Loss or Dilution—A New Diagnostic Method to Assess the Impact of Dilution on Standard Laboratory Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Sampling and Laboratory Tests
2.3. Data Collection, Anesthesia and Cardiopulmonary Bypass Management
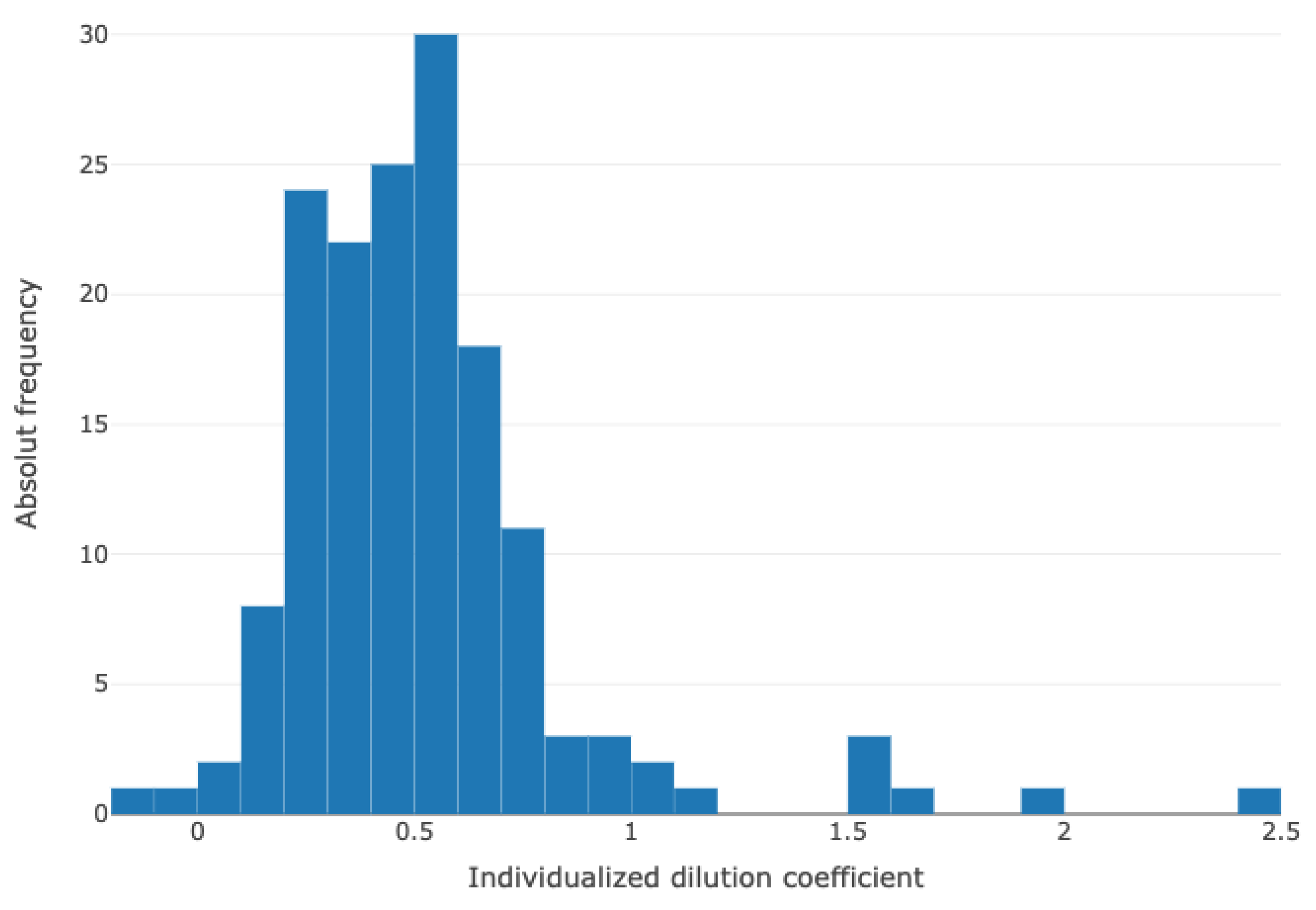
2.4. Calculations of Blood Volume and the Individual Dilutional Coefficients
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Surgical Procedure and Fluid Management
3.3. Calculation of Individual Dilution-Related Volume
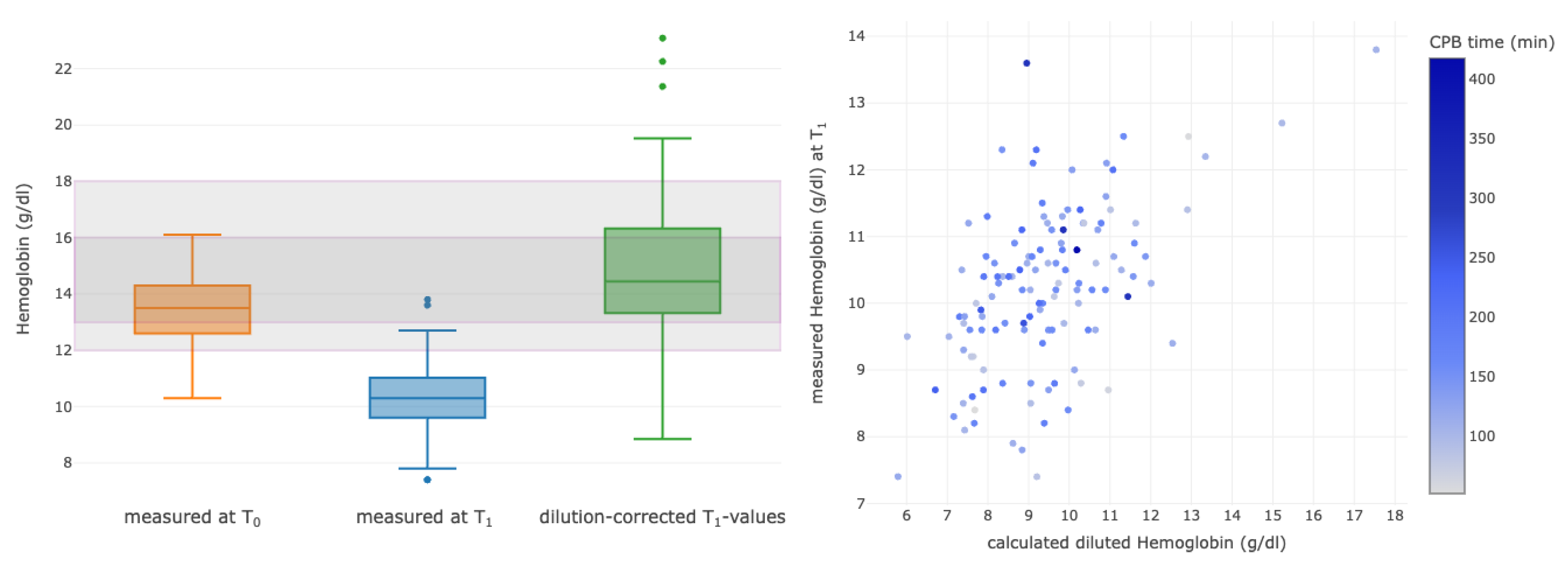
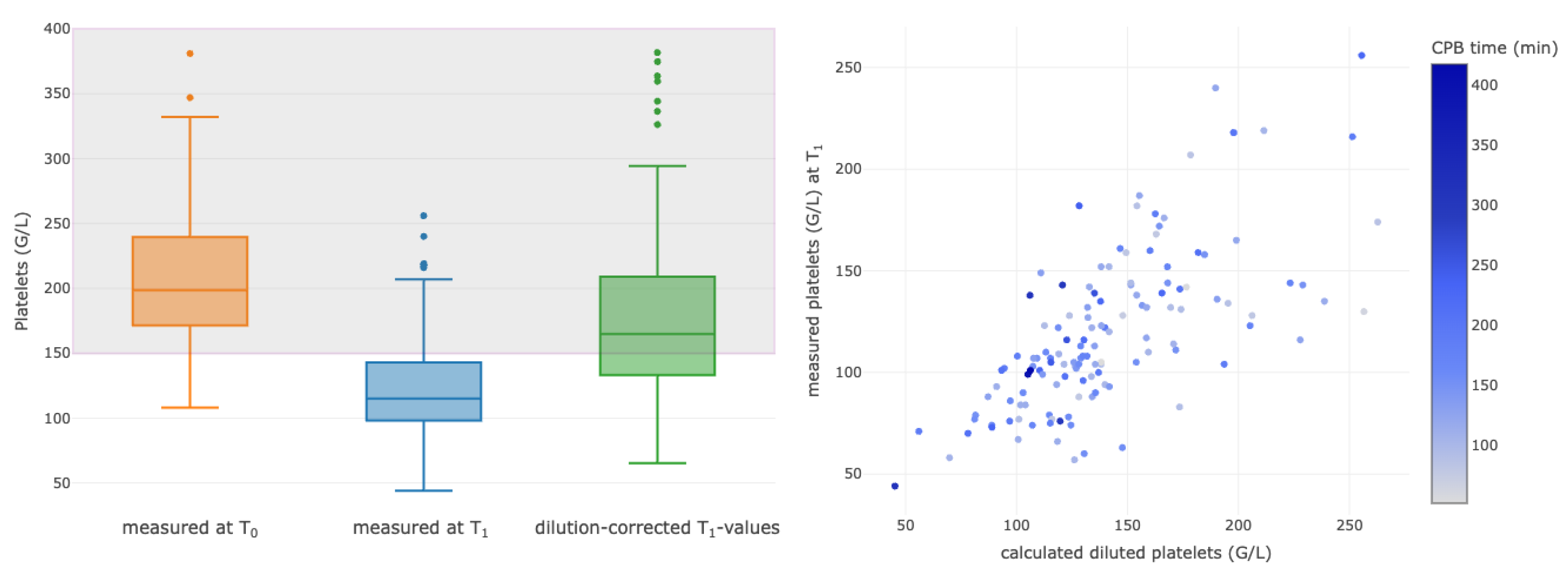
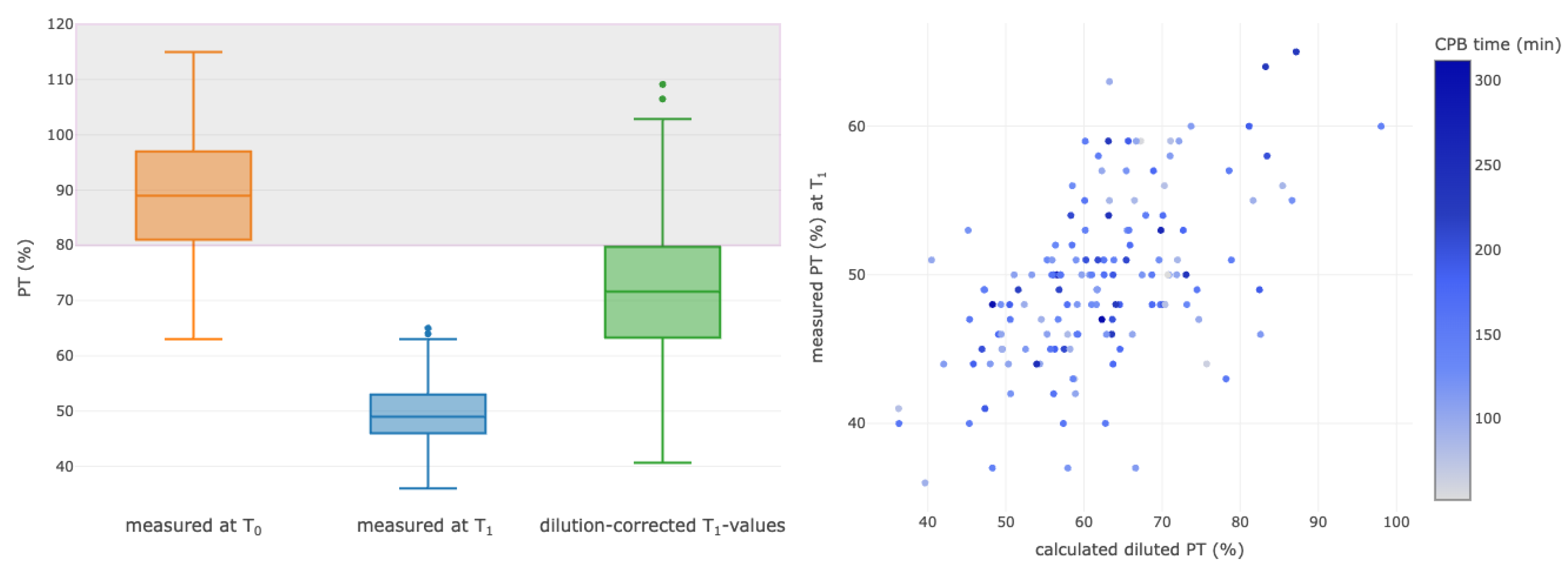
3.4. Correlations between Selected Calculated Diluted and Measured Parameters
4. Discussion
4.1. Future Perspectives
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, A.G.; Friesenecker, B.; McCarthy, M.; Sakai, H.; Intaglietta, M. Plasma viscosity regulates capillary perfusion during extreme hemodilution in hamster skinfold model. Am. J. Physiol.-Heart Circ. Physiol. 1998, 275, H2170–H2180. [Google Scholar]
- Murphy, G.S.; Hessel, E.A.I.; Groom, R.C. Optimal Perfusion During Cardiopulmonary Bypass: An Evidence-Based Approach. Anesth. Analg. 2009, 108, 1394. [Google Scholar]
- Habib, R.H.; Zacharias, A.; Schwann, T.A.; Riordan, C.J.; Durham, S.J.; Shah, A. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: Should current practice be changed? J. Thorac. Cardiovasc. Surg. 2003, 125, 1438–1450. [Google Scholar]
- Grist, G.; Whittaker, C.; Merrigan, K.; Fenton, J.; Worrall, E.; O’Brien, J.; Lofland, G. The Correlation of Fluid Balance Changes During Cardiopulmonary Bypass to Mortality in Pediatric and Congenital Heart Surgery Patients. J. Extra Corpor. Technol. 2011, 43, 215–226. [Google Scholar]
