Figure 1.
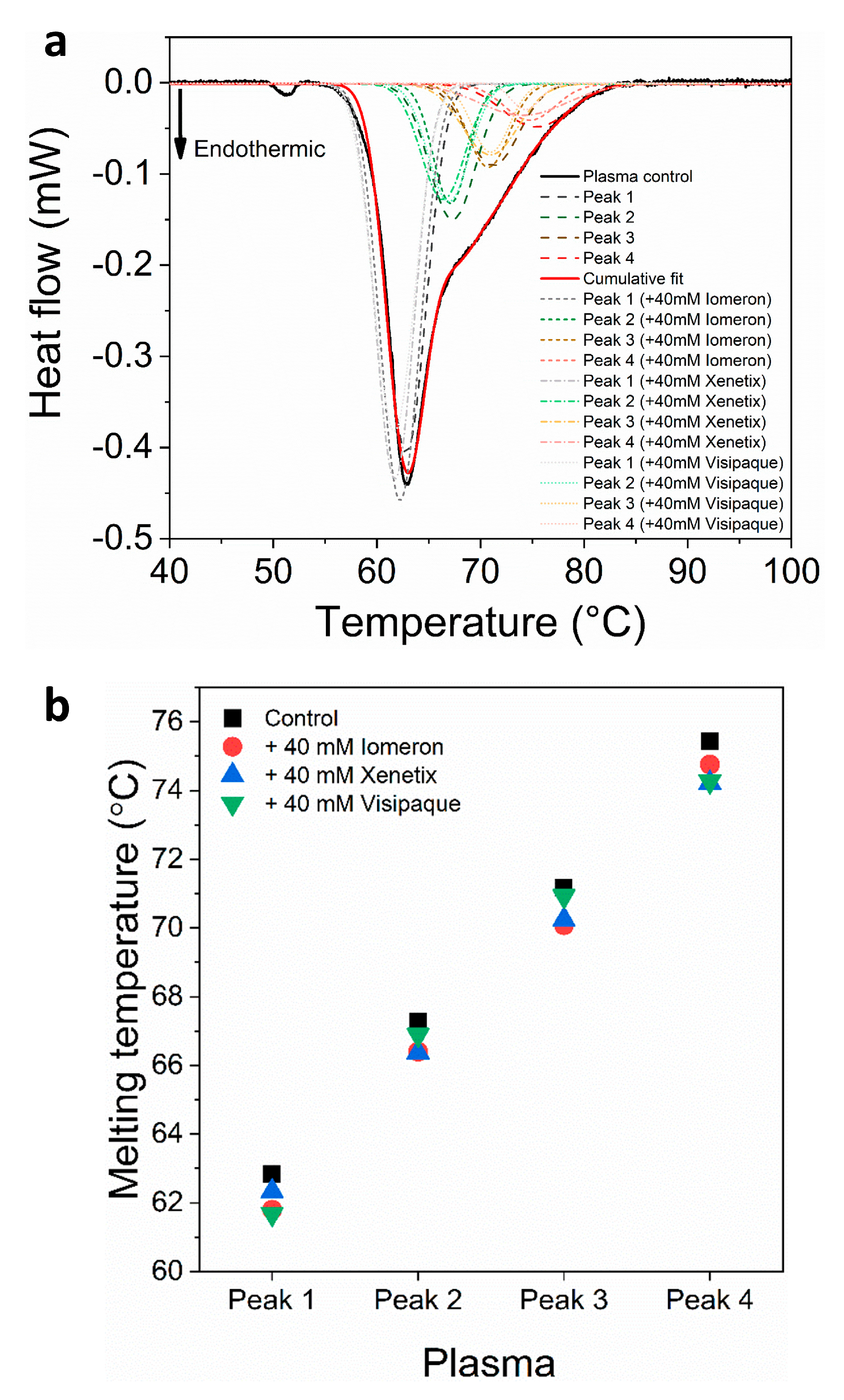
(a) Deconvoluted thermal denaturation curves of human blood plasma in the absence (experimental curve—solid black, cumulative fit—solid red and dashed lines) and in the presence of 40 mM Iomeprol, Iobitridol and Iodixanol (short dashed, short dashed dot and short dotted lines), respectively. (b) Schematic representation of the deconvoluted thermal transition peaks of anticoagulated plasma as a function of the melting temperature in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque.
Figure 1.
(a) Deconvoluted thermal denaturation curves of human blood plasma in the absence (experimental curve—solid black, cumulative fit—solid red and dashed lines) and in the presence of 40 mM Iomeprol, Iobitridol and Iodixanol (short dashed, short dashed dot and short dotted lines), respectively. (b) Schematic representation of the deconvoluted thermal transition peaks of anticoagulated plasma as a function of the melting temperature in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque.
Figure 2.
