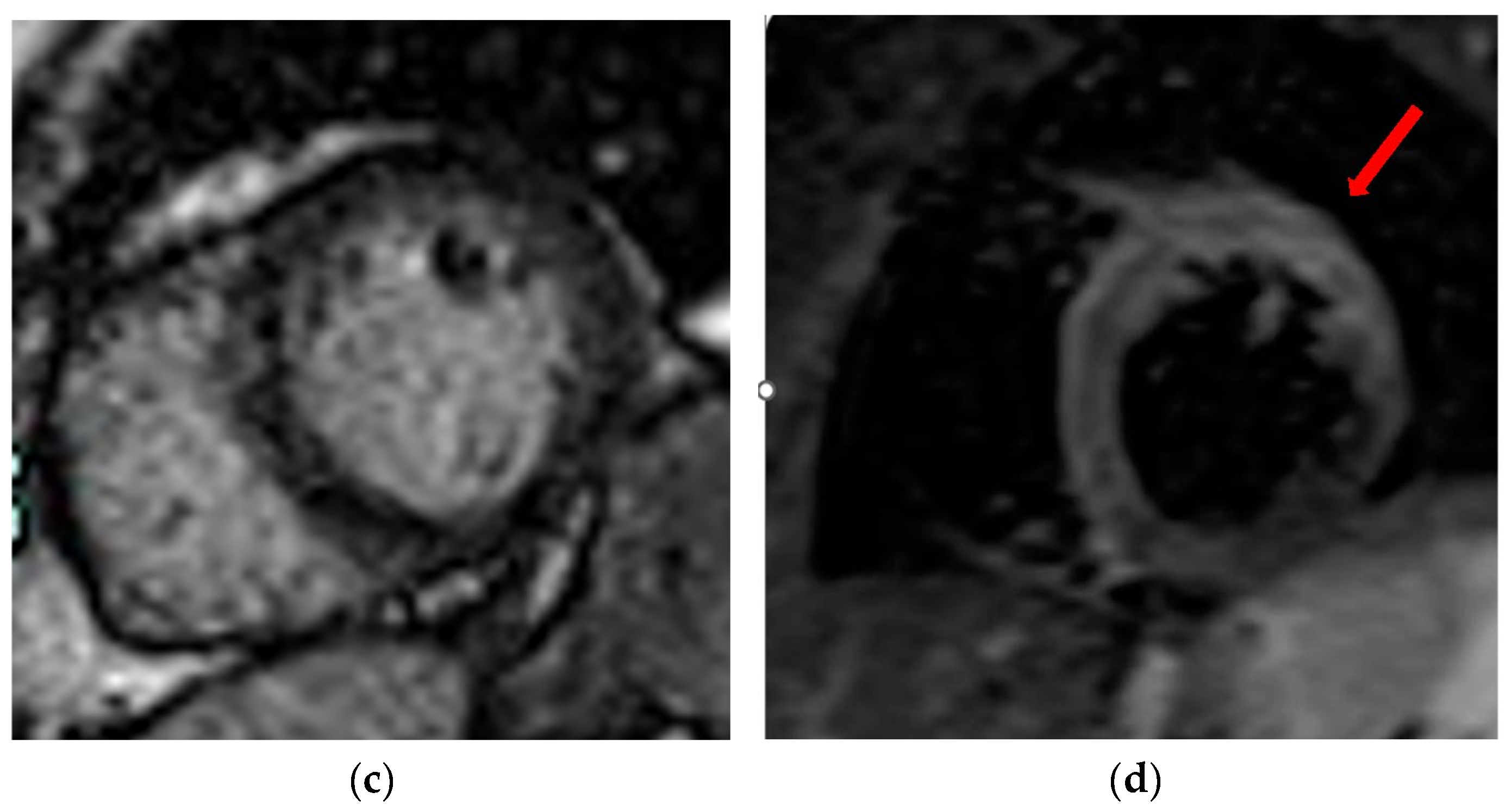
| Takotsubo Syndrome |
|
|
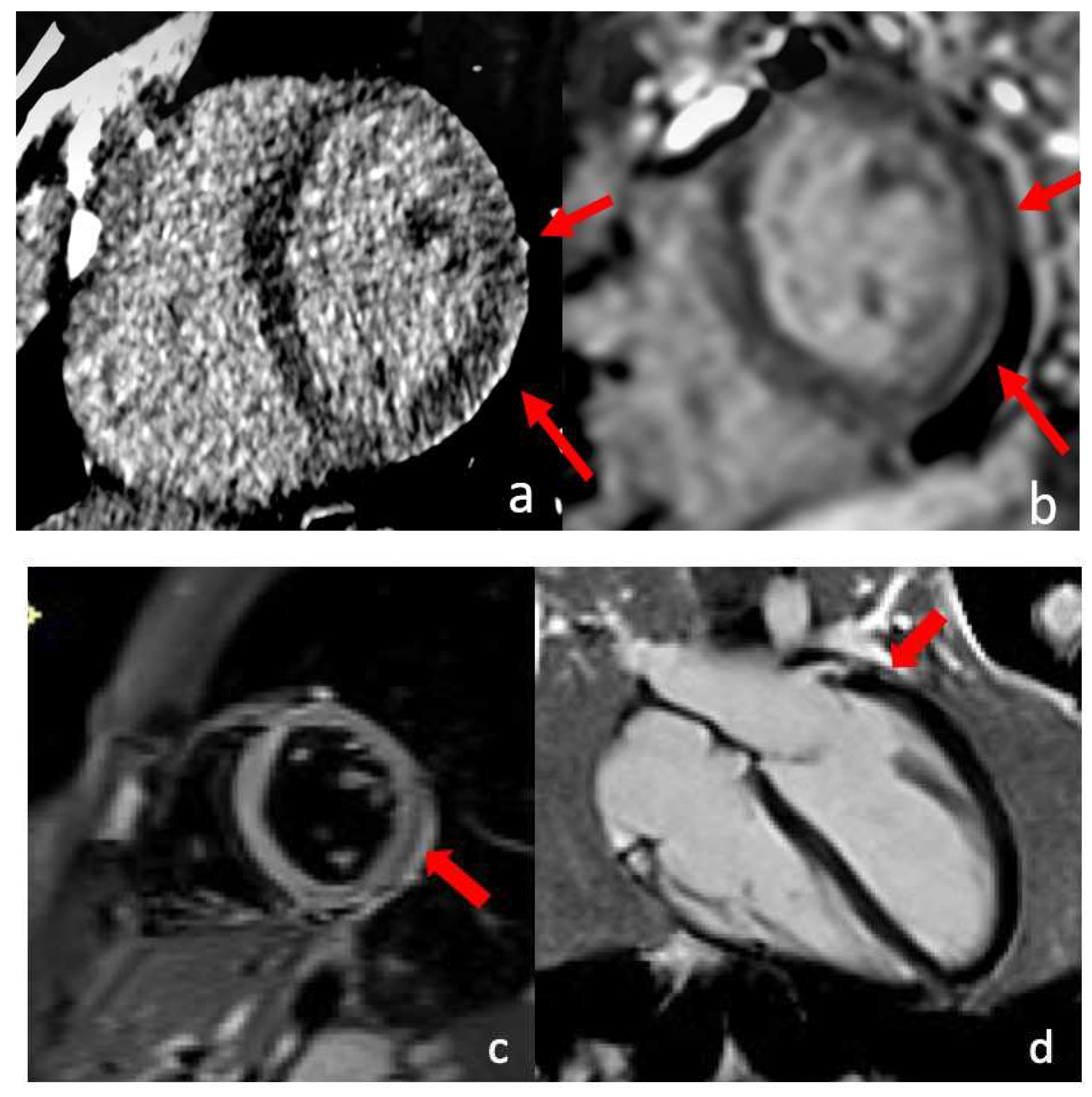
| Myocarditis |
|
|
| Pericarditis |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Aversana, F.; Tedeschi, C.; Comune, R.; Gallo, L.; Ferrandino, G.; Basco, E.; Tamburrini, S.; Sica, G.; Masala, S.; Scaglione, M.; et al. Advanced Cardiac Imaging and Women’s Chest Pain: A Question of Gender. Diagnostics 2023, 13, 2611. https://doi.org/10.3390/diagnostics13152611
Dell’Aversana F, Tedeschi C, Comune R, Gallo L, Ferrandino G, Basco E, Tamburrini S, Sica G, Masala S, Scaglione M, et al. Advanced Cardiac Imaging and Women’s Chest Pain: A Question of Gender. Diagnostics. 2023; 13(15):2611. https://doi.org/10.3390/diagnostics13152611
Chicago/Turabian StyleDell’Aversana, Federica, Carlo Tedeschi, Rosita Comune, Luigi Gallo, Giovanni Ferrandino, Emilia Basco, Stefania Tamburrini, Giacomo Sica, Salvatore Masala, Mariano Scaglione, and et al. 2023. "Advanced Cardiac Imaging and Women’s Chest Pain: A Question of Gender" Diagnostics 13, no. 15: 2611. https://doi.org/10.3390/diagnostics13152611
APA StyleDell’Aversana, F., Tedeschi, C., Comune, R., Gallo, L., Ferrandino, G., Basco, E., Tamburrini, S., Sica, G., Masala, S., Scaglione, M., & Liguori, C. (2023). Advanced Cardiac Imaging and Women’s Chest Pain: A Question of Gender. Diagnostics, 13(15), 2611. https://doi.org/10.3390/diagnostics13152611