- Habib, R.H.; Zacharias, A.; Schwann, T.A.; Riordan, C.J.; Engoren, M.; Durham, S.J.; Shah, A. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: Implications on operative outcome. Crit. Care Med. 2005, 33, 1749. [Google Scholar] [CrossRef] [PubMed]
- Karkouti, K.; Beattie, W.S.; Wijeysundera, D.N.; Rao, V.; Chan, C.; Dattilo, K.M.; Djaiani, G.; Ivanov, J.; Karski, J.; David, T. Hemodilution during cardiopulmonary bypass is an independent risk factor for acute renal failure in adult cardiac surgery. J. Thorac. Cardiovasc. Surg. 2005, 129, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.J.; Reeves, B.C.; Rogers, C.A.; Rizvi, S.I.A.; Culliford, L.; Angelini, G.D. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007, 116, 2544–2552. [Google Scholar]
- Engoren, M.C.; Habib, R.H.; Zacharias, A.; Schwann, T.A.; Riordan, C.J.; Durham, S.J. Effect of blood transfusion on long-term survival after cardiac operation. Ann. Thorac. Surg. 2002, 74, 1180–1186. [Google Scholar]
- Kuduvalli, M.; Oo, A.Y.; Newall, N.; Grayson, A.D.; Jackson, M.; Desmond, M.J.; Fabri, B.M.; Rashid, A. Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery☆. Eur. J. Cardio-Thorac. Surg. 2005, 27, 592–598. [Google Scholar] [CrossRef]
- Koch, C.; Li, L.; Figueroa, P.; Mihaljevic, T.; Svensson, L.; Blackstone, E.H. Transfusion and Pulmonary Morbidity After Cardiac Surgery. Ann. Thorac. Surg. 2009, 88, 1410–1418. [Google Scholar]