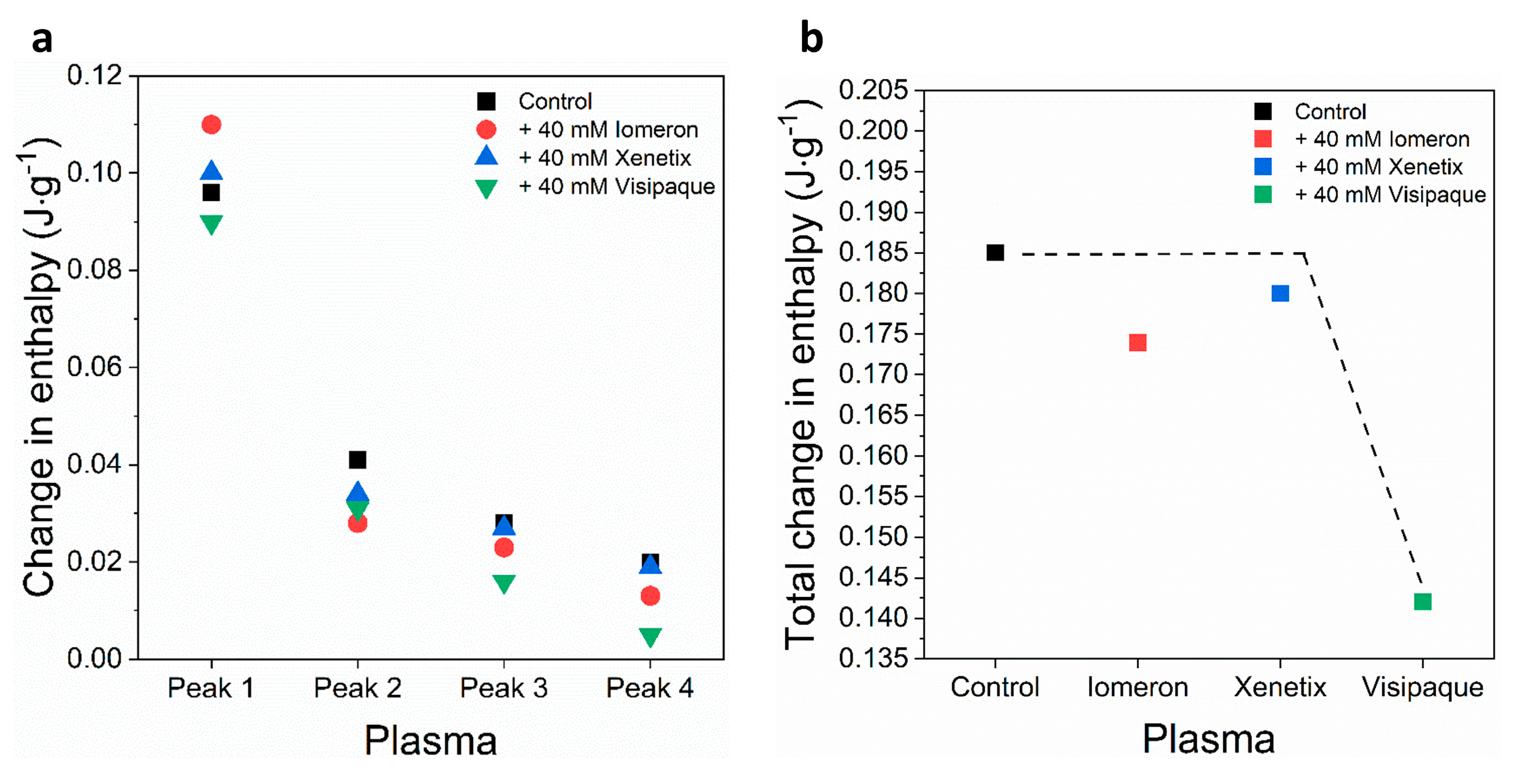
Schematic representation of the deconvoluted thermal transition peak of anticoagulated plasma as a function of the (a) calculated enthalpy change and (b) total enthalpy change in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque. The black dotted line represents the highest total change in enthalpy.
Figure 2.
Schematic representation of the deconvoluted thermal transition peak of anticoagulated plasma as a function of the (a) calculated enthalpy change and (b) total enthalpy change in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque. The black dotted line represents the highest total change in enthalpy.
Figure 3.
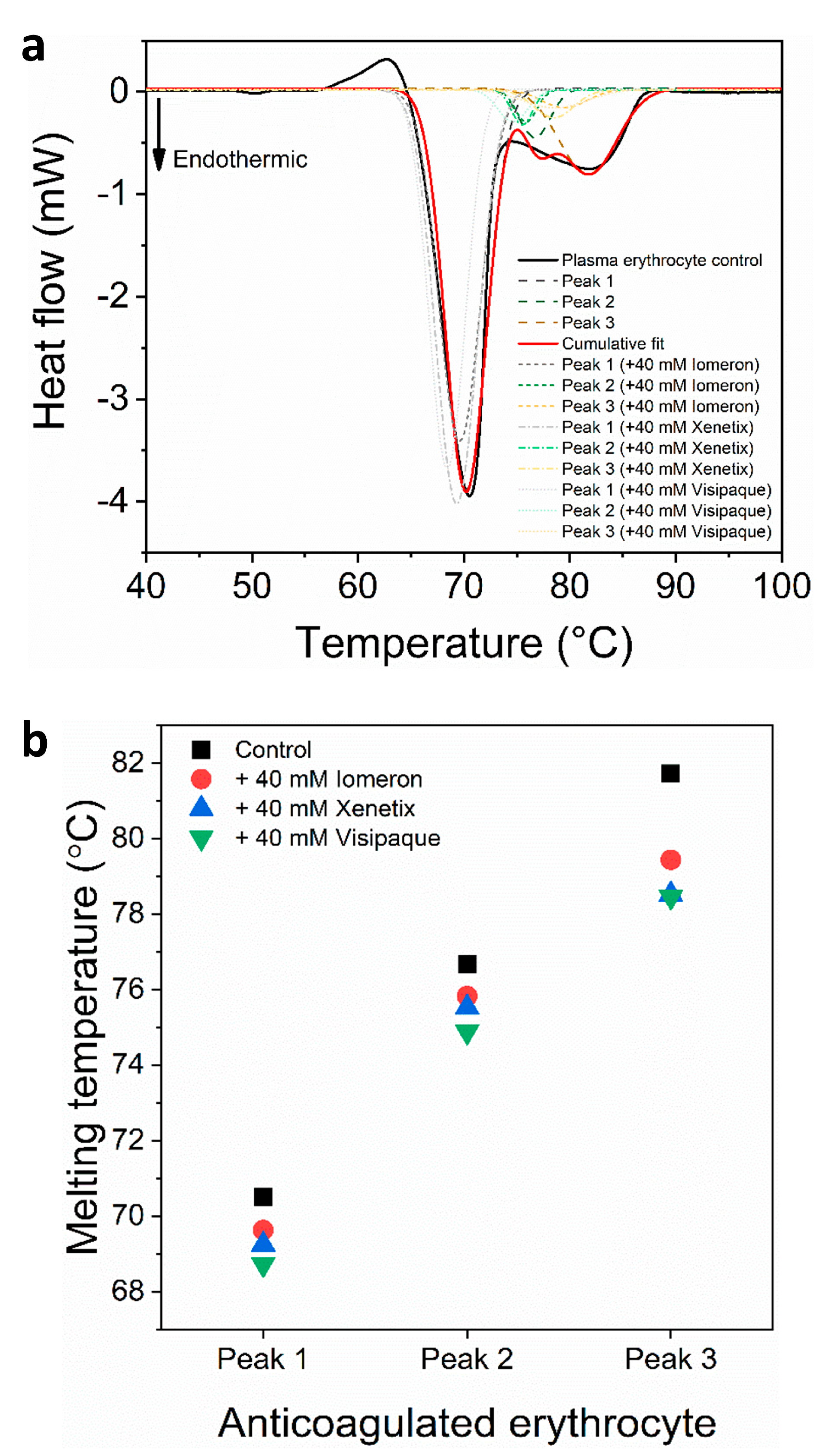
(a) Deconvoluted thermal denaturation curves of human erythrocytes of anticoagulated blood in the absence (experimental curve—solid black, cumulative fit—solid red and dashed lines) and in the presence of 40 mM Iomeprol, Iobitridol and Iodixanol (short dashed, short dashed dot and short dotted lines), respectively. (b) Schematic representation of the deconvoluted thermal transition peaks of anticoagulated erythrocyte as a function of the melting temperature in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque.
Figure 3.
(a) Deconvoluted thermal denaturation curves of human erythrocytes of anticoagulated blood in the absence (experimental curve—solid black, cumulative fit—solid red and dashed lines) and in the presence of 40 mM Iomeprol, Iobitridol and Iodixanol (short dashed, short dashed dot and short dotted lines), respectively. (b) Schematic representation of the deconvoluted thermal transition peaks of anticoagulated erythrocyte as a function of the melting temperature in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque.
Figure 4.
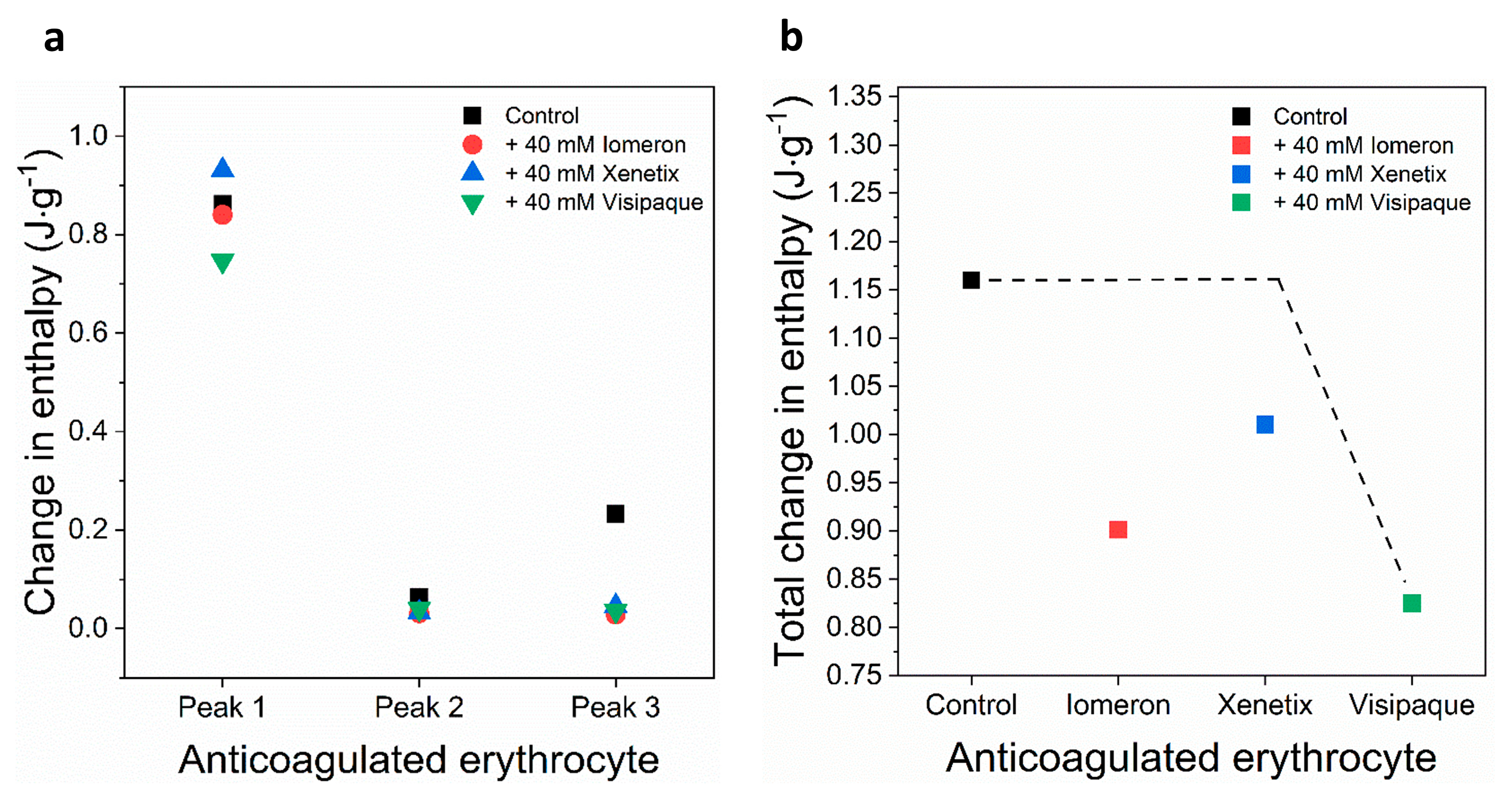
Schematic representation of the deconvoluted thermal transition peaks of anticoagulated erythrocyte as a function of the (a) calculated enthalpy change and (b) total enthalpy change in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque. The black dotted line represents the highest total change in enthalpy.
Figure 4.
Schematic representation of the deconvoluted thermal transition peaks of anticoagulated erythrocyte as a function of the (a) calculated enthalpy change and (b) total enthalpy change in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque. The black dotted line represents the highest total change in enthalpy.