- Koch, C.G.; Li, L.; Duncan, A.I.; Mihaljevic, T.; Loop, F.D.; Starr, N.J.; Blackstone, E.H. Transfusion in Coronary Artery Bypass Grafting is Associated with Reduced Long-Term Survival. Ann. Thorac. Surg. 2006, 81, 1650–1657. [Google Scholar] [PubMed]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–745. [Google Scholar]
- Nadler, S.B.; Hidalgo, J.H.; Bloch, T. Prediction of blood volume in normal human adults. Surgery 1962, 51, 224–232. [Google Scholar]
- Swaminathan, M.; Phillips-Bute, B.G.; Conlon, P.J.; Smith, P.K.; Newman, M.F.; Stafford-Smith, M. The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann. Thorac. Surg. 2003, 76, 784–791, discussion 792. [Google Scholar] [CrossRef]
- Ranucci, M.; Carboni, G.; Cotza, M.; Bianchi, P.; Di Dedda, U.; Aloisio, T. Hemodilution on Cardiopulmonary Bypass as a Determinant of Early Postoperative Hyperlactatemia. PLoS ONE 2015, 10, e0126939. [Google Scholar] [CrossRef] [PubMed]
- Erpicum, M.; Dardenne, N.; Hans, G.; Larbuisson, R.; Defraigne, J.O. Prediction of the post-dilution hematocrit during cardiopulmonary bypass. Are new formulas needed? Perfusion 2016, 31, 458–464. [Google Scholar] [CrossRef]
- Hajjar, L.A.; Vincent, J.L.; Galas, F.R.; Nakamura, R.E.; Silva, C.M.; Santos, M.H.; Fukushima, J.; Kalil Filho, R.; Sierra, D.B.; Lopes, N.H.; et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA 2010, 304, 1559–1567. [Google Scholar] [CrossRef]
- Vivacqua, A.; Koch, C.G.; Yousuf, A.M.; Nowicki, E.R.; Houghtaling, P.L.; Blackstone, E.H.; Sabik, J.F. Morbidity of Bleeding After Cardiac Surgery: Is It Blood Transfusion, Reoperation for Bleeding, or Both? Ann. Thorac. Surg. 2011, 91, 1780–1790. [Google Scholar] [CrossRef]