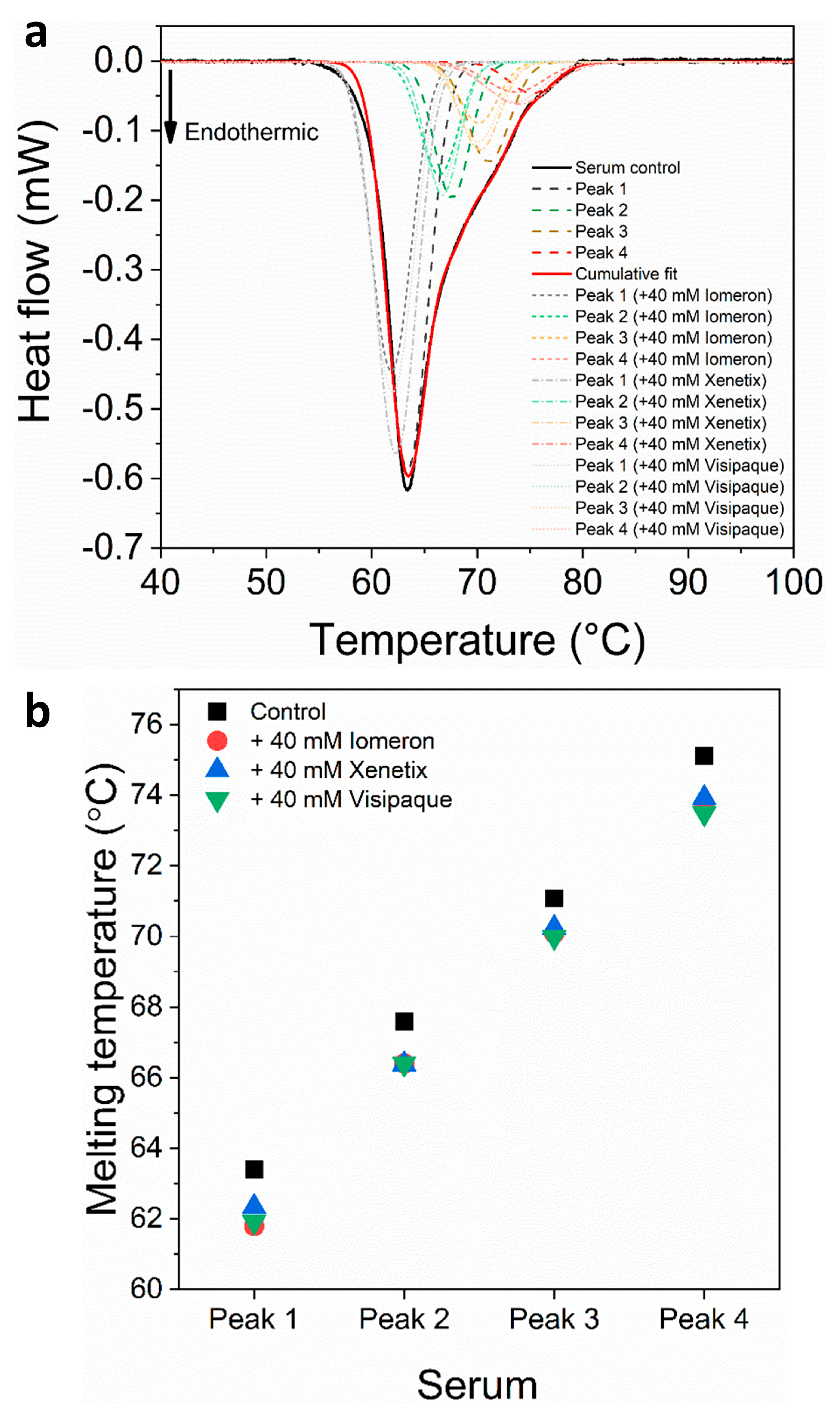
Figure 5.
(a) Deconvoluted thermal denaturation curves of human blood serum in the absence (experimental curve—solid black, cumulative fit—solid red and dashed lines) and in the presence of 40 mM Iomeprol, Iobitridol and Iodixanol (short dashed, short dashed dot and short dotted lines), respectively. (b) Schematic representation of the deconvoluted thermal transition peaks of non-anticoagulated serum as a function of the melting temperature in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque.
Figure 5.
(a) Deconvoluted thermal denaturation curves of human blood serum in the absence (experimental curve—solid black, cumulative fit—solid red and dashed lines) and in the presence of 40 mM Iomeprol, Iobitridol and Iodixanol (short dashed, short dashed dot and short dotted lines), respectively. (b) Schematic representation of the deconvoluted thermal transition peaks of non-anticoagulated serum as a function of the melting temperature in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque.
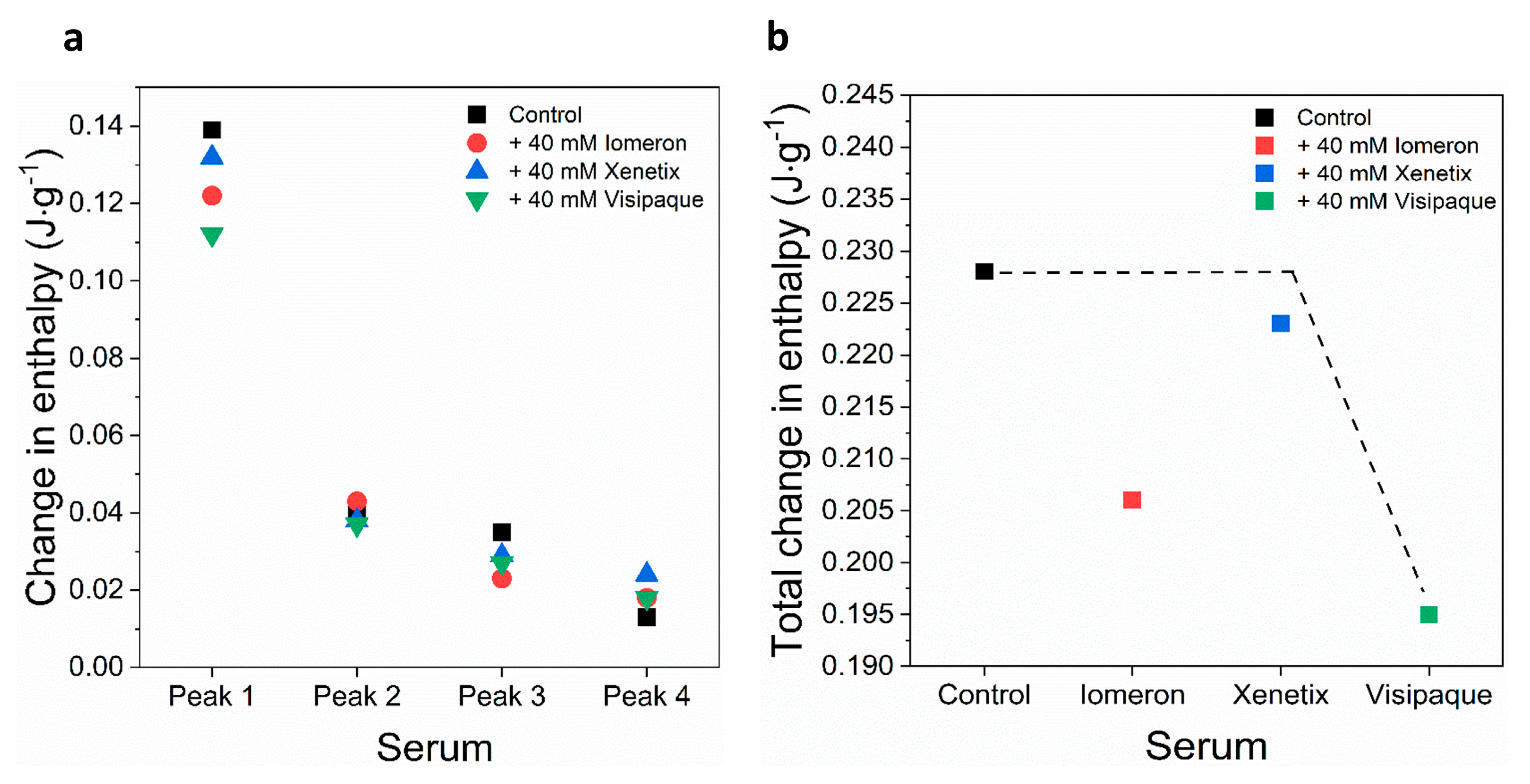
Figure 6.
Schematic representation of the deconvoluted thermal transition peaks of non-anticoagulated serum as a function of the (a) calculated enthalpy change and (b) total change in enthalpy in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque. The black dotted line represents the highest total change in enthalpy.
Figure 6.
Schematic representation of the deconvoluted thermal transition peaks of non-anticoagulated serum as a function of the (a) calculated enthalpy change and (b) total change in enthalpy in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque. The black dotted line represents the highest total change in enthalpy.
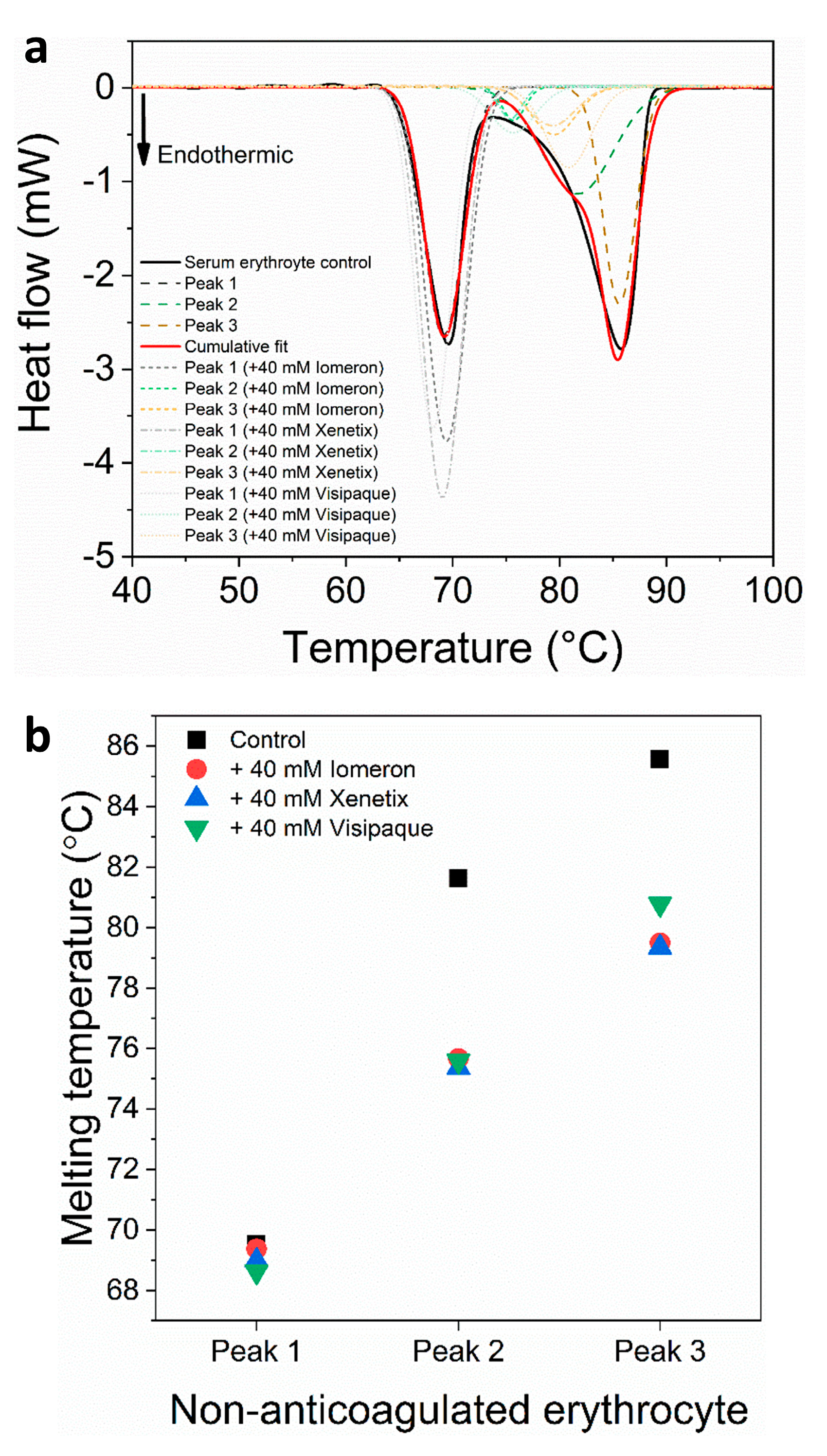
Figure 7.