- Kougias, P.; Sharath, S.; Barshes, N.R.; Chen, M.; Mills, J.L., Sr. Effect of postoperative anemia and baseline cardiac risk on serious adverse outcomes after major vascular interventions. J. Vasc. Surg. 2017, 66, 1836–1843. [Google Scholar]
- El-Sayed, H.; Goodall, S.R.; Hainsworth, R. Re-evaluation of Evans blue dye dilution method of plasma volume measurement. Clin. Lab. Haematol. 1995, 17, 189–194. [Google Scholar]
- Elhassan, M.G.; Chao, P.W.; Curiel, A. The Conundrum of Volume Status Assessment: Revisiting Current and Future Tools Available for Physicians at the Bedside. Cureus 2021, 13, e15253. [Google Scholar] [PubMed]
- Singh, S.; Kuschner, W.G.; Lighthall, G. Perioperative intravascular fluid assessment and monitoring: A narrative review of established and emerging techniques. Anesthesiol. Res. Pract. 2011, 2011, 231493. [Google Scholar] [PubMed]
- Rajsic, S.; Breitkopf, R.; Bachler, M.; Treml, B. Diagnostic Modalities in Critical Care: Point-of-Care Approach. Diagnostics 2021, 11, 2202. [Google Scholar]
- Woods, S.E.; Noble, G.; Smith, M.J.; Hasselfeld, K. The Influence of Gender in Patients Undergoing Coronary Artery Bypass Graft Surgery: An Eight-Year Prospective Hospitalized Cohort Study. J. Am. Coll. Surg. 2003, 196, 428–434. [Google Scholar]
- Giacinto, O.; Satriano, U.; Nenna, A.; Spadaccio, C.; Lusini, M.; Mastroianni, C.; Nappi, F.; Chello, M. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Lamke, L.O.; Nilsson, G.E.; Reithner, H.L. Insensible perspiration from the skin under standardized environmental conditions. Scand. J. Clin. Lab. Investig. 1977, 37, 325–331. [Google Scholar] [CrossRef]