(a) Deconvoluted thermal denaturation curves of human erythrocyte of non-anticoagulated blood in the absence (experimental curve—solid black, cumulative fit—solid red and dashed lines) and in the presence of 40 mM Iomeprol, Iobitridol and Iodixanol (short dashed, short dashed dot and short dotted lines), respectively. (b) Schematic representation of the deconvoluted thermal transition peaks of non-anticoagulated erythrocyte as a function of the melting temperature in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque.
Figure 7.
(a) Deconvoluted thermal denaturation curves of human erythrocyte of non-anticoagulated blood in the absence (experimental curve—solid black, cumulative fit—solid red and dashed lines) and in the presence of 40 mM Iomeprol, Iobitridol and Iodixanol (short dashed, short dashed dot and short dotted lines), respectively. (b) Schematic representation of the deconvoluted thermal transition peaks of non-anticoagulated erythrocyte as a function of the melting temperature in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque.
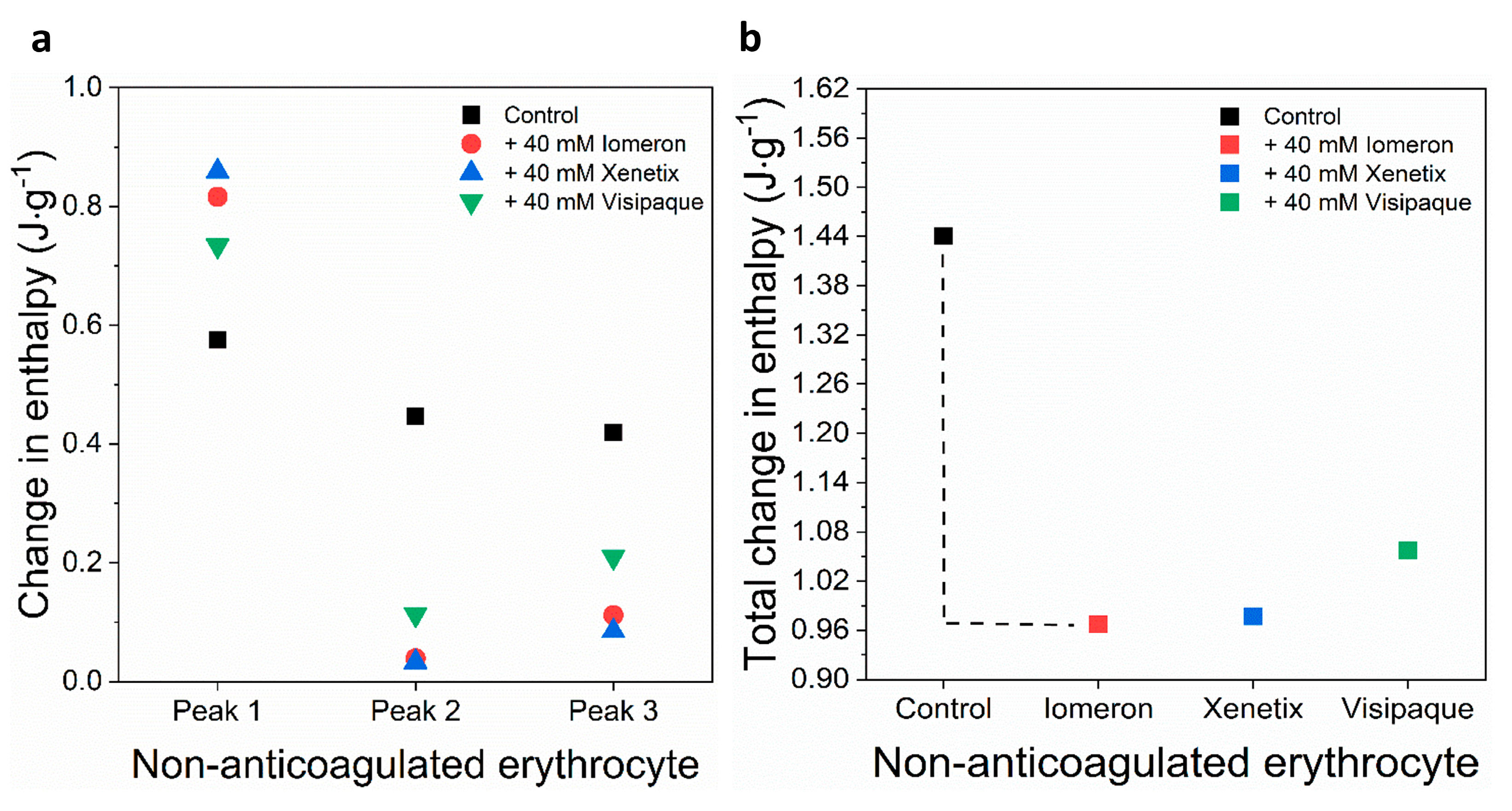
Figure 8.
Schematic representation of the deconvoluted thermal transition peaks of non-anticoagulated erythrocyte as a function of the (a) calculated enthalpy change and (b) total change in enthalpy in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque. The black dotted line represents the highest total change in enthalpy.
Figure 8.
Schematic representation of the deconvoluted thermal transition peaks of non-anticoagulated erythrocyte as a function of the (a) calculated enthalpy change and (b) total change in enthalpy in the absence and presence of 40 mM Iomeron, Xenetix and Visipaque. The black dotted line represents the highest total change in enthalpy.
Table 1.
The melting temperatures of the deconvoluted curves of anticoagulated plasma in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the melting temperatures of the control (Tmax1–4(c)), Iomeprol (Tmax1–4(i)), Xenetix (Tmax1–4(x)) and Visipaque (Tmax1–4(v)) treated samples.
Table 1.
The melting temperatures of the deconvoluted curves of anticoagulated plasma in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the melting temperatures of the control (Tmax1–4(c)), Iomeprol (Tmax1–4(i)), Xenetix (Tmax1–4(x)) and Visipaque (Tmax1–4(v)) treated samples.
| Blood | Melting Temperature of Anticoagulated Plasma (°C) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| Tmax1(c) | Tmax2(c) | Tmax3(c) | Tmax4(c) | Tmax1(i) | Tmax2(i) | Tmax3(i) | Tmax4(i) | Tmax1(x) | Tmax2(x) | Tmax3(x) | Tmax4(x) | Tmax1(v) | Tmax2(v) | Tmax3(v) | Tmax4(v) |
|---|
| | 62.9 | 67.3 | 71.2 | 75.4 | 61.8 | 66.4 | 70.1 | 74.8 | 62.3 | 66.4 | 70.2 | 74.2 | 61.7 | 66.9 | 70.9 | 74.3 |
Table 2.
Change in the enthalpy of the deconvoluted DSC plots of anticoagulated plasma in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the enthalpy changes of the control (ΔH1-4(c)), Iomeprol (ΔH1–4(i)), Xenetix (ΔH1–4(x)) and Visipaque (ΔH1–4(v)) treated samples.
Table 2.
Change in the enthalpy of the deconvoluted DSC plots of anticoagulated plasma in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the enthalpy changes of the control (ΔH1-4(c)), Iomeprol (ΔH1–4(i)), Xenetix (ΔH1–4(x)) and Visipaque (ΔH1–4(v)) treated samples.
| Blood | Change in Enthalpy of Anticoagulated Plasma (J·g−1) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| ΔH1(c) | ΔH2(c) | ΔH3(c) | ΔH4(c) | ΔH1(i) | ΔH2(i) | ΔH3(i) | ΔH4(i) | ΔH1(x) | ΔH2(x) | ΔH3(x) | ΔH4(x) | ΔH1(v) | ΔH2(v) | ΔH3(v) | ΔH4(v) |
|---|
| | 0.09 | 0.04 | 0.02 | 0.02 | 0.11 | 0.02 | 0.02 | 0.01 | 0.10 | 0.03 | 0.02 | 0.01 | 0.09 | 0.03 | 0.01 | 0.005 |
Table 3.
Summary of the total enthalpy change of the deconvoluted thermal transition curves of anticoagulated plasma in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque.
Table 3.
Summary of the total enthalpy change of the deconvoluted thermal transition curves of anticoagulated plasma in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque.
| Blood | Change in Total Enthalpy of Anticoagulated Plasma (J·g−1) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| ΔHtotal(c) | ΔHtotal(i) | ΔHtotal(x) | ΔHtotal(v) |
|---|
| | 0.18 | 0.17 | 0.18 | 0.14 |
Table 4.
The melting temperatures of the deconvoluted curves of anticoagulated erythrocyte components in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the melting temperatures of the control (Tmax1–3(c)), Iomeprol (Tmax1–3(i)), Xenetix (Tmax1–3(x)) and Visipaque (Tmax1–3(v)) treated samples.
Table 4.
The melting temperatures of the deconvoluted curves of anticoagulated erythrocyte components in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the melting temperatures of the control (Tmax1–3(c)), Iomeprol (Tmax1–3(i)), Xenetix (Tmax1–3(x)) and Visipaque (Tmax1–3(v)) treated samples.
| Blood | Melting Temperature of Anticoagulated Erythrocyte (°C) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| Tmax1(c) | Tmax2(c) | Tmax3(c) | Tmax1(i) | Tmax2(i) | Tmax3(i) | Tmax1(x) | Tmax2(x) | Tmax3(x) | Tmax1(v) | Tmax2(v) | Tmax3(v) |
|---|
| | 70.5 | 76.7 | 81.7 | 69.6 | 75.8 | 79.4 | 69.3 | 75.5 | 78.5 | 68.7 | 74.9 | 78.5 |
Table 5.
Change in the enthalpy of the deconvoluted DSC plots of anticoagulated blood components in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the enthalpy changes of the control (ΔH1–3(c)), Iomeprol (ΔH1–3(i)), Xenetix (ΔH1–3(x)) and Visipaque (ΔH1–3(v)) treated samples.
Table 5.
Change in the enthalpy of the deconvoluted DSC plots of anticoagulated blood components in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the enthalpy changes of the control (ΔH1–3(c)), Iomeprol (ΔH1–3(i)), Xenetix (ΔH1–3(x)) and Visipaque (ΔH1–3(v)) treated samples.
| Blood | Change in Enthalpy of Anticoagulated Erythrocyte (J·g−1) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| ΔH1(c) | ΔH2(c) | ΔH3(c) | ΔH1(i) | ΔH2(i) | ΔH3(i) | ΔH1(x) | ΔH2(x) | ΔH3(x) | ΔH1(v) | ΔH2(v) | ΔH3(v) |
|---|
| | 0.86 | 0.06 | 0.23 | 0.84 | 0.03 | 0.02 | 0.93 | 0.03 | 0.04 | 0.74 | 0.04 | 0.03 |
Table 6.
Summary of the total enthalpy change of the deconvoluted thermal transition curves of anticoagulated erythroctyte in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque.
Table 6.
Summary of the total enthalpy change of the deconvoluted thermal transition curves of anticoagulated erythroctyte in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque.
| Blood | Change in Total Enthalpy of Anticoagulated Erythrocyte (J·g−1) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| ΔHtotal(c) | ΔHtotal(i) | ΔHtotal(x) | ΔHtotal(v) |
|---|
| | 1.16 | 0.90 | 1.01 | 0.82 |
Table 7.