- Reithner, L.; Johansson, H.; Strouth, L. Insensible perspiration during anaesthesia and surgery. Acta Anaesthesiol. Scand. 1980, 24, 362–366. [Google Scholar] [CrossRef]
- Reithner, L. Insensible water loss from the respiratory tract in patients with fever. Acta Chir. Scand. 1981, 147, 163–167. [Google Scholar]
- Lamke, L.O.; Nilsson, G.; Reithner, L. The influence of elevated body temperature on skin perspiration. Acta Chir. Scand. 1980, 146, 81–84. [Google Scholar]

| Characteristics | Patient Data (n = 174) |
|---|---|
| Age (years) | 67 (59–74) |
| Female sex (%) | 52 (29.9) |
| Height (cm) | 174.5 (168–180) |
| Weight (kg) | 80 (70–90) |
| Body mass index (kg/m2) | 26.1 (23.7–29.1) |
| Euroscore II | 1.44 (0.86–2.52) |
| Valve surgery | 97 (56%) |
| Coronary artery bypass graft surgery | 41 (24%) |
| Combined CABG with valve surgery | 36 (20%) |
| CPB duration (minutes) | 143 (111–177) |
| Laboratory measurements | |
| Hemoglobin T0 (g/dL) | 12.9 (11.6–14.2) |
| Prothrombin time T0 (%) | 89 (81–97) |
| Platelet count T0 (G/L) | 198 (168–237) |
| Hemoglobin T1 (g/dL) | 10.1 (9.4–10.8) |
| Prothrombin time T1 (%) | 49 (45–52) |
| Platelet count T1 (G/L) | 113 (94–143) |
| Fluid Balance | Patient Data (n = 174) |
|---|---|
| Infusions | |
| Colloids (mL) | 500 (500–500) |
| Crystalloids (mL) | 520 (500–900) |
| CPB positive fluid balance change (mL) | 2790 (1949–3548) |
| Packed red blood cells units (280 mL each) | |
| One concentrate | 16 (9%) |
| Two concentrates | 13 (7%) |
| ≥Three concentrates | 8 (5%) |
| Platelet concentrate units (300 mL each) | 13 (8%) |
| Desmopressin | 9 (5%) |
| Diuresis (estimated in mL) | 1016 (712–1541) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Innerhofer, N.; Rajsic, S.; Ronzani, M.; Breitkopf, R.; Gollmann Tepeköylü, C.; Velik-Salchner, C.; Schlosser, L.; Fries, D.; Streif, W.; Schirmer, M.; et al. Loss or Dilution—A New Diagnostic Method to Assess the Impact of Dilution on Standard Laboratory Parameters. Diagnostics 2023, 13, 2596. https://doi.org/10.3390/diagnostics13152596
Innerhofer N, Rajsic S, Ronzani M, Breitkopf R, Gollmann Tepeköylü C, Velik-Salchner C, Schlosser L, Fries D, Streif W, Schirmer M, et al. Loss or Dilution—A New Diagnostic Method to Assess the Impact of Dilution on Standard Laboratory Parameters. Diagnostics. 2023; 13(15):2596. https://doi.org/10.3390/diagnostics13152596
Chicago/Turabian StyleInnerhofer, Nicole, Sasa Rajsic, Marco Ronzani, Robert Breitkopf, Can Gollmann Tepeköylü, Corinna Velik-Salchner, Lisa Schlosser, Dietmar Fries, Werner Streif, Michael Schirmer, and et al. 2023. "Loss or Dilution—A New Diagnostic Method to Assess the Impact of Dilution on Standard Laboratory Parameters" Diagnostics 13, no. 15: 2596. https://doi.org/10.3390/diagnostics13152596
APA StyleInnerhofer, N., Rajsic, S., Ronzani, M., Breitkopf, R., Gollmann Tepeköylü, C., Velik-Salchner, C., Schlosser, L., Fries, D., Streif, W., Schirmer, M., & Martini, J. (2023). Loss or Dilution—A New Diagnostic Method to Assess the Impact of Dilution on Standard Laboratory Parameters. Diagnostics, 13(15), 2596. https://doi.org/10.3390/diagnostics13152596