The melting temperatures of the deconvoluted curves of non-anticoagulated serum in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the melting temperatures of the control (Tmax1–4(c)), Iomeprol (Tmax1–4(i)), Xenetix (Tmax1–4(x)) and Visipaque (Tmax1–4(v)) treated samples.
Table 7.
The melting temperatures of the deconvoluted curves of non-anticoagulated serum in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the melting temperatures of the control (Tmax1–4(c)), Iomeprol (Tmax1–4(i)), Xenetix (Tmax1–4(x)) and Visipaque (Tmax1–4(v)) treated samples.
| Blood | Melting Temperature of Non-Anticoagulated Serum (°C) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| Tmax1(c) | Tmax2(c) | Tmax3(c) | Tmax4(c) | Tmax1(i) | Tmax2(i) | Tmax3(i) | Tmax4(i) | Tmax1(x) | Tmax2(x) | Tmax3(x) | Tmax4(x) | Tmax1(v) | Tmax2(v) | Tmax3(v) | Tmax4(v) |
|---|
| | 63.4 | 67.6 | 71.1 | 75.1 | 61.8 | 66.4 | 70.1 | 73.7 | 62.3 | 66.4 | 70.2 | 73.9 | 61.9 | 66.4 | 70.0 | 73.5 |
Table 8.
Change in the enthalpy of the deconvoluted DSC plots of non-anticoagulated serum in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the enthalpy changes of the control (ΔH1–4(c)), Iomeprol (ΔH1–4(i)), Xenetix (ΔH1–4(x)) and Visipaque (ΔH1–4(v)) treated samples.
Table 8.
Change in the enthalpy of the deconvoluted DSC plots of non-anticoagulated serum in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the enthalpy changes of the control (ΔH1–4(c)), Iomeprol (ΔH1–4(i)), Xenetix (ΔH1–4(x)) and Visipaque (ΔH1–4(v)) treated samples.
| Blood | Change in Enthalpy of Non-Anticoagulated Serum (J·g−1) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| ΔH1(c) | ΔH2(c) | ΔH3(c) | ΔH4(c) | ΔH1(i) | ΔH2(i) | ΔH3(i) | ΔH4(i) | ΔH1(x) | ΔH2(x) | ΔH3(x) | ΔH4(x) | ΔH1(v) | ΔH2(v) | ΔH3(v) | ΔH4(v) |
|---|
| | 0.13 | 0.04 | 0.03 | 0.01 | 0.12 | 0.04 | 0.02 | 0.01 | 0.13 | 0.03 | 0.02 | 0.02 | 0.11 | 0.03 | 0.02 | 0.01 |
Table 9.
Summary of the total enthalpy change of the deconvoluted thermal transition curves of non-anticoagulated serum in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque.
Table 9.
Summary of the total enthalpy change of the deconvoluted thermal transition curves of non-anticoagulated serum in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque.
| Blood | Change in Total Enthalpy of Non-Anticoagulated Serum (J·g−1) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| ΔHtotal(c) | ΔHtotal(i) | ΔHtotal(x) | ΔHtotal(v) |
|---|
| S | 0.22 | 0.20 | 0.22 | 0.19 |
Table 10.
The melting temperatures of the deconvoluted curves of non-anticoagulated erythrocytes in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the melting temperatures of the control (Tmax1–3(c)), Iomeprol (Tmax1–3(i)), Xenetix (Tmax1–3(x)) and Visipaque (Tmax1–3(v)) treated samples.
Table 10.
The melting temperatures of the deconvoluted curves of non-anticoagulated erythrocytes in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the melting temperatures of the control (Tmax1–3(c)), Iomeprol (Tmax1–3(i)), Xenetix (Tmax1–3(x)) and Visipaque (Tmax1–3(v)) treated samples.
| Blood | Melting Temperature of Non-Anticoagulated Erythrocyte (°C) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| Tmax1(c) | Tmax2(c) | Tmax3(c) | Tmax1(i) | Tmax2(i) | Tmax3(i) | Tmax1(x) | Tmax2(x) | Tmax3(x) | Tmax1(v) | Tmax2(v) | Tmax3(v) |
|---|
| | 69.6 | 81.6 | 85.6 | 69.4 | 75.7 | 79.5 | 69 | 75.4 | 79.4 | 68.6 | 75.6 | 80.8 |
Table 11.
Change in the enthalpy of the deconvoluted DSC plots of non-anticoagulated erythrocyte in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the enthalpy changes of the control (ΔH1–3(c)), Iomeprol (ΔH1–3(i)), Xenetix (ΔH1–3(x)) and Visipaque (ΔH1–3(v)) treated samples.
Table 11.
Change in the enthalpy of the deconvoluted DSC plots of non-anticoagulated erythrocyte in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque. The abbreviations correspond to the enthalpy changes of the control (ΔH1–3(c)), Iomeprol (ΔH1–3(i)), Xenetix (ΔH1–3(x)) and Visipaque (ΔH1–3(v)) treated samples.
| Blood | Change in Enthalpy of Non-Anticoagulated Erythrocyte (J·g−1) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| ΔH1(c) | ΔH2(c) | ΔH3(c) | ΔH1(i) | ΔH2(i) | ΔH3(i) | ΔH1(x) | ΔH2(x) | ΔH3(x) | ΔH1(v) | ΔH2(v) | ΔH3(v) |
|---|
| | 0.57 | 0.44 | 0.41 | 0.81 | 0.03 | 0.11 | 0.85 | 0.03 | 0.08 | 0.73 | 0.11 | 0.21 |
Table 12.
Summary of the total enthalpy change of the deconvoluted thermal transition curves of non-anticoagulated erythrocyte in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque.
Table 12.
Summary of the total enthalpy change of the deconvoluted thermal transition curves of non-anticoagulated erythrocyte in the absence and presence of 40 mM Iomeprol, Xenetix and Visipaque.
| Blood | Change in Total Enthalpy of Non-Anticoagulated Erythrocyte (J·g−1) |
|---|
| Control | Iomeron | Xenetix | Visipaque |
|---|
| ΔHtotal(c) | ΔHtotal(i) | ΔHtotal(x) | ΔHtotal(v) |
|---|
| | 1.44 | 0.96 | 0.97 | 1.05 |